Vitamin D deficiency is a global health problem. Nutritional rickets is only the tip of iceberg representing widespread vitamin D deficiency with important public health implications(Reference Aparna, Muthathal and Nongkynrih1). There is a bouquet of treatment regimens for nutritional rickets incorporating vitamin D3 and/or Ca. Till not so long ago, rickets was treated with mega doses (300 000–600 000 IU) of vitamin D3 (Reference Mittal, Rai and Shah2–Reference Bothra, Gupta and Jain5). Since then, the therapeutic efficacy and safety of lower doses of vitamin D3 (90 000–300 000 IU) for treatment of rickets have also been shown(Reference Cesur, Caksen and Gündem6–Reference Akcam, Yildiz and Yilmaz10). The Global Consensus Guidelines recommend that daily oral vitamin D3 at doses of 2000–4000 IU for at least 12 weeks duration may be used for treating nutritional rickets(Reference Munns, Shaw and Kiely11). Daily dosage is considered to be more physiological and safer, although poor compliance and higher cost can be major concerns. However, studies directly comparing the therapeutic efficacy and safety of daily v. depot vitamin D3 for rickets in doses recommended by Global Consensus are scant. Stogmann et al.(Reference Mittal, Yadav and Khadgawat8) reported comparable efficacy of 400 000 IU vitamin D3 (given staggered as 200 000 IU on day 1 and day 3, orally) and daily 9600 IU of vitamin D3 given as drops for 18 d for rickets. Similarly, Akcam et al.(Reference Stögmann, Sacher and Blümel9) found a comparable response in terms of radiological healing of rickets in children who received single dose of 600 000 IU of oral vitamin D3 or 20 000 IU/d of oral vitamin D3 for 30 d.
Hence, we conducted this trial to compare the efficacy of a low dose oral vitamin D3 given as depot v. daily oral vitamin D3 for 12 weeks’ duration in under-five children with nutritional rickets in terms of improvement in serum 25-hydroxyvitamin D (25(OH)D) levels and radiological healing. We also intended to compare the two regimens in terms of improvement measured by proportion of children with hypocalcaemia, hypophosphataemia, raised serum alkaline phosphatase (ALP) and raised parathormone (PTH) following treatment.
Methods
This open label, randomised placebo-controlled study was conducted at a tertiary care hospital in Delhi, India, between November 2018 and April 2020. The study was approved by the Institutional Ethics Committee of the University College of Medical Sciences, New Delhi (IECHR/2018/36/116).
Sample size calculation
Sample size estimation was based on the study by Wadia et al. (Reference Wadia, Soon and Chivers12) wherein depot oral vitamin D3 therapy was compared with daily oral vitamin D3 in children with vitamin D insufficiency. The mean difference of serum 25(OH)D levels between baseline and after 16 weeks of therapy was 36 nmol/l in the daily group and 23 nmol/l in the depot group with standard deviation of change (post minus pre) in both groups of 17 nmol/l. To detect a difference of change in mean serum 25(OH)D levels of 13 nmol/l after 12 weeks of therapy between both groups at 80 % power and 5 % type I error with 1:1 ratio and two-sided, a sample size of twenty-eight per group was needed. Considering attrition of 15 %, a sample size of thirty-three per group was estimated.
Enrolment
We assessed children aged 3 months to 5 years in the paediatric out-patient department for eligibility. Children with clinical (wrist widening, knock knees, bowed legs, frontal bossing, short stature, rachitic rosary, etc.), biochemical (raised serum ALP with/without hypocalcaemia and/or hypophosphataemia) and radiological evidence of rickets were eligible for inclusion. Radiological rickets was diagnosed based on Thacher score (TS) of radiographs of both knees and wrists(Reference Thacher, Fischer and Pettifor13); scoring was done by a radiologist who was blinded for treatment allocation. TS ≥ 1·5 was considered as radiological rickets. Critically ill children, those having known malabsorption disorders, liver or renal insufficiency, or hypercalcaemia, were excluded. Children with a history of having received vitamin D, Ca supplements or drugs affecting vitamin D metabolism (anticonvulsants, steroids, cancer chemotherapy) in previous 6 months were also excluded. Informed written consent was obtained from the child’s caregiver.
Baseline assessment
We obtained a detailed history and performed a complete physical examination including anthropometric measurements. We estimated weight for age Z score, height for age Z score, weight for height Z score and mid-upper arm circumference Z score using AnthroCal software(Reference Nagori14) and WHO reference charts(15). We assessed serum Ca, phosphate, albumin, ALP, 25(OH)D and PTH levels at baseline. Radiographs of both wrists and knees were performed at baseline for all participants to assess severity of rickets.
Randomisation and allocation concealment
We randomised sixty-six consecutive children with nutritional rickets to two groups (daily or depot vitamin D3 regimens) using a computer-generated block randomisation sequence using eleven blocks of size 6 each at www.randomization.com. The randomisation code was generated and concealed by a person not associated with the study. Allocation to either group was done using a sealed envelope technique.
Intervention, monitoring and follow-up
Children in the ‘daily’ group (n 33) received oral vitamin D3 given as drops (3–12 months: 2000 IU (2·5 ml once daily), 12 months to 5 years: 4000 IU (5 ml once daily); Vitanova® drops, 800 IU/ml; Zuventus Healthcare Limited) for 12 weeks. Children in ‘depot’ group (n 33) received a single oral dose of vitamin D3 granules (3–12 months: 60 000 IU (1 sachet), 12 months to 5 years: 150 000 IU (2·5 sachets); Vitanova® granules, one sachet = 60 000 IU, Zuventus Healthcare Limited) dissolved completely in 50 ml of milk under supervision. To enable accurate dispensing of vitamin D dose, we used a high precision (nearest 0·01 g) table top digital weighing scale (Mettler Toledo ®) to divide one sachet into two, where ever necessary. For exclusively breastfed babies, we counselled the mothers to express their breast milk and vitamin D3 granules were added to about 50 ml of expressed breast milk and then fed by cup/spoon. All children received daily oral Ca for 12 weeks (3–12 months: 250 mg; 12 months to 5 years: 500 mg; Syrup Coralium®; 5 ml = 200 mg of elemental Ca, Zuventus Healthcare Limited). If any child vomited the oral drug within 15 min of administration, it was noted and the drug was administered again per orally. Caregivers in the ‘daily’ group were provided with extra doses as buffer to cover for any wastage due to spillage or vomiting of the drug and they were asked to note the number of times they had to readminister the dose. One research team member (RS) was entrusted with the responsibility of ensuring compliance by contacting caregivers telephonically at least once a week; report of any adverse events was also made. Compliance was checked by asking the parents to return the empty bottles of medicine to RS during their scheduled hospital visit. At the 4 weeks of follow-up visit, we assessed serum Ca, phosphate, albumin and ALP. We measured serum Ca, phosphate, albumin, ALP, 25(OH)D, PTH levels and performed radiographic assessment of both wrists and knees, at the 12 weeks follow-up.
Definition and measurement of outcomes
The primary outcome was the change in serum 25(OH)D following therapy at 12 weeks. The secondary outcomes were proportion of children with radiological healing (TS < 1·5), vitamin D deficiency, hypocalcaemia (serum Ca < 8·5 mg/dl)(Reference Fong and Khan16), hypophosphataemia (serum phosphorous < 3·8 mg/dl)(Reference Lo, Kliegman, Stanton and St Geme17), hypercalcaemia (serum Ca > 10·8 mg/dl)(Reference Dewan, Gupta, Menon and Ramji18), raised serum ALP (> 420 IU/l in infants, > 320 IU/l in 12 months to 5 years)(Reference Lo, Kliegman, Stanton and St Geme17) and hyperparathyroidism (serum PTH > 65 ng/l)(Reference Dewan, Gupta, Menon and Ramji18) at 12 weeks. Vitamin D status ascertained by serum 25(OH)D levels was defined as: deficiency < 30 nmol/l, severe deficiency < 12·5 nmol/l, insufficient 30–50 nmol/l, sufficient 50 – 250 nmol/l and toxicity > 250 nmol/l(Reference Munns, Shaw and Kiely11).
Laboratory assessment
A venous blood sample (3 ml) was collected in vacutainers, spun and the serum stored at −20°C. Serum 25(OH)D and PTH levels were estimated after thawing the stored sera using RIA-based kits (Beckman Coulter India Pvt. Ltd). Serum Ca, P, ALP and albumin were estimated using Beckman Coulter Unicel Dxc 600 automatic analyser. Serum Ca was corrected for serum albumin (corrected Ca (mg/dl) = measured Ca (mg/dl) + 0·8 ×(4·0 – serum albumin (g/dl))(Reference Orrell19).
Statistical analysis
The analysis was done using Statistical Package for the Social Sciences software version 26(20). Shapiro–Wilk test was used to test for normal distribution of data. Means and standard deviations of age, change in serum 25(OH) vitamin D, serum 25(OH) vitamin D, Ca, phosphorus, ALP and PTH levels were compared between both groups using unpaired t test. Median (interquartile range (IQR)) was compared between the two groups using Mann–Whitney U test. Proportions of children with radiological healing, vitamin D deficiency, hypocalcaemia, hypophosphataemia, raised ALP and hyperparathyroidism between the two groups were compared using χ 2 test or Fisher’s exact test. Change in serum 25(OH)D levels within a group was assessed using paired t test. P value < 0·05 was considered as significant. A sensitivity analysis was performed to compare the response to daily v. depot vitamin D supplements in the subgroup of vitamin D deficient children.
Results
Figure 1 depicts the enrolment of participants in the trial.
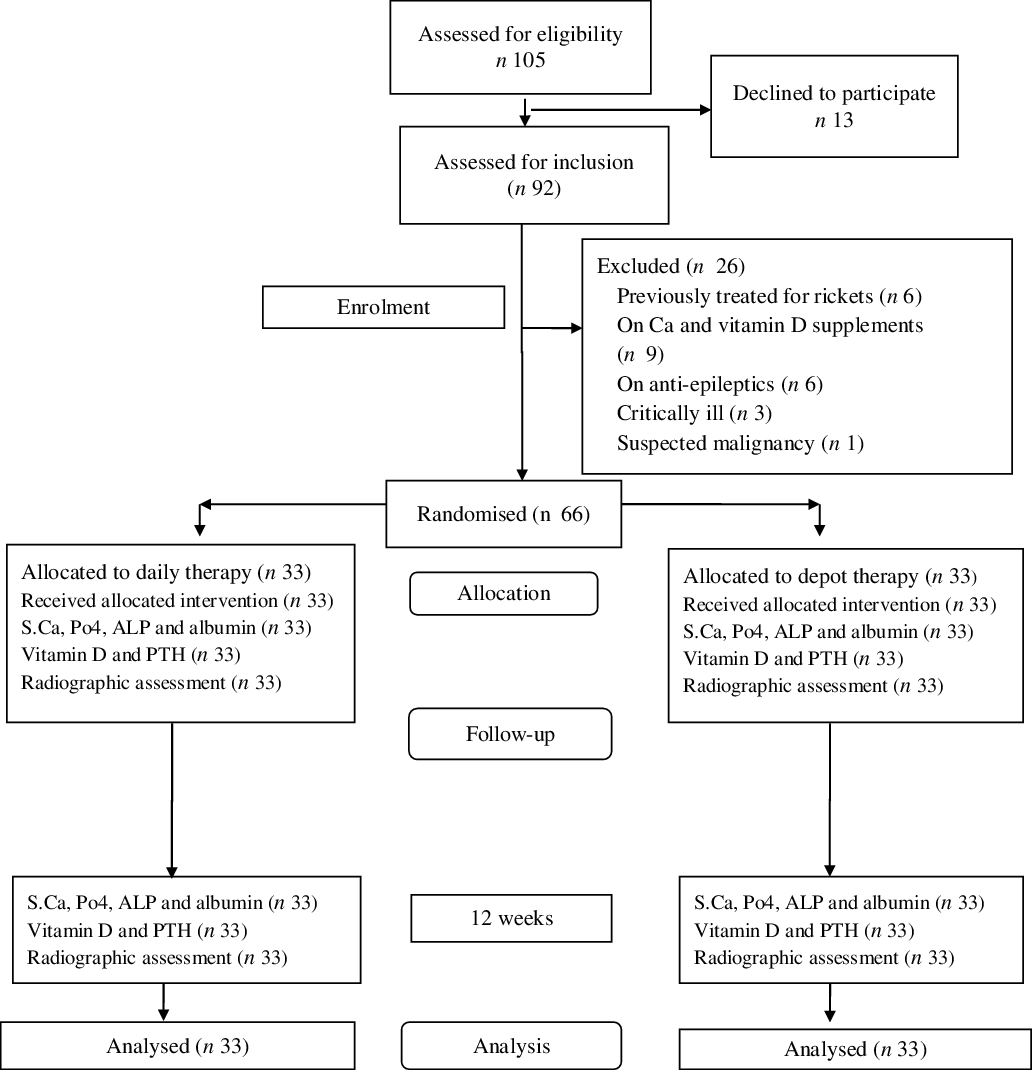
Fig. 1. Flow of participants in the study entitled ‘Low Dose Depot Oral Vitamin D3 v. Daily Oral Vitamin D3 for Treating Nutritional Rickets: a Randomised Clinical Trial’.
Baseline characteristics
Between December 2018 and November 2019, we enrolled sixty-six children (forty-six boys, twenty girls) with mean age 20·9 (sd 10·6, range = 9 to 60) months; thirty-three each allocated to either treatment arm. Twenty-one percent of participants were aged ≤ 12 months. Overall, 77·2 % of participants had delay in gross motor milestones, 27·2 % had irritability, 15·1 % had bony pains and 12·1 % had convulsions at the time of presentation to the hospital. The clinical signs on examination included wrist widening (90·9 %), frontal bossing (65·1 %), protuberant abdomen (42·4 %), rachitic rosary (36·3 %), bowing of legs (36·3 %), delayed closure of fontanelles (16·6 %) and knock knees (3 %). The baseline characteristics of sixty-six children enrolled are shown in Table 1. Sixty percent of children were vitamin D deficient at presentation; 40 % of these had severe vitamin D deficiency. Children receiving daily and depot vitamin D3 had comparable means and standard deviations levels of baseline serum Ca, phosphorus, ALP, 25(OH)D and PTH as shown in Table 1. The median TS of children in both groups at enrolment was statistically comparable (daily 8 (IQR 4·5–10); depot 8 (IQR 4·2–10), P = 0·62).
Table 1. Baseline characteristics of children receiving low dose depot oral vitamin D3 v. daily oral vitamin D3 for treating nutritional rickets
(Mean values and standard deviations; numbers and percentages)

IQR, interquartile range.
* P value > 0·05 for tests of differences between the thirty-three children each in daily and depot vitamin D3 group for all parameters.
Follow-up
Both groups showed a good biochemical response to treatment at 4 weeks with a significant (P < 0·001) rise in mean levels of serum Ca (daily 9·3 (sd 0·6) mg/dl; depot 9·3 (sd 0·5) mg/dl, P = 0·93), phosphorus (daily 3·9 (sd 0·8) mg/dl; depot 4·4 (sd 0·9) mg/dl, P = 0·02) and a significant (P < 0·001) fall in serum ALP levels (daily 399 (sd 182) IU/l; depot 323 (sd 142) IU/l, P = 0·03) compared with baseline estimates.
At 12 weeks, the serum Ca, phosphorous, ALP and PTH had normalised in almost all children, and the proportions of children with hypocalcaemia, hypophosphataemia, raised ALP, raised PTH or vitamin D deficiency were comparable in both the groups as shown in Table 2. At 12 weeks, the means and standard deviations serum 25(OH)D levels increased significantly in both groups (P < 0·001); the rise was comparable in both groups (daily 90·5 (sd 86·5) nmol/l; depot 82·7 (sd 77) nmol/l; P = 0·70). No child had severe vitamin D deficiency, although two children continued to have vitamin D deficiency and another three had vitamin D insufficiency. At 12 weeks, the median (IQR) TS decreased significantly in both groups (P < 0·001); the median TS was comparable in both groups (daily 0 (IQR 0–0); depot 0 (IQR 0–0); P = 0·44). Radiographs at 12 weeks revealed healing (TS < 1·5) in 93·9 % children (31 per group) and 90·9 % children had TS of 0 (31 in daily group and 29 in depot group). Two children in the depot group had TS of 1 each, three children (two in depot group and one in daily group) had TS of 2 each and one child in daily group had TS score of 4·5, although none of them had evidence of vitamin D deficiency, hypocalcaemia or hypophosphataemia. Overall, twenty-six children in the daily group and twenty-eight children in the depot group had neither biochemical nor radiological evidence of rickets (P = 0·52).
Table 2. Laboratory characteristics of children in daily and depot oral vitamin D3 at 12 weeks
(Mean values and standard deviations; numbers and percentages)

ALP, alkaline phosphatase; TS, Thacher score.
* P values for tests of differences between the thirty-three children each in daily and depot vitamin D3 group.
A sensitivity analysis done in vitamin D deficient subgroup did not reveal any significant difference in therapeutic effects of either regimen as depicted in Table 3.
Table 3. Laboratory characteristics of children with vitamin D deficiency in daily and depot groups at 0, 4 and 12 weeks
(Mean values and standard deviations)

ALP, alkaline phosphatase.
* P values for tests of differences between the 19 vitamin D deficient children in the daily and 21 vitamin D deficient children in the depot vitamin D3 groups.
Compliance and adverse effects
All children were compliant with therapy, and there was no loss to follow-up. Hypervitaminosis D (serum 25(OH)D > 250 nmol/l) was seen in three children in the daily group and one child in the depot group, although they remained asymptomatic.
Discussion
We found that oral low dose depot of vitamin D3 (60 000 IU in children aged 3–12 months and 150 000 IU in children aged 1–5 years) is an effective and safe alternative to daily oral vitamin D3 given for 12 weeks’ duration for treating nutritional rickets in under-five children without any increased risk of hypercalcaemia. Both the regimens achieved comparable clinical, biochemical and radiological resolution without any adverse effects of therapy.
At 4 weeks follow-up, we found that both groups showed significant rise in serum Ca and phosphorus and fall in serum ALP; the serum phosphorus levels being significantly higher in the depot group compared with daily group. At 12 weeks, however, the serum phosphorus was comparable in both treatment groups and hence this early rise in serum phosphorus in the depot group may be of little clinical benefit.
The increase in mean serum 25(OH)D levels was comparable in both groups despite the fact that the cumulative vitamin D3 dose in the daily group is twice that in the depot group. A subgroup analysis of the effect of the two treatment strategies in vitamin D deficient children with rickets showed that both regimens were equally effective in terms of biochemical and radiological resolution of rickets as well as increase in serum 25(OH)D levels. In contrast, another study(Reference Wadia, Soon and Chivers12) has shown that in vitamin D deficient children, daily oral vitamin D3 supplements have fared better than low dose oral depot (100 000–200 000 IU) vitamin D3 doses in achieving and maintaining normalcy in serum vitamin D levels, although they increase the risk for hypervitaminosis D and hypercalcaemia.
We found that three children in the daily group developed hypervitaminosis D and one child in the depot group developed hypervitaminosis D, although these children were asymptomatic and without concomitant hypercalcaemia. Similar observations have been reported previously with the use of low dose oral vitamin D bolus in rickets(Reference Mittal, Yadav and Khadgawat8,Reference Akcam, Yildiz and Yilmaz10) . None of the children in either groups had serum 25(OH)D levels exceeding 350 mmol/l reiterating the safety of both regimens. No child developed hypercalcaemia in either groups. Previously, hypercalcaemia was shown to be more likely with the use of higher oral depot doses of vitamin D (600 000 IU and 300 000 IU) compared with low dose (150 000 IU) oral vitamin D bolus(Reference Cesur, Caksen and Gündem6).
We found that four children (two per group) had TS ≥ 1·5 at the end of 12 weeks which might suggest the need for continued supplementation of vitamin D3 and Ca beyond 12 weeks in rickets. Contrasting to our figures of 91 %, Chatterjee et al. (Reference Chatterjee, Gupta and Sharma21) showed that only 47 % of children with rickets who received 600 000 IU of parenteral vitamin D3 had TS of 0 after 12 weeks in rickets. This emphasises that radiological healing may take more than 12 weeks and continued therapy may be needed in a few cases of rickets.
The strengths of our study include a head-to-head comparison of a single low dose depot oral vitamin D3 with daily vitamin D3 therapy. A comprehensive assessment of clinical, biochemical and radiological parameters in a homogenous cohort empowers our study. A robust follow-up and good compliance by all participants was possible in our study as one of the research team members was dedicated to carrying out follow-up. Limitations of our study include the fact that we did not estimate hypercalciuria, another marker of safety profile of vitamin D supplementation. Only 21 % of our participants were aged ≤ 12 months and hence focused studies on infants evaluating efficacy of these regimens would be preferred. A prolonged follow-up at 6–12 months post-treatment would be preferred to assess complete radiological resolution in the children with less than complete healing at 12 weeks and whether serum 25(OH)D sufficiency is sustained in the children post-treatment.
Conclusions
A low dose oral depot vitamin D3 is an effective regimen to treat nutritional rickets in under-five children. Compared with the daily oral vitamin D3 regimen, it offers the advantage of convenience and ease of administration.
Acknowledgements
The authors are grateful to Zuventus Healthcare Limited for providing the vitamin D drops and sachets and oral Ca carbonate syrup for this trial, without any preconditions.
This work was supported by the Indian Council of Medical Research: Grant Award Number: 3/2/July-2019/PG-Thesis-HRD. No author has any financial relationship relevant to this article to disclose.
R. S.: study design, acquisition of data and analysis, drafting the manuscript, approved the final version. P. D.: conceptualised the study, study design, acquisition of data, analysis and interpretation of data, drafted the article and revised it critically for important intellectual content, and approved of the final version. S. G.: study design, analysis and interpretation of data, critical inputs while drafting the manuscript and approved of the final version. S. M.: study design, laboratory support, data collection, analysis and interpretation of data, critical inputs while drafting the manuscript and approved the final version. S. B.: study design, laboratory support, data collection, analysis and interpretation of data, critical inputs while drafting the manuscript and approved the final version. P. G.: study design, analysis and interpretation of data, critical inputs while drafting and revising the manuscript and approved the final version. The authors declare that there are no conflicts of interest.







