Introduction
New technologies, leading to the miniaturization of electronic devices, have resulted in the increased use of tantalum. Use of tantalum-based capacitors, in particular, is on the increase in automotive electronics, mobile phones, personal computers and wireless devices (Mackay and Simandl, Reference Mackay and Simandl2014). On the other hand, there is an increasing interest in Ta in terms of the ethical aspects involved in its extraction. A significant amount of the Ta produced comes from non-sustainable operations. Around 62% of the world's tantalum supply comes from Africa, particularly from Burundi, Democratic Republic of Congo (DRC) and Rwanda (Roskill, 2016), where its exploitation can cause conflicts (such as war) and involves child labour (Smith et al., Reference Smith, Cazier, Fox and Kitunda2012). Brazil and China also contribute to the global tantalum supply, with ~19% of the supply coming from the Pitinga and Mibra mines in Brazil, and ~12% of supply coming from the Yichun granite in China. The tantalum market has important ethical aspects that should be taken into account when considering sustainable exploitation (Bleischwitz et al., Reference Bleischwitz, Dittrich and Pierdicca2012) and new sources should be developed to diversify geographic sources of supply for strategic reasons (Mackay and Simandl, Reference Mackay and Simandl2014).
Traditionally, the majority of tantalum was exploited from pegmatites. However, rare-metal granites also contain significant amounts of this metal (Černý et al., Reference Černý, Blevin, Cuney and London2005; Linnen et al., Reference Linnen, Samson, Williams-Jones, Chakhmouradian, Holland and Turekian2014) and have been studied extensively (Cuney et al., Reference Cuney, Marignac and Weisbrod1992; Helba et al., Reference Helba, Trumbull, Morteani, Khalil and Arslan1997; Belkasmi and Cuney, Reference Belkasmi and Cuney1998; Rub et al., Reference Rub, Štemprok and Rub1998; Belkasmi et al., Reference Belkasmi, Cuney, Pollard and Bastoul2000; Huang et al., Reference Huang, Wang, Chen, Hu and Liu2002; Stepanov et al., Reference Stepanov, Mavrogenes, Meffre and Davidson2014; Melcher et al., Reference Melcher, Graupner, Gäbler, Sitnikova, Henjes-Kunst, Oberthür, Gerdes and Dewaele2015). The scarcity of this metal makes it essential that further research, on granites, be done.
In addition, several aspects related to the genesis of Ta ore deposits remain unsolved. The transport and fractionation of Ta in the magmatic-hydrothermal transition, and the possible influence of subsolidus processes in Ta precipitation, in pegmatites and granites, have generated considerable controversy. Many authors consider that Ta has an affinity for melt, as supported by experimental results (Linnen and Keppler, Reference Linnen and Keppler1997; Linnen, Reference Linnen1998; London, Reference London2008) and field evidence in pegmatites (Dewaele et al., Reference Dewaele, Hulsbosch, Cryns, Boyce, Burgess and Muchez2015) and granites (Huang et al., Reference Huang, Wang, Xie, Zhu, Erdmann, Che and Zhang2015; López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017). Other cases provide evidence that late exsolved hydrothermal fluids during the late stages of magmatic crystallization are responsible for the Ta-richest mineralization (Belkasmi and Cuney, Reference Belkasmi and Cuney1998; Dostal et al., Reference Dostal, Kontak, Gerel, Shellnutt and Fayek2015; Neiva et al., Reference Neiva, Gomes and Silva2015; Zhu et al., Reference Zhu, Wang, Che, Zhu, Wei and Huang2015). This hypothesis was maintained by evidence from the textures of Nb-Ta oxide minerals from granites and pegmatites (Zhu et al., Reference Zhu, Wang, Che, Zhu, Wei and Huang2015). Therefore, the study of Nb-Ta mineralization in granites is of great interest from both an economical and scientific point of view.
The rare-metal granite at Penouta is a Sn, Ta-rich leucogranite that belongs to the Sn-W European Variscan belt. In the Iberian Massif this belt contains abundant W, Sn and Ta deposits (e.g. Gonzalo Corral and Gracía Plaza, Reference Gonzalo Corral and Gracía Plaza1985; Charoy and Noronha, Reference Charoy and Noronha1996; Llorens and Moro, Reference Llorens and Moro2010, Reference Llorens and Moro2012; Canosa et al., Reference Canosa, Martín-Izard and Fuertes-Fuente2012; Chicharro et al., Reference Chicharro, Martín-Crespo, Gómez-Ortiz, López-García, Oyarzun and Villaseca2015; Llorens González et al., Reference Llorens González, García Polonio, López Moro, Fernández-Fernández, Sans Contreras and Moro Benito2017; López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017).
The Penouta deposit was exploited from Roman times until 1985 (IGME, 1976). The re-opening of this deposit is now being investigated. The measured and indicated resources are reported to be 95.5 Mt with an average grade of 77 ppm Ta and 443 ppm Sn (Llorens González et al., Reference Llorens González, García Polonio, López Moro, Fernández-Fernández, Sans Contreras and Moro Benito2017). The aim of the present study was to present a mineralogical and geochemical study of Sn and Nb-Ta minerals in this granite in order to determine the influence of magmatic and hydrothermal processes on the formation of this mineralization.
Geological setting
The Penouta deposit is located in the Central Iberian Zone of the Iberian Variscan Massif, near the contact with the Asturoccidental–Leonese Zone (Fig. 1), according to the classification of Julivert et al. (Reference Julivert, Fontboté, Ribeiro and Nabais Conde1972). In the Central Iberian Zone the Variscan orogeny generated most of the extant structures, the internal deformation and the metamorphism. During the first Variscan deformation phase (D1), a low-grade slaty cleavage (S1) and recumbent folds were developed in the northern domain of the CIZ. The second Variscan deformation event yielded thrusting toward the external zones, crustal anatexis and a pervasive subhorizontal tectonic foliation (S2) linked to extension in the internal domains. The Variscan D3 phase led to upright folds, open to tight folds and occasionally a crenulation cleavage (S3) associated with subvertical shear zones with a dextral wrench component (Iglesias and Choukroune, Reference Iglesias and Choukroune1980).
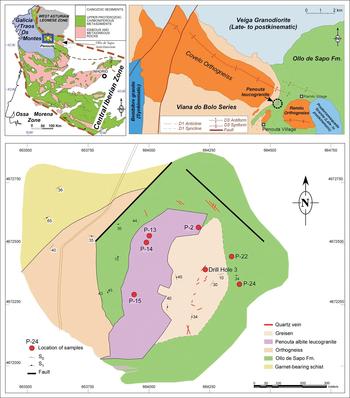
Fig. 1. Detailed geological map of the Penouta deposit showing the locations of the samples. Upper left: location of Penouta in the Iberian Variscan Massif; upper right: geological sketch of the Ollo de Sapo Anticlinorium in its western region (modified from López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017).
The Penouta deposit is located in the hinge of a D1 Variscan antiform (Fig. 1) that is obliterated by the D3 Ollo de Sapo anticlinorium. This megastructure follows a NW–SE direction (Fig. 1) and forms a continuous outcrop that extends ~300 km, from the island of Coelheira (Lugo) to the point where it disappears under the Tertiary materials of the Duero Basin (Arribas and Mangas, Reference Arribas and Mangas1991; Arias et al., Reference Arias, Farias and Marcos2002) (Fig. 1).
The metamorphic rocks of the area consist of a lower part which crops out in the core of a gneiss dome around the Viana do Bolo village, the so-called Viana do Bolo Series (Ferragne, Reference Ferragne1972); this is overlain by the Ollo de Sapo Formation. The Viana do Bolo Series constitutes the oldest material in the area and is a metamorphic complex consisting of quartzites, followed by mica-schists with garnet and Ca-silicate rocks which crop out NE of Penouta, and orthogneisses deformed during the Variscan orogeny, which crop out in the Covelo and Ramilo localities, near Penouta (Díez Montes, Reference Díez Montes2006) (Fig. 1). The lower part of the series is considered to be Lower Cambrian (Arias et al., Reference Arias, Farias and Marcos2002) but the age of the upper part is not well known and a range of ages from Middle Cambrian to the lowermost Ordovician has been reported (Díez et al., Reference Díez, Martínez Catalán and Bellido Mulas2010). These materials are overlain by the Ollo de Sapo Formation, which is a volcanogenic sequence consisting of fine- to coarse-grained massive gneisses with ocellar texture.
With regard to igneous rocks in the area, synkinematic granites are scarce and crop out close to Viana do Bolo village (Bembibre granite), whereas late to post-kinematic granodiorites (Veiga Granodiorite) and granites (Pradorramisquedo granite) are more important volumetrically (Fig. 1).
The Penouta deposit is a greisenized cupola with four units: albite leucogranite, aplite-pegmatite dykes, greisen and quartz veins hosted in the metamorphic country rock (Llorens González et al., Reference Llorens González, García Polonio, López Moro, Fernández-Fernández, Sans Contreras and Moro Benito2017). The Penouta stock is a leucogranitic body that intruded the Viana do Bolo Series and Ollo de Sapo Formation. Study of the outcrops has revealed that the granite intruded following planar anisotropies developed in the country rock corresponding to the regional D2 Variscan fabric. There are no ages available for the Penouta granite and only a relative dating can be inferred from structural and contact relationships. The lack of foliation and significant internal deformation in this body are features typical of the late- to post-Variscan, similar to the Veiga and Pradorramisquedo plutons (Fig. 1), which have been inferred to be related to a strike-slip shear zone (Vegas et al., Reference Vegas, Aranguren, Cuevas and Tubía2001), this stage being similar in age (~308 Ma, Gutiérrez-Alonso et al., Reference Gutiérrez-Alonso, Collins, Fernández-Suárez, Pastor-Galán, González-Clavijo, Jourdan, Weil and Johnston2015) to other Sn-bearing granites dated recently in the CIZ (e.g. Logrosán Sn-(W) ore deposit, 308 ± 1 Ma, Chicharro et al., Reference Chicharro, Martín-Crespo, Gómez-Ortiz, López-García, Oyarzun and Villaseca2015).
The Penouta albite granite is similar in composition to other albite granites in the Iberian Massif, most of them located in the innermost part of the Iberian Variscan Belt, namely, in the Central Iberian Zone. Most of these albite leucogranites were affected by an intense albitization, kaolinitization and greisenization (Mangas and Arribas, Reference Mangas and Arribas1991; Clauer et al., Reference Clauer, Fallick, Galán, Aparicio, Miras, Fernández-Caliani and Aubert2015), probably related to fluid saturation in the apical zone of the leucogranite (López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017).
Analytical methods
Granite samples were obtained from the ancient open pit of the Penouta mine (Fig. 1), corresponding to the apical zone. Whole-rock samples were analysed by Activation Laboratories (ACTLABS), Ontario, Canada. Major elements were analysed by X-ray fluorescence (XRF). Minor elements were measured using inductively coupled plasma-mass spectrometry (ICP-MS) from acid digestion of fused glass beads. Fusion was obtained using lithium or sodium borate. More details of sample preparation and analysis can be found in ACTLABS (2017).
Petrographic and mineralogical characterizations were carried out by powder X-ray diffraction (XRD), optical microscopy and scanning electron microscopy (SEM). The XRD spectra were measured from powdered samples in a Bragg-Brentano PANAnalytical X'Pert Diffractometer (graphite monochromator, automatic gap, Kα-radiation of Cu at λ = 1.54061 Å, powered at 45 kV–40 mA, scanning range 4–100° with a 0.017°2θ step scan and a 50 s measuring time. Identification and Rietveld semi-quantitative evaluation of phases were done using the PANanalytical X'Pert HighScore software.
Scanning electron microscopy with energy-dispersive spectral analysis (SEM–EDS) was used in back-scattered electron (BSE) mode. Electron microprobe analysis was used to establish the chemistry of the minerals. Analyses were carried out using a JEOL JXA-8230 electron microprobe located at the Centres Cientifics i Tecnològics de la Universitat de Barcelona. Analyses were conducted at an accelerating voltage of 20 kV, an electron beam current of 20 nA, and a beam diameter of 2 μm. Each element was counted for 5 s, except Ti, Sc and Pb, which were counted for 10 s and F for 15 s. Standards used were: Nb (NbLα), Ta (TaLα), Fe2O3 (FeKα), rhodonite (MnKα), rutile (TiKα), ThO2 (ThMα), UO2 (UMβ), Sn (SnLα), W (WLα), Sc (ScKα), albite (NaKα), apatite (FKα), wollastonite (CaKα), galena (PbMα), ZrO2 (ZrLα) and YAG (YLα). The detection limits are 0.17 wt.% U, 0.1 wt.% Y, Th and W, 0.06 wt.% Ta, Sn and Nb and <0.03 wt.% for other elements. The structural formulae were calculated on the basis of 24 oxygens and 12 cations per formula unit (apfu) for CGM. The number of cations was fixed by a method of charge balance by conversion of part of Fe2+ to Fe3+ as proposed by Ercit et al. (Reference Ercit, Hawthorne and Černý1992a). The structural formula of tapiolite and cassiterite were calculated on the basis of 6 and 4 oxygens, respectively. The structural formula of microlite was calculated on the basis of a fully occupied B site (Nb + Ta + W + Ti = 2 apfu) and OH− was calculated by charge-balancing to the anion total of 7 (Černý et al., Reference Černý, Chapman and Ferreira2004). The wodginite formula was calculated according to the general formula ABC 2O8 (Z = 4); A = Mn2+, Fe2+, Li, □, B = Sn4+, Ti, Fe3+, Ta; C = Ta, Nb (Ercit et al. Reference Ercit, Hawthorne and Černý1992a,Reference Ercit, Černý, Hawthorne and McGammonb,Reference Ercit, Černý and Hawthornec). When the C site had >2 apfu, the excess was corrected by extraction of Ta to the B site. The Fe3+ was calculated for the B site (Ti + Sn4+ + Zr + Hf + Fe3+) fully occupied, with 1 apfu.
Mineral liberation analysis (MLA) was performed on a polished section prepared with a concentrated sample at the University of Tasmania using an FEI MLA 650 ESEM to obtain textural and compositional information from a large number of particles.
Results
Petrography and geochemistry of the leucogranite
The Penouta leucogranite is a phaneritic inequigranular rock, with fine to medium grain size. It consists of quartz, albite, white mica and microcline, as essential constituents. The XRD analyses indicate that quartz varies from 15 to 67 wt.%, albite from 2 to 40 wt.%, muscovite from 8 to 35 wt.%, microcline from 4 to 18 wt.%, and there is sporadic kaolinite.
Accessory minerals are spessartine, almandine, tourmaline, cassiterite, Nb-Ta oxides, beryl, apatite, monazite, xenotime, zircon, rutile, pyrite and native bismuth, fluorite, uraninite, Fe, Mn oxides, stannite, molybdenite, chalcopyrite and arsenopyrite.
Quartz occurs at least in two generations, the first of which is euhedral to subhedral crystals in a snowball texture, 0.8–2 mm in size, similar to other rare-metal granites (Charoy and Noronha, Reference Charoy and Noronha1996; Helba et al., Reference Helba, Trumbull, Morteani, Khalil and Arslan1997; Abdalla et al., Reference Abdalla, Helba and Mohamed1998; Huang et al., Reference Huang, Wang, Chen, Hu and Liu2002; Zhu et al., Reference Zhu, Wang, Che, Zhu, Wei and Huang2015). Quartz phenocrysts are rich in mineral inclusions consisting mainly of albite but also of other minerals, such as muscovite, arranged concentrically along growth planes (Fig. 2). The second generation of quartz consists of anhedral grains of <3 mm in size. Zircon also occurs as euhedral crystals that are typically zoned.
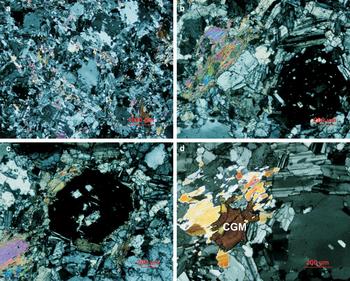
Fig. 2. Transmitted light photomicrographs of the Penouta granite: (a) general view of the porphyritic texture; (b,c) details of the snowball texture of quartz; (d) columbite-group crystal (CGM) associated with muscovite.
The feldspars are mainly albite. Most of the K-feldspar found is microcline with euhedral crystals, up to 2 mm in size, which often exhibit tartan twinning. Preliminary microprobe analyses of microcline yield up to 1.1 wt.% of Rb2O and up to 0.17 wt.% of Cs2O.
Two generations of white micas are distinguished. The first generation consists of Rb- and Cs-poor small crystals and the second generation is consists of much larger crystals, of ~0.5–1 mm in size, and shows textures of replacement. EDS analyses reveal greater contents of incompatible elements in this this second generation, as Rb2O and Cs2O.
A widespread albitization and kaolinitization is common throughout the leucogranite outcrops. Kaolinite represents ~5–10 wt.% of all minerals, but locally can reach up to 50 wt.%.
The major- and trace-element compositions of the Penouta leucogranite and the associated greisen from the open pit are given in Table 1. The leucogranite is peraluminous, with an A/(CNK) (Al2O3/CaO + Na2O + K2O) index of 1.8, and P-poor, with 0.03–0.07 wt.% P2O5. However, in the greisen this can reach as much as 0.32 wt.% P2O5. The SiO2 content varies between 69 and 75 wt.%, whereas in the kaolinitic alteration and in the greisen it is less, up to 67.39 wt.%. The Na2O content of leucogranite varies between 4 and 6 wt.%.
Table 1. Representative chemical composition of the Penouta leucogranite and the associated greisen.
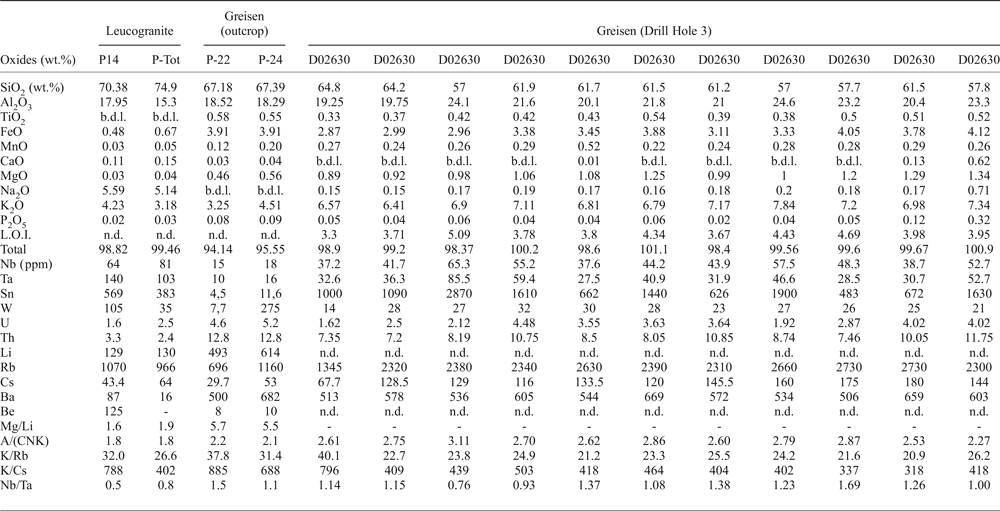
b.d.l.: below detection limit; n.d.: not determined; A/(CNK): molar Al2O3/(CaO + Na2O + K2O).
The Li content in the leucogranite is 80–226 ppm, whereas in the greisen it is greater, between 420 and 614 ppm. The Rb content is 900–1500 ppm in the leucogranite and 696–2730 in the greisen and Cs is 30–65 ppm and 29.7–180 ppm, respectively. The Ta and Nb contents are less in the greisen relative to the granite; in the leucogranite the Ta contents are 81–140 ppm, Nb = 51–81 ppm and Sn up to 569 ppm and in the greisen, Ta is 6.3–85.5 ppm, Nb = 9–65.3 ppm and Sn = 4.5–1900 ppm.
The Mg/Li ratio is a good indicator of the degree of fractionation of granites and pegmatites, being in the range 1.7–50 for fertile granites (Selway et al., Reference Selway, Breaks and Tindle2005). This ratio in the Penouta leucogranite ranges from 0.8 to 1.9, indicative of a fertile granite (Černý, Reference Černý, Moller, Černý and Saupe1989; Selway et al., Reference Selway, Breaks and Tindle2005).
According to Černý (Reference Černý, Moller, Černý and Saupe1989) and Selway et al. (Reference Selway, Breaks and Tindle2005) the K/Rb in fertile granites is 42–270, and the K/Cs 1600–15,400. These criteria are exceeded by the results from the Penouta leucogranite for which K/Rb is 26.6–31.9 and K/Cs is 402–788, both indicative of even higher fertility. In addition the greisen has a Nb/Ta ratio >1.
Mineralogy and chemistry of Nb-Ta-Sn oxides
Columbite-group minerals are the most abundant Nb-Ta rich phases in the Penouta leucogranite although tapiolite, microlite and wodginite occur also. Cassiterite can also contain significant amounts of Nb and Ta. Although Nb-Ta oxide minerals can occur in the greisen and pegmatites from the Penouta deposit (Llorens González et al., Reference Llorens González, García Polonio, López Moro, Fernández-Fernández, Sans Contreras and Moro Benito2017), they occur mainly in the leoucogranite, and only these are presented here.
Columbite-group minerals
Columbite-group minerals occur as platy crystals usually <200 µm in size, with an average size of 80 μm. Crystals usually constitute isolated grains but are occasionally associated with quartz, muscovite, plagioclase, cassiterite, zircon and other Nb-Ta rich minerals such as wodginite and microlite.
These minerals usually exhibit a zoned texture with Nb-rich cores and Ta-rich rims (Figs 3, 4). Occasionally this zonation is progressive, but in other cases there is a sharp boundary between the zones, so that the Ta-rich phase could be considered as an overgrowth. Moreover, these rims are partially dissolved (Fig. 3). Oscillatory zoning also occurs, with multiple bright Ta-rich, and dark Nb-rich bands. Reverse zoning, where the core is Ta-rich and rims are Nb-rich (Lahti, Reference Lahti1987) is quite abundant. This zoning is relatively common in Nb-Ta-group minerals (Belkasmi et al., Reference Belkasmi, Cuney, Pollard and Bastoul2000; Neiva et al., Reference Neiva, Gomes, Ramos and Silva2008; Abdalla et al., 1998; Anderson et al., Reference Anderson, Lentz, McFarlane and Falck2013). Irregular patchy zoning is also present, which is usual in CGM from rare-metal granites (Tadesse and Zerihun, Reference Tadesse and Zerihun1996) and pegmatitic occurrences (Alfonso et al., Reference Alfonso, Corbella and Melgarejo1995; Tindle and Breaks, Reference Tindle and Breaks2000; Uher et al., Reference Uher, Žitňan and Ozdín2007). In other cases these minerals exhibit convoluted zoning or are homogeneous. Zoning often shows a complex combination of different types, such as oscillatory and patchy. Patchy zonation has been interpreted as evidence for replacement involving partial resorption of an early columbite and the generation of a more Ta-rich composition usually along the margins of crystals (Abdalla et al., Reference Abdalla, Helba and Mohamed1998).
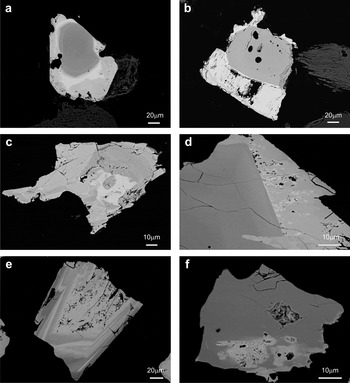
Fig. 3. Back-scattered SEM images of CGM from the Penouta leucogranite: (a) typical zoned crystal with a Nb-rich core (dark) and a Ta-rich rim (bright); (b) columbite crystal with an overgrowth of tantalite; (c) columbite-tantalite with patchy zoning (d) Columbite with a rim of tantalite; (e) reverse oscillatory zoning, with a dissolution texture in the innermost Ta-rich phase; (f) columbite with a Ta replacement.
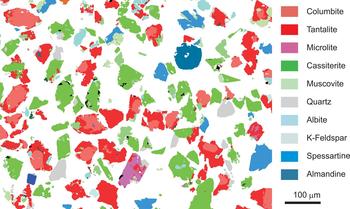
Fig. 4. MLA map from a concentrate of Nb-Ta oxide minerals from Penouta. The common texture of CGM, i.e. a Nb-rich core and a Ta-rich rim, is observed and the association of columbite–tantalite with quartz and muscovite is noted.
Many of the CGM exhibit corrosion or dissolution with sponge-like textures that affect the columbite rims, and the tantalite rims in particular. This corrosion destroys columbite that is replaced by tantalite. Similar dissolution textures are evident in other Nb-Ta minerals from pegmatites (e.g. Wise and Brown, Reference Wise and Brown2010; Dill et al., Reference Dill, Dohrmann, Kaufhold and Balaban2015).
The chemical composition of 490 points of CGM from ~130 crystals was determined (Table 1). Atomic compositions and ratios were calculated and plotted on the columbite quadrilateral, where most compositions are manganotantalite and manganocolumbite (Fig. 5). The Mn/(Mn + Fe) ratio varies between 0.33 and 0.97 and the Ta/(Ta + Nb) ratio between 0.07 and 0.93. These values are typical of highly evolved magmatic systems. Two clusters can be observed, both at Mn/(Mn + Fe) = 0.8, one at Ta/(Ta + Nb) = 0.1–0.2 and the other at Ta/(Ta + Nb) = 0.5–0.8, that correspond to the cores and rims of the crystals, respectively. The TiO2 content is <0.5 wt.%, except in one crystal, where TiO2 ranges from 2.17 to 2.76 wt.%. This crystal is also relatively Y-rich, varying from 1.09 to 1.30 wt.% Y2O3.
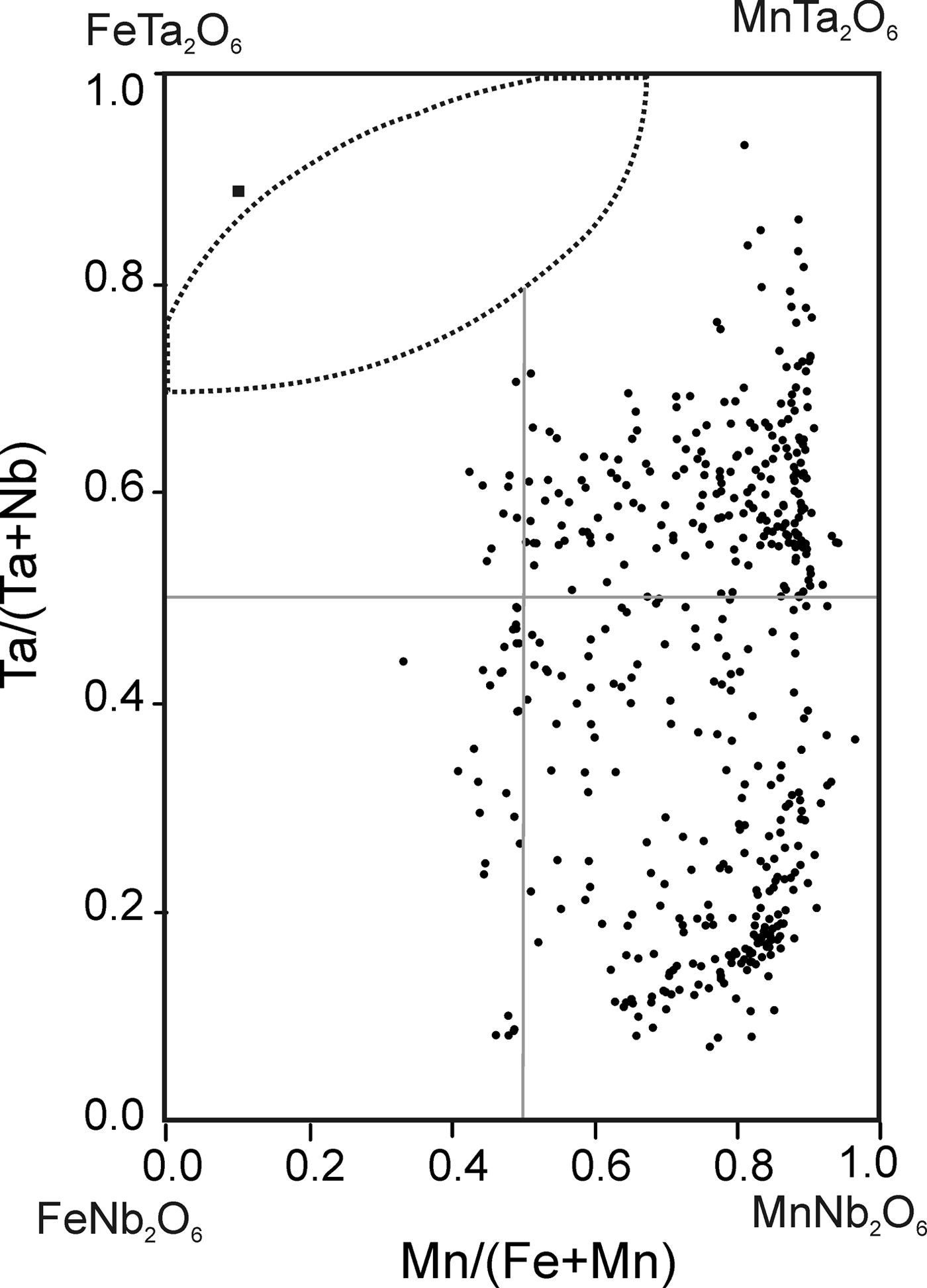
Fig. 5. Composition of the CGM (dots) and tapiolite (square) from the Penouta leucogranite in the columbite quadrilateral (atomic ratios).
Columbite-group minerals are relatively poor in minor elements (Table 2). Microprobe analyses reveal variable contents of Sn and W, usually <1 wt.%, although they can occasionally reach 2.71 and 2.54 wt.%, respectively. Ca, Y, U and Th are negligible. The ZrO2 content is up to 0.28 wt.% and HfO2 up to 0.16 wt.%. Sb and Bi are below the detection limits of the EMPA.
Table 2. Representative chemical composition of CGM and tapiolite from the Penouta leucogranite.
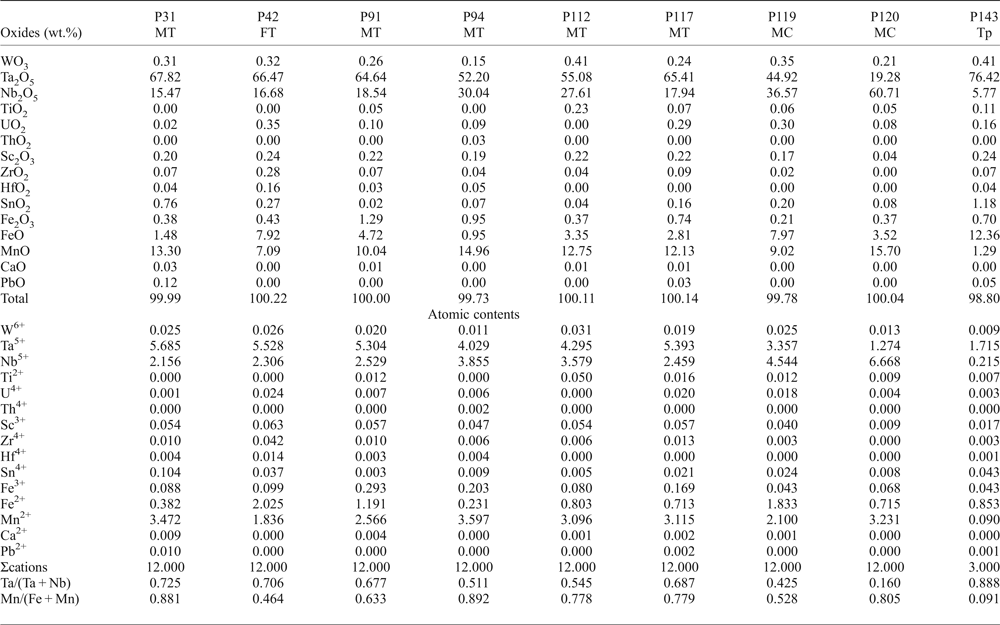
MT – manganotantalite; FT – ferrotantalite; MC – manganocolumbite; Tp – tapiolite.
Ferrotapiolite
Tapiolite is rare; only one crystal of 10 µm was detected as an inclusion in cassiterite. Its chemical composition shows a Ta/(Ta + Nb) ratio of 0.89 and Mn/(Mn + Fe) ratio of 0.11 (Table 2).
Microlite
Primary euhedral microlite crystals are rare and only a few micrometres in size. This mineral usually occurs as a late phase associated with CGM. In most cases microlite is enclosed in tantalite and cassiterite. According to the MLA data only 12 wt.% of the microlite crystals are ‘free’, whereas 24 wt.% were in contact with tantalite and 14 wt.% with cassiterite. Representative compositions of microlite are given in Table 3. Microlite is always Ta-rich, with Ta/(Ta + Nb) values varying between 0.91 and 0.99. Its composition ranges from 64.00 to 80.27 wt.% of Ta2O5 and 2.36 to 16.92 wt.% of Nb2O5; TiO2 reaches 0.25 wt.% and SnO2 varies between 0.38 and 3.44 wt.% . The occupation of the A site varies considerably; Ca is the most abundant cation, up to 11.89 wt.%, but U and Pb can also be significant, with up to 6.01 and 8.46 wt.%, respectively. Plumbomicrolite occurs as a thin rim around microlite (Fig. 6). Na2O contents reach 5.86 wt.%, whereas SnO reaches 3 wt.%. Fluorine is present in small amounts, up to 0.17 wt.%. Antimony and Bi were not detected.
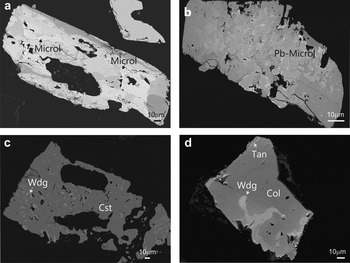
Fig. 6. Back-scattered SEM images of Na-Ta oxides from the Penouta leucogranite: (a) columbite–tantalite crystal with microlite (Microl) enclosed (bright); (b) microlite replacement of columbite with bright rims composed of plumbomicrolite (Pb-Microl); (c) wodginite (Wdg) inclusions in cassiterite (Cst); (d) wodginite replacement in CGM, with a Nb-rich core (dark), and a Ta-rich rim (Tan) (bright).
Table 3. Representative chemical composition (wt.%) of microlite from the Penouta leucogranite.
Wodginite
Wodginite occurs associated with cassiterite and tantalite (Fig. 6) as late replacements or inclusions. The differentiation between ixiolite and wodginite is based on the structure, but stoichiometric criteria can also be used (Wise et al., Reference Wise, Černý and Falster1998). As shown in Table 4, most analyses fit well with the formula of wodginite. The B and C sites are completed and only A-site values are slightly less than that of the ideal formula (Table 4). This may be due to the presence of Li or vacancies (Ercit et al., Reference Ercit, Hawthorne and Černý1992a).
Table 4. Representative chemical composition (wt.%) of wodginite from the Penouta leucogranite.
Table 5. Representative chemical composition (wt.%) of cassiterite from the Penouta leucogranite.
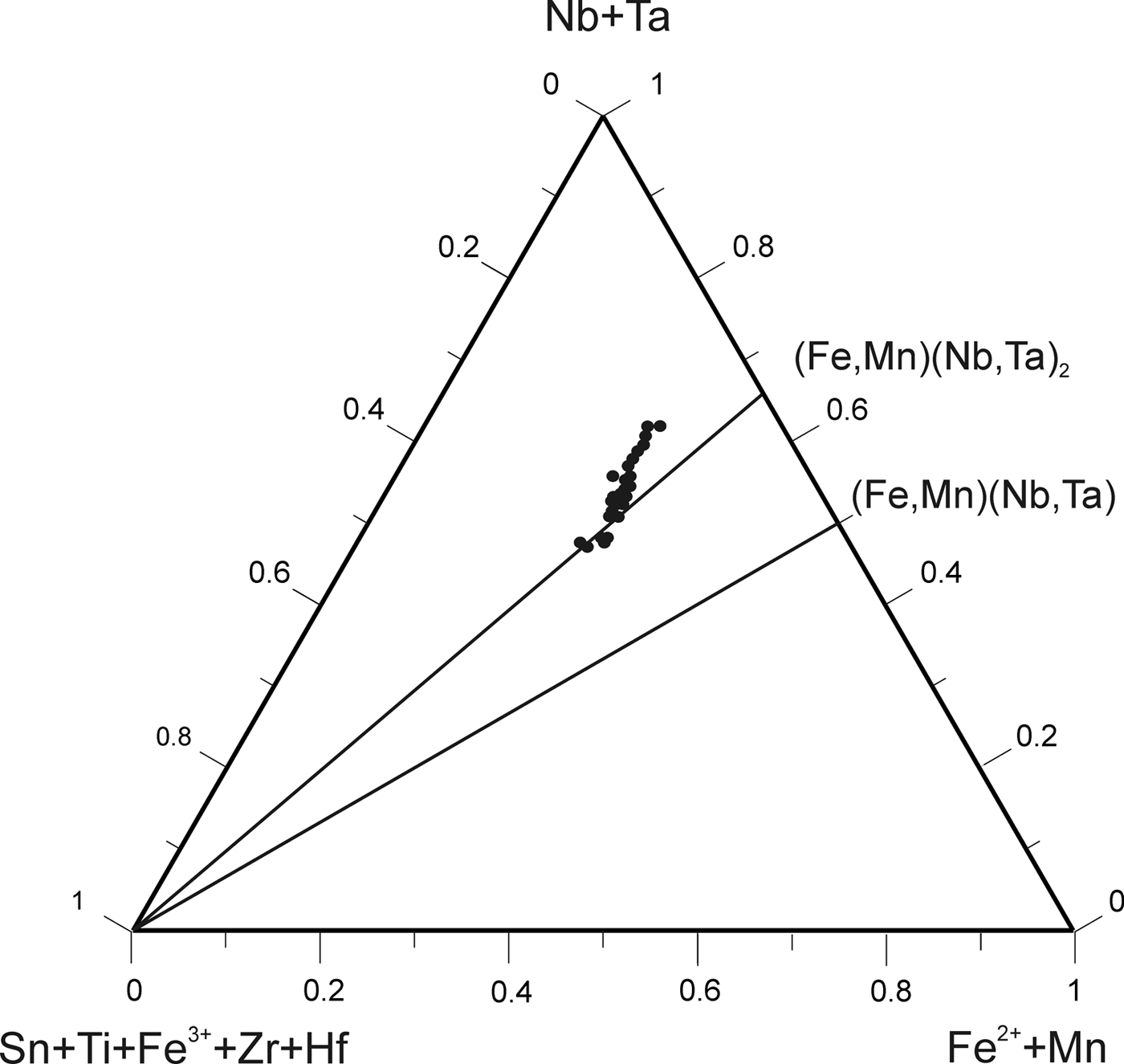
According to the classification criteria of Ercit et al. (Reference Ercit, Černý, Hawthorne and McGammon1992b), most of the wodginite group minerals analysed are wodginite and only some compositions are of ferrowodginite. Ta2O5 ranges from 48.36 to 65.35 wt.%., SnO2 from 14.63 to 19.37, MnO from 4.36 to 10.91. W is usually <1 wt.% but can reach 4.19 wt.%. The W content is correlated with the total Fe (Fig. 7). Hf and Zr are present in relatively large amounts, up to 1.34 wt.% of HfO2 and 1.29 wt.% of ZrO2. The average Zr/Hf ratio is ~0.7. Such large amounts have only been reported by Černý et al. (Reference Černý, Ercit, Smeds, Groat and Chapman2007) for granitic pegmatites. In contrast with Černý et al. (Reference Černý, Ercit, Smeds, Groat and Chapman2007) there is a slight correlation here between the Ta/(Ta + Nb) and Hf/(Hf + Zr) (Fig. 8).
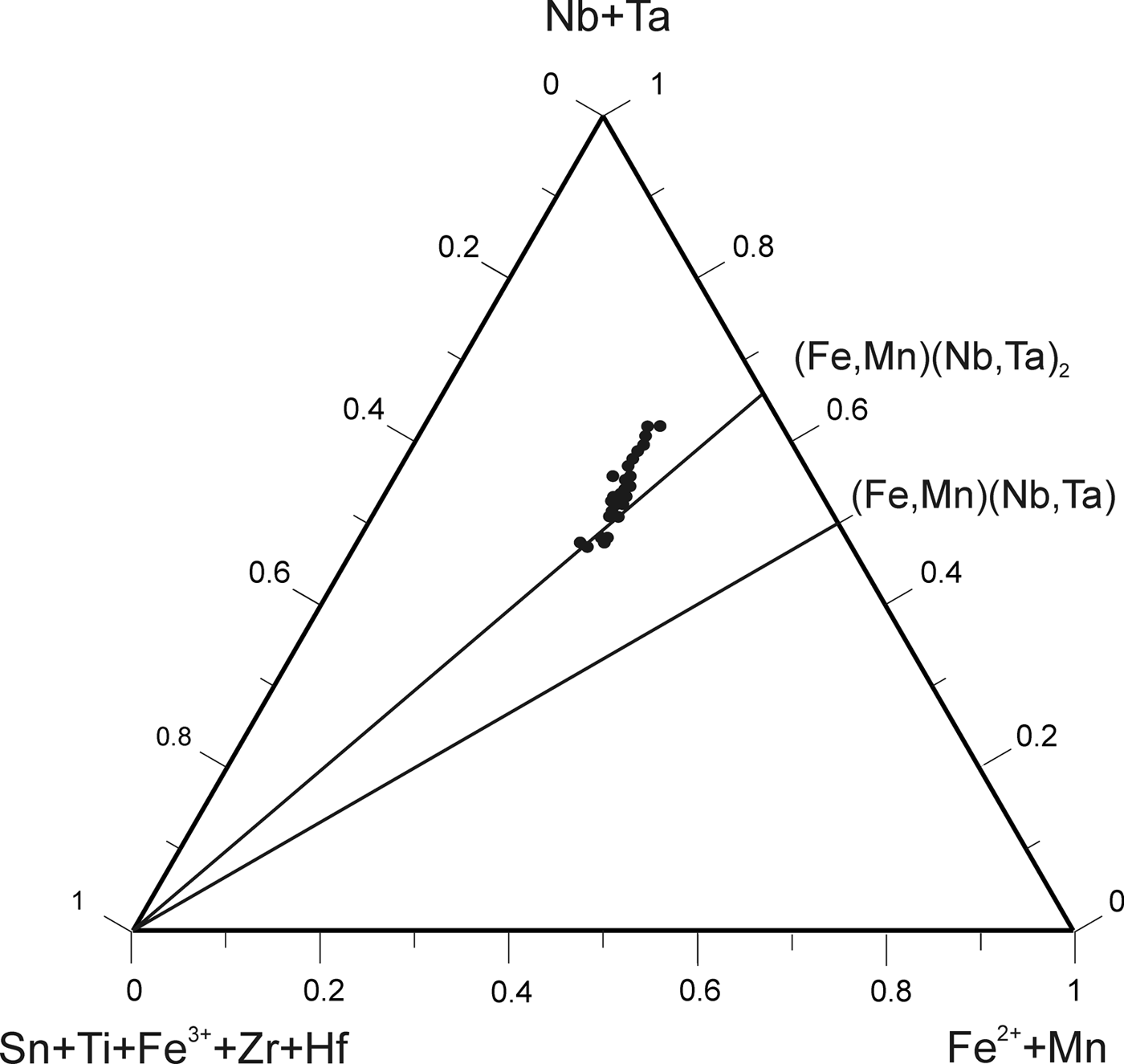
Fig. 7. Compositions of wodginite-group minerals in the (Nb,Ta)–(Sn,Ti,Fe3+)–(Fe,Mn) diagram (atomic ratios).
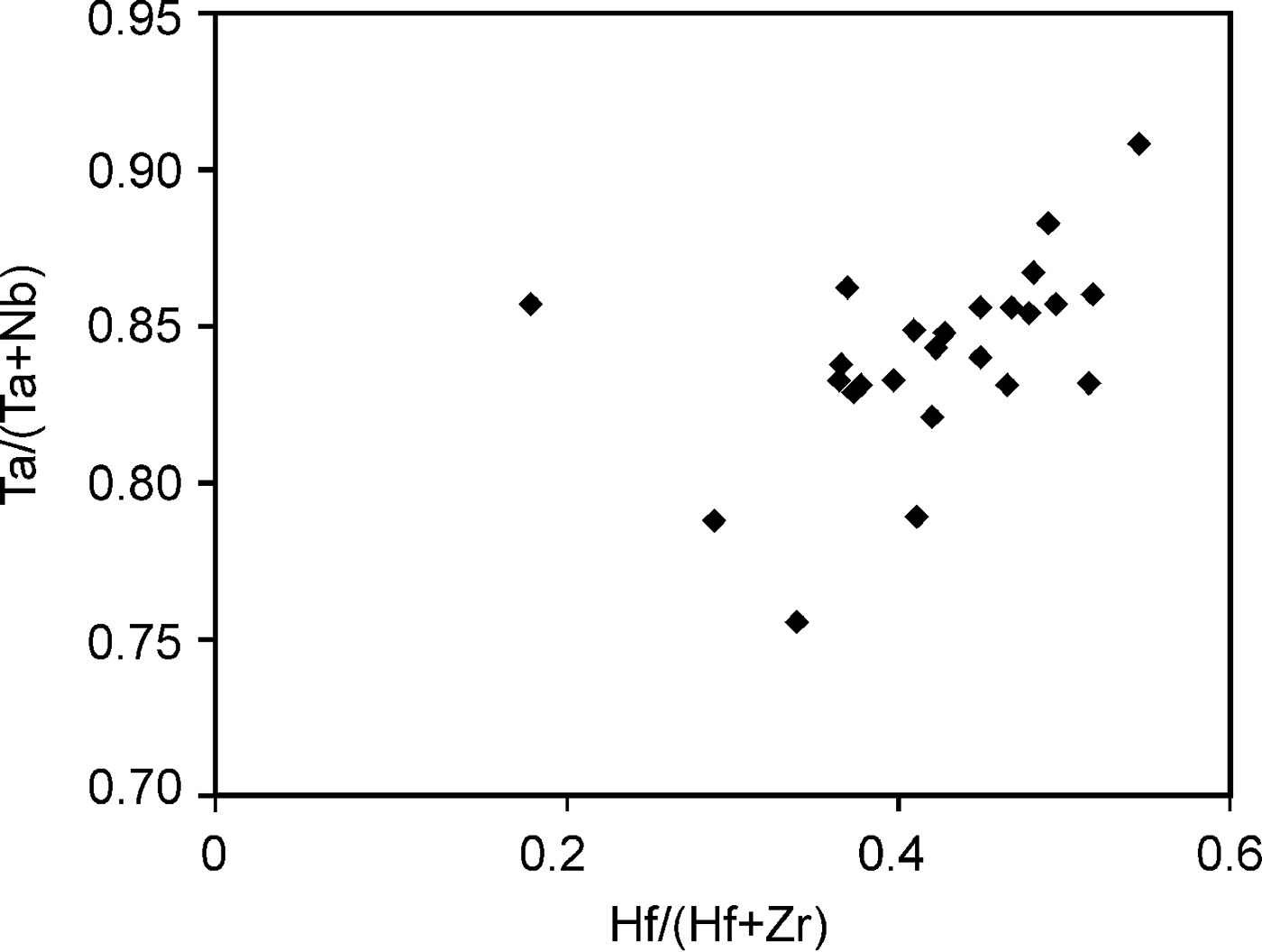
Fig. 8. Correlation between the Ta/(Ta + Nb) and Hf/(Hf + Zr) in wodginite from Penouta.
Cassiterite
Cassiterite is the main oxide mineral in the Penouta leucogranite. It occurs as subhedral to euhedral crystals usually up to 200 µm long. Under BSE, cassiterite seems to be homogeneous in appearance; the chemical composition varies, however. Two generations can be recognized. The first generation consists of homogeneous crystals of nearly pure composition, whereas, in the second generation, Ta can reach 8.51 wt.% Ta2O5, and the Nb content is up to 1.94 wt.% Nb2O5. As usual in greisens and veins from granites, Fe is dominant over Mn (Černý and Ercit, Reference Černý, Ercit, Moller, Černý and Saupe1989), reaching 1.45 wt.% and 0.20 wt.%, respectively. In granitic pegmatites the presence of Nb and Ta within cassiterite is attributed to the typical substitution scheme (Fe,Mn)2+ + 2(Nb,Ta)5+ ↔ 3(Sn,Ti)4+, or tapiolite substitution (Černý et al., Reference Černý, Roberts, Ercit and Chapman1985), in which case the (Fe + Mn)/(Nb + Ta) ratio is in a 1 : 2 proportion (Černý et al., Reference Černý, Chapman and Ferreira2004). Most analyses from the Penouta cassiterite show the 1 : 2 ratio to be attributed to the tapiolite substitution (Fig. 9a).
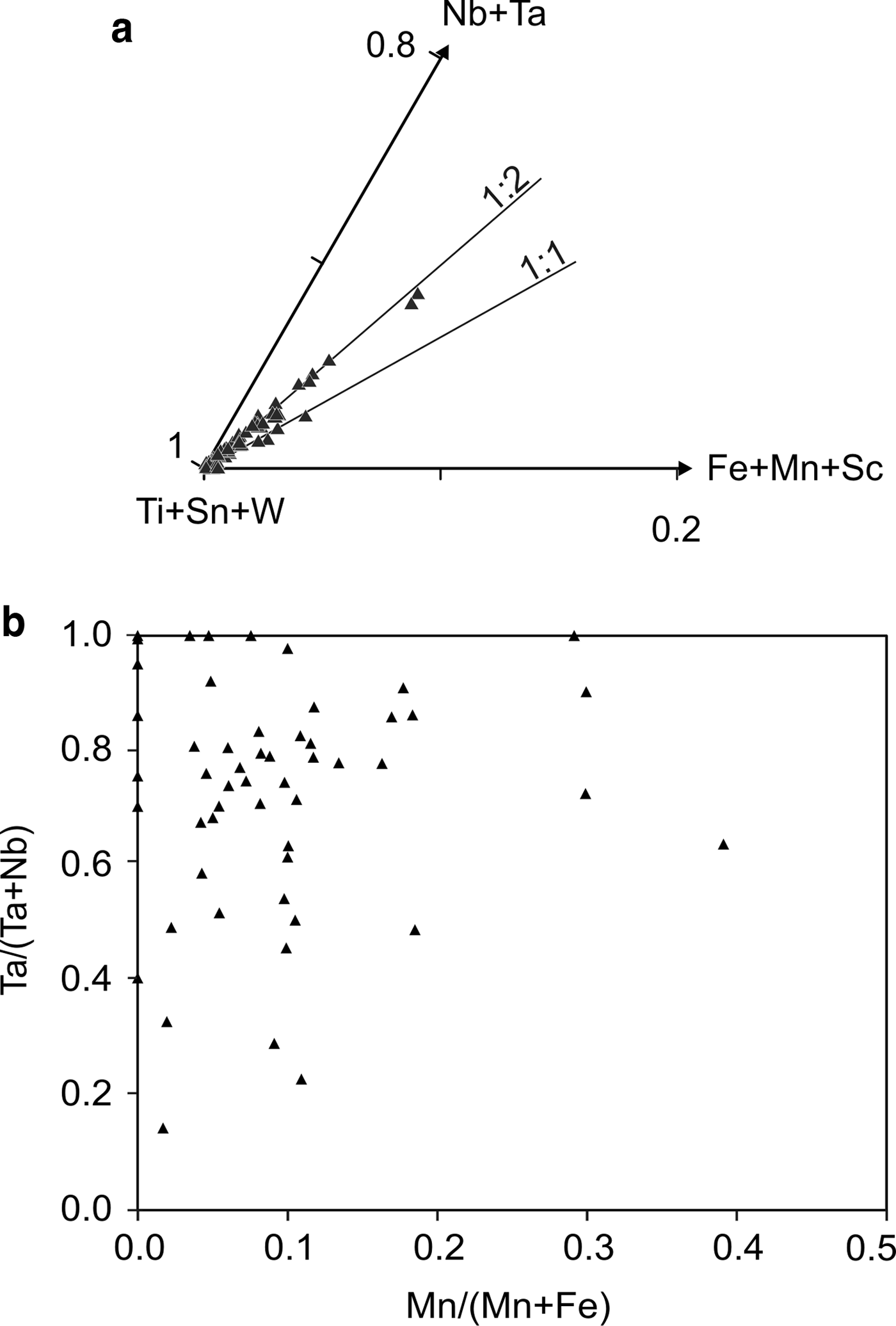
Fig. 9. Cassiterite composition from the Penouta leuogranite: (a) plot in the (Ti + Sn + W)–(Fe + Mn + Sc)–(Nb + Ta) triangle; (b) in the columbite quadrilateral (atomic ratios).
The cassiterite grains typically contain inclusions of Nb-Ta-rich minerals (Spilde and Shearer, Reference Spilde and Shearer1992). Similarly, wodginite inclusions have also been found in other deposits (Masau et al., Reference Masau, Černý and Chapman2000; Černý et al., Reference Černý, Ercit, Smeds, Groat and Chapman2007; Rao et al., Reference Rao, Wang, Hu and Zhang2009). Masau et al. (Reference Masau, Černý and Chapman2000) described Hf-Zr-rich wodginite formed by exsolution from cassiterite, which was favoured by its high Zr and Hf contents. In the present study the Hf and Zr contents are relatively high in wodginite, suggesting a possible similar origin. However, inclusions of CGM in cassiterite constitute discrete grains probably trapped when cassiterite formed after CGM, as in other occurrences (Martins et al., Reference Martins, Lima, Simmons, Falster and Noronha2011).
Discussion
Two aspects cause a great deal of controversy in the genesis of Nb-Ta deposits, both pegmatites and granites: (1) the rare-metal enrichment of the melts; and (2) the fractionation between Nb and Ta, with special emphasis on the magmatic vs. hydrothermal enrichment of the late Ta-rich phases.
In the genesis of the of the Penouta deposit, three stages can be distinguished: (1) a primary magmatic stage; (2) a late metasomatic stage with albitization and muscovitization; and (3) an epigenetic or supergene stage. The existence of these stages has been supported by field and geochemical evidence and also by a fluid-inclusion study carried out by Mangas and Arribas (Reference Mangas and Arribas1991).
Magmatic crystallization of CGM and related textures
Whole-rock Ta distribution in the Penouta granite exhibits a progressive Ta enrichment upwards in the granite sheet. Evidence of the saturation of columbite-tantalite was not attained; if it had been, a depletion in Ta contents would have occurred once this mineral phase reached its saturation, as CGM contain Ta, an essential structural constituent (ESC) in these mineral phases (López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017). There is petrographical evidence, however, of the occurrence of CGM from the bottom to the top of the Penouta granite body and an external origin (assimilated or residual minerals) can be ruled out. Both observations suggest that CGM grew within trapped intercumulus melt, where the crystallization of major rock-forming minerals incompatible with Ta and Nb (e.g. quartz, alkali feldspar and albite) increased the concentration of Ta and Nb in the shrinking residual melt until saturation occurred. Crystallization of CGM in the Penouta granite seems to have occurred far from the liquidus of the parental magma, but related to local saturation. In a scenario of local saturation, Nb tends to crystallize prior to Ta due to the lower solubility of Nb in peraluminous melts (Keppler, Reference Keppler1993; Linnen and Keppler, Reference Linnen and Keppler1997; Linnen, Reference Linnen1998; Stepanov et al., Reference Stepanov, Mavrogenes, Meffre and Davidson2014), resulting in a normal zoning that is very common in columbite–tantalites from the Penouta leucogranite (e.g. Fig. 3). CGM from the Penouta leucogranite also exhibit often-complex zoning patterns (e.g. oscillatory zoning), that could be explained in a magmatic scenario. Extrinsic mechanisms, such as temperature or water-pressure changes (Shore and Fowler, Reference Shore and Fowler1996) or intrinsic mechanisms, such as non-equilibrium concentration gradients (Bacon, Reference Bacon1989) have been proposed to explain such rhythmic zoning.
The primary character of CGM is also in line with their core Ta/Nb variation observed from bottom to top in the granite (Fig. 10). Because CGM phases were saturated only locally in a trapped melt, the Ta/Nb variation of cores has to be a consequence of the fractionation process of the liquidus mineral phases (e.g. quartz, alkali feldspar, albite, muscovite and garnet). The distribution coefficients of these liquidus minerals for Ta and Nb are typically incompatible except for muscovite, which would fractionate Nb relative to Ta (D Nb = 3.5; D Ta = 0.4; Raimbault and Burnol, Reference Raimbault and Burnol1998), contributing to the increase in the Ta/Nb ratio as the melt evolves. Whole-rock ratio variations in Ta/Nb from the Penouta granite have been modelled recently, taking into account, among others factors, muscovite as a fractionated mineral phase, resulting in a good match between modelled and real melt evolution (López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017). Consequently, the involvement of muscovite as a fractionated liquidus mineral phase could explain core Ta/Nb variations of CGM from bottom to top in the Penouta granite and the magmatic origin of normal zoning of CGM.
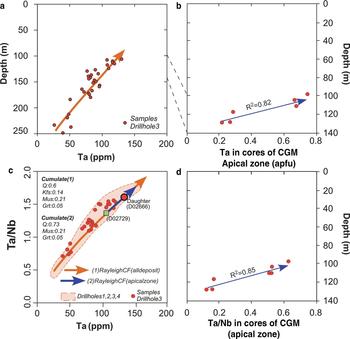
Fig. 10. Variation with depth in the Penouta deposit: (a) Ta content in granite; (b) Ta content in cores of CGM; (c) Ta vs. Ta/Nb; and (d) Ta/Nb variation observed from bottom to top in the granite.
Overprints on CGM
One of the most striking features of back-scattering images of CGM is the occurrence of a large number of micro holes, frequently grouped, and within areas with a marked Nb depletion and Ta enrichment relative to surrounding blackish areas of the crystal (Fig. 3). These textures have been cited in many other occurrences (e.g. Galliski et al., Reference Galliski, Márquez-Zavalia, Černý, Martínez and Capman2008; Wise and Brown, Reference Wise and Brown2010; Zhu et al., Reference Zhu, Wang, Che, Zhu, Wei and Huang2015), are known as ‘sponge-like’ textures and resemble textures related to overprinting processes in an environment enriched in fluids during their formation. However, the origin of this fluid is an open issue but could be linked to the evolution of the magma itself or to processes unrelated to the magmatic process.
(1) Processes related to the evolution of the melt
Experimental and fluid/melt inclusion studies have demonstrated that during the last stage of magmatic crystallization, an exsolution process can occur, resulting in up to three products, namely, a silicate melt, vapour and a hydrosaline phase, the latter being described either as a fluid (Roedder, Reference Roedder1992; Kamenetsky et al., Reference Kamenetsky, Naumovc, Davidsona, van Achterberghd and Ryan2004; Alfonso and Melgarejo, Reference Alfonso and Melgarejo2008; Tartèse and Boulvais, Reference Tartèse and Boulvais2010; Chicharro et al., Reference Chicharro, Martín-Crespo, Gómez-Ortiz, López-García, Oyarzun and Villaseca2015; Webster et al., Reference Webster, Vetere, Botcharnikov, Goldoff, McBirney and Doherty2015; Assadzadeh et al., Reference Assadzadeh, Samson and Gagnon2017) or as a melt (Badanina et al., Reference Badanina, Veksler, Thomas, Syritso and Trumbull2004; Veksler, Reference Veksler2004; Thomas and Davidson, Reference Thomas and Davidson2013; Huang et al., Reference Huang, Wang, Xie, Zhu, Erdmann, Che and Zhang2015).
The hydrosaline phase was defined as a melt rich in fluxing elements such as Cl, Li, B and/or F; which are fluxing elements with low solubility in aluminosilicate melt and which fractionate to the hydrosaline phase, causing its low viscosity (Veksler, Reference Veksler2004; Linnen and Cuney, Reference Linnen, Cuney, Linnen and Samson2005). In such conditions this fluid/melt could easily move and produce metasomatism during its interaction with the early formed minerals and consequently the formation of the sponge-like textures. The existence of an exsolution process resulting in a hydrosaline phase can be checked by the mineralogical and geochemical fingerprints left by this process.
(a) The role played by B-enriched hydrosaline fluids
A B-enriched hydrosaline fluid can be practically ruled out in the Penouta deposit, because tourmaline is a very accessory mineral and also by the deposit type itself. Boron-bearing melts provide evidence of explosive processes such as the formation of breccia pipes and stockworks (Pollard et al., Reference Pollard, Pichavant and Charoy1987), whereas in the Penouta leucogranite the mineralization is disseminated, indicating a passive crystallization of the magma.
(b) The role played by F-enriched hydrosaline fluids
The occurrence of a F-enriched hydrosaline fluid/melt in the apical zone, where the sponge-like textures of CGM occur, should trigger the following mineralogical and geochemical fingerprints according to the distribution coefficients between silicate and a fluoride-enriched hydrosaline melt compiled by Veksler (Reference Veksler2004): (1) Na would be fractionated into the hydrosaline melt and K would remain in the aluminosilicate melt; (2) Ta and Hf appear to be much less compatible with F hydrosaline melts than Nb and Zr, resulting in lower Zr/Hf and Nb/Ta ratios in the residual silicate melt and the opposite in the hydrosaline melt; (3) Y and REE are highly fractionated in the F hydrosaline fluid/melt, resulting in a depleted aluminosilicate melt in these elements.
The evolution of Zr/Hf and Nb/Ta ratios, together with Y and REE depletion in the silica melt and the Na enrichment upwards in the granite are consistent with whole-rock evolutions observed in the Penouta granite on a deposit scale (see López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017). However, most of these geochemical constraints can also be explained in terms of fractional crystallization involving quartz, muscovite, alkali feldspar, zircon, monazite and xenotime as fractionated mineral phases (López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017). A similar conclusion can be drawn for the albite enrichment of the apical zone, since a change in the ternary cotectic in the system Qz–Ab–Or can be yielded as a consequence of F enrichment during melt evolution, resulting in a change in the crystallization of quartz by albite as liquidus phases (Manning, Reference Manning and Evans1982). Moreover, the exsolution of the hydrosaline fluid/melt in the terms mentioned above requires a melt enriched in F (Gramenitskiy and Shekina, Reference Gramenitskiy and Shekina1994). Evidence has been found of some enrichment in F in the most apical zone of the Penouta leucogranite, where scarce fluorite and scarce, F-enriched apatite occur (Llorens González et al., Reference Llorens González, García Polonio, López Moro, Fernández-Fernández, Sans Contreras and Moro Benito2017); the rest of the deposit is heavily depleted in F, and the only mineral phase containing this element is apatite, an accessory mineral, as can be justified by the extremely low whole-rock P2O5 contents (~0.02%, Table 1). Besides, the fluorine enrichment in the apical zone could also be justified in terms of the melt evolution itself, as F is an incompatible element (London et al., Reference London, Hervig and Morgan1988) tending to concentrate in the residual melt.
Furthermore, a scenario of exsolution of a F-enriched hydrosaline fluid/melt triggering an enrichment in Ta in the residual silicate melt could explain the normal zoning of CGM, but it remains unclear whether Ta enrichment, or Nb depletion, which accompany the micro holes in the sponge-like textures are consistent with the exsolution process itself, as the exsolved fluid is enriched in Nb and not in Ta. The latter, linked to the scant evidence of F enrichment in the Penouta deposit, the possibility of a fractional crystallization process as an alternative to explain Nb/Ta, Zr/Hf ratios, and the evolution of Y and REE lead us to conclude that the formation of an F-enriched, hydrosaline, low-viscosity melt cannot be justified easily in the Penouta leucogranite on a deposit scale, as for other rare-metal granites that are F, P-rich, e.g. those from the Krásno-Horní Slavkov ore district (René and Škoda, Reference René and Škoda2011) or in ongoites from the Xianghualing tin district (Huang et al. Reference Huang, Wang, Chen, Hu and Liu2002). In the Penouta deposit, the exsolution of a F-bearing melt could only be feasible at a very specific level where the melt, by maximum degree of evolution, would have a significant amount of F (e.g. in the most apical zone where fluorite occurs).
(c) The role played by Cl-bearing fluids
Another possible explanation for the sponge-like textures related to the melt evolution is a Cl-enriched fluid/vapour. The existence of such a fluid is checked easily by an anomalous enrichment in elements that can be mobilized with Cl, as is the case for Sn. This separated Cl-bearing fluid/vapour seems to have occurred in the central part of the Penouta granite body, where there is an enrichment of Sn and Be, and a lack of concomitant enrichment in other elements typically of the evolution of the melt (e.g. Ta, Y and Gd) which would support the notion that degassing processes occurred (see trend II in Fig. 11). Similar conclusions can be drawn for the upper part of the granite body, where some samples of the leucogranite exhibit a dramatic Sn enrichment collinear with the Sn enrichment trend of the central part of the granite (Fig. 11). There is evidence of this separated Cl-bearing fluid in the greisen, where a strong enrichment in Sn occurs with the smallest increase in Ta (trend III, Fig. 11), supporting the notion that Ta in the greisen is hosted exclusively by cassiterite, whereas Ta in the leucogranite is hosted by CGM and to a lesser extent by cassiterite (trends I and II in Fig. 10). This fluid/vapour as a metasomatizing agent would probably behave differently in the central part relative to the upper level of the granite. In the central part, the fluid separated from lower levels was probably resorbed in the hot melt when it reached the central part, and only geochemical fingerprints can be found of this process. In contrast, in the upper level the fluid found a solid rock (country rock) and solidified granite, enhancing metasomatic processes on the solidified granite in the most apical zone and the formation of the greisen on the cooler country rock. The different behaviours of the exsolved fluid depending on the area where it is produced could explain that CGM with sponge-like textures are specifically located in the upper part of the granite and are missing in the central part of the Penouta granite.
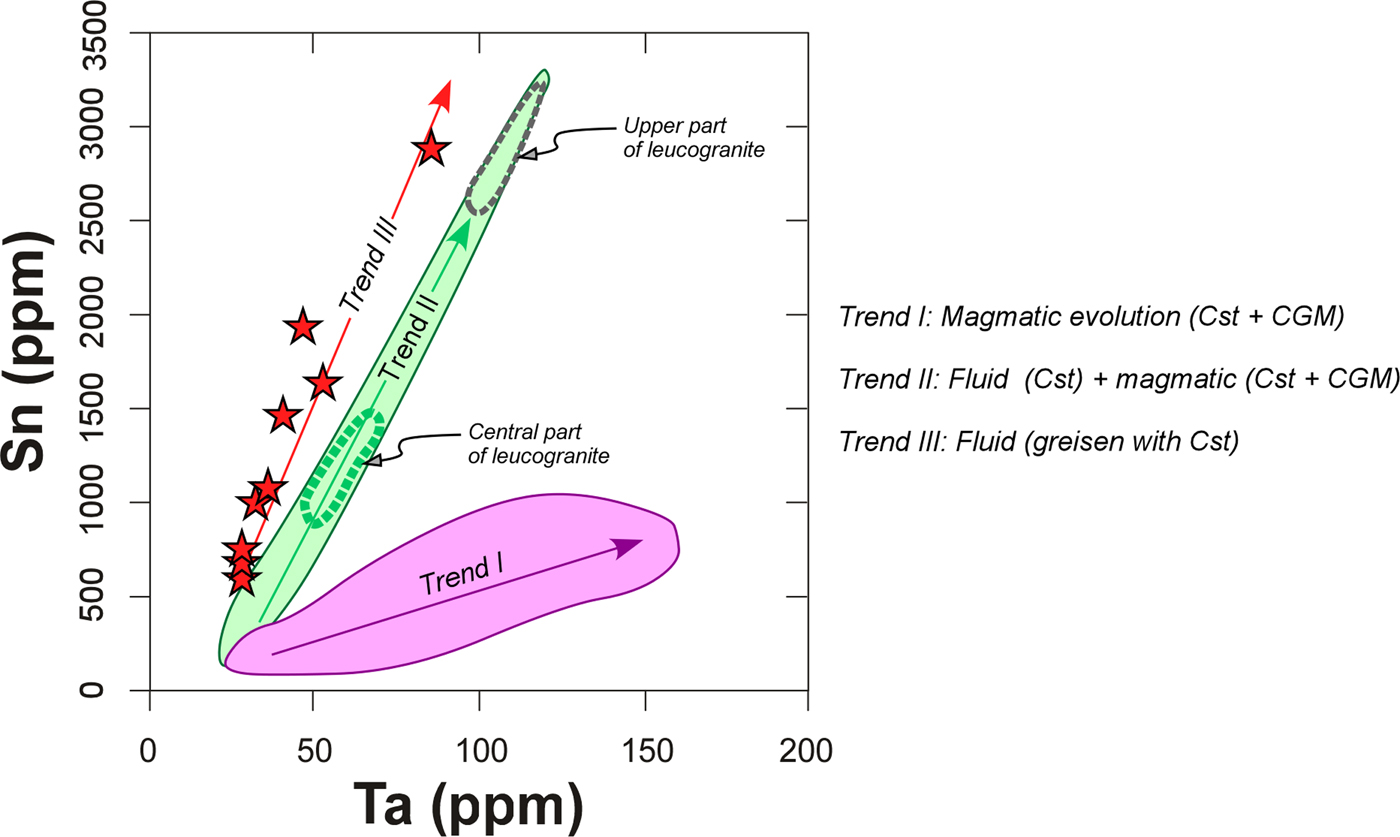
Fig. 11. Trends in variation of the Ta vs. Sn contents in the Penouta deposit.
Experimental constraints seem to support the suggestion that the mobility of Nb might be greater than that of the Ta in an aqueous solution at very low pH (2) and high-temperature conditions (>200°C) (Zaraisky et al., Reference Zaraisky, Korzhinskaya and Kotova2010; Ballouard et al., Reference Ballouard, Marc Poujol, Boulvais, Branquet, Tartèse and Vigneresse2016b; Timofeev et al., Reference Timofeev, Migdisov and Williams-Jones2017). Therefore, the existence of a Cl-Sn-Be-bearing fluid/vapour separated from the melt could replace previous CGM grains, contribute to the developing of sponge-like textures and the formation of Nb-depleted areas around micro holes as long as this fluid had adequate acidity conditions.
(2) Processes unrelated to the evolution of the melt
It cannot be ruled out that the sponge-like textures could be related to a fluid unrelated to the magmatic evolution, as the Ta-rich rims in CGM could be much younger than the cores (see the geochronological results obtained by Melcher et al., Reference Melcher, Graupner, Gäbler, Sitnikova, Henjes-Kunst, Oberthür, Gerdes and Dewaele2015), but no tests on the geochronological constraints have been carried out on the Penouta granite to check this possibility. If a long time gap between the formation of the cores and the rims was revealed then the formation of kaolinite might explain this texture, as microthermometry constraints indicate that this process is related to a fluid unrelated to magmatic evolution (Mangas and Arribas, Reference Mangas and Arribas1991). Besides, the mineral where these textures occur is located at the top of the Penouta granite.
Ta-rich mineral formation
Ta-rich minerals, such as microlite, tapiolite and wodginite, are extremely scarce in the deposit and occur specifically in the apical zone of the leucogranite. Texturally, they seem to be late mineral phases, nucleated on previous CGM crystals and to resemble secondary minerals (see Fig. 6).
A magmatic scenario, in which a melt evolves upwards, fractionating white mica, could lead to a residual liquid rich in Ta (see above), the nucleation of rims of tantalite and ultimately to the formation of other more Ta-enriched minerals. However, the interstitial and secondary aspect of most of these minerals seems to involve an environment with fluids for their formation. It is likely, therefore, that the formation of Ta-rich minerals is related to a fluid capable of leaching more Nb than Ta from previous CGM crystals, in a similar fashion to that proposed for the formation of the sponge-like textures. The difference between the formation of sponge-like textures and Ta-rich minerals could be a consequence of more fluid–rock interaction in cases where the Ta-rich minerals were formed.
Two points to take into account here are the availability of Nb and Ta and the mobility of the hydrosaline phase. Firstly, enrichment in volatile elements (Li, P, F) in the melt increase the solubility of Na and Ta (Li et al., Reference Li, Huang, He, Li, Li, Yu and Cheng2015). These volatile elements increase in the late stages of magmatic crystallization, which could lead to the start of the dissolution of early-formed columbite.
With regard to mobility, the phase responsible for the metasomatic transformations must be able to move easily. The hydrosaline phase was defined as a melt rich in fluxing elements such as Cl, Li and F; these are fluxing elements which are poorly soluble in aluminosilicate melt and fractionate to the hydrosaline phase causing its low viscosity (Veksler, Reference Veksler2004; Linnen and Cuney, Reference Linnen, Cuney, Linnen and Samson2005). In such conditions this magma could move easily and produce metasomatism during its interaction with the early-formed minerals. The metasomatic changes cause albitization and the replacement of Nb-rich oxide minerals by Ta-rich phases such as tantalite, microlite and wodginite. Hydrosaline melts could then be responsible for metasomatic events such as albitization and Ta replacements. The occurrence of fluorite in the apical part of the Penouta deposit suggests a fluorine-rich magma but it was probably released from the magma to the host rocks. Two other elements that play a role in increasing the solubility of H2O (and thereby in reducing the viscosity) are P and B (Holtz, et al., Reference Holtz, Dingwell and Behrens1993; London, Reference London2015). The Penouta leucogranite is poor in B, and so the formation of a hydrosaline, low-viscosity melt cannot be justified easily as in other rare-metal granites that are F, P-rich, such as those from the Krásno-Horní Slavkov ore district (René and Škoda, Reference René and Škoda2011) or in ongoites from the Xianghualing tin district (Huang et al. Reference Huang, Wang, Chen, Hu and Liu2002).
The overgrowths of tantalite on columbite from the Penouta leucogranite, as well as in other granites (Dostal et al., Reference Dostal, Kontak, Gerel, Shellnutt and Fayek2015) could be interpreted differently from the Ta-rich rims that formed in a continuous mineral crystallization, however. Melcher et al. (Reference Melcher, Graupner, Gäbler, Sitnikova, Henjes-Kunst, Oberthür, Gerdes and Dewaele2015) dated grains of CGM which had Ta-rich overgrowths and found different ages, 2000 Ma and 2487 Ma, respectively. Similar overgrowths were attributed to the Ta-rich crystallization from a hydrothermal late fluid in granites, e.g. the Nb-Ta-Sn-W-Zr mineralization of the Songshugang granite (Zhu et al., Reference Zhu, Wang, Che, Zhu, Wei and Huang2015), or in pegmatites (Neiva et al., Reference Neiva, Gomes and Silva2015). These overgrowths could then have been formed during the magmatic hydrothermal transition (Ballouard et al., Reference Ballouard, Marc Poujol, Boulvais, Branquet, Tartèse and Vigneresse2016a,Reference Ballouard, Marc Poujol, Boulvais, Branquet, Tartèse and Vigneresseb).
Moreover, the existence of a fluid/vapour separated from the melt cannot be ruled out in the Penouta granite (López Moro et al., Reference López Moro, García Polonio, Llorens González, Sanz Contreras, Fernández-Fernández and Moro Benito2017). The occurrence of aplite-pegmatites in the upper margin of the granite body, together with the development of the greisen in the country rock, support the separation of a fluid/vapour in the apical part of the granite, where the samples studied were collected. This fluid could contribute to the development of sponge-like textures in some columbite–tantalite crystals and might also favour the leaching of Nb in the crystal rim due to the preferential mobilization of Nb relative to Ta in a fluid (Zaraisky et al., Reference Zaraisky, Korzhinskaya and Kotova2010; Ballouard et al., Reference Ballouard, Marc Poujol, Boulvais, Branquet, Tartèse and Vigneresse2016b), resulting in zoned crystals with a Ta-rich rim.
Conclusions
Tantalum in the Sn-Ta Penouta deposit occurs in a highly evolved leucogranite. The main Ta ore in the upper level of the granite is manganocolumbite and manganotantalite with minor amounts of microlite, wodginite and tapiolite, the latter two are mentioned at this location for the first time.
Ta and Ta/Nb variations in the cores of CGM from the Penouta granite are good tools for deciphering the evolution of the melt, revealing an evolution of the melt upwards, which is concomitant with the evolution of the melt inferred by whole-rock geochemistry. However, the whole-rock geochemistry shows that CGM did not fractionate Ta or Nb in the melt, as they probably precipitated in a trapped liquid. As a result, variations of Ta and Nb in cores of CGM depended on the fractionation of essential minerals, especially muscovite, which is able to fractionate Nb relative to Ta. This observation, together with the existence of normal and oscillatory zoning in CGM, indicates that these phases crystallized from a melt.
Similarly to other rare-metal leucogranites, a subsequent important fluid activity occurred in the Penouta albite leucogranite, resulting in the development of sponge-like textures and depletions of Nb in the CGM. Experimental constraints seem to suggest that a fluid under certain conditions of acidity and temperature could cause partial digestion of the CGM and greater solubility of Nb than Ta. There is evidence of Cl-Sn-Be-bearing fluids exsolved from the melt, probably with very-low pH conditions, affecting the central and the upper levels of the leucogranite and the country rock (greisen) that is most likely to explain the formation of sponge-like textures and the leaching of Nb in CGM. Similarly, the formation of interstitial Ta-enriched minerals (microlite, tapiolite and wodginite) could respond to the same phenomenon as sponge-like textures, but with a greater degree of fluid–rock interaction.
Acknowledgements
This work is part of the project H2020-642201-OptimOre financed by the European Union. Ana Domingo and Eva Prats assisted with the SEM study and Xavier Llovet with the microprobe analyses. The authors thank ‘Strategic Minerals Spain’ for supporting the sampling. They are also grateful to the Guest Prinicpal Editor J. Bowles, Guest Associate Editor E. Deady, and to R.A. Shaw and two other anonymous reviewers for their valuable comments.