CVD is the leading cause of mortality in developed nations, with 40 % of all deaths in the USA attributable to CVD. The annual healthcare burden of treatment and management of CVD is >$656 billion and is projected to increase as the population ages( Reference Mozaffarian, Benjamin and Go 1 ). As such, identification of modifiable risk factors and non-pharmacological interventions are important for CVD prevention. Increasing dairy product intake is an emerging lifestyle factor that is associated with a decreased CVD risk( Reference Crichton and Alkerwi 2 , Reference Markey, Vasilopoulou and Givens 3 ). Long-term dairy product consumption is associated with lower blood pressure in healthy, aged individuals( Reference Wang, Manson and Buring 4 ), but its cardioprotective activities are mediated independent of its blood pressure-lowering effect( Reference Ballard and Bruno 5 – Reference De Mattia, Bravi and Laurenti 14 ). A putative mechanism through which dairy product consumption may benefit vascular function is the antioxidant properties of dairy peptides. Administration of dairy peptides in animal models reduces markers of inflammation and attenuates measures of oxidative stress, including total antioxidant capacity( Reference Xia, Liu and Wu 9 , Reference Jain, Velusamy and Croad 12 , Reference Tsai, Cui and Syed 13 ), suggesting that these mechanisms may decrease lifetime risk of cardiovascular morbidity and mortality.
A high dietary Na intake is independently associated with elevations in arterial blood pressure( Reference Elliott, Stamler and Nichols 15 ) as well as increased cardiovascular morbidity and mortality( Reference O’Donnell, Mente and Yusuf 16 ). Animal studies of the vascular effects of high dietary Na implicate endothelium-derived oxidative stress, particularly the production of superoxides, in reduced nitric oxide (NO) bioavailability and endothelial dysfunction( Reference Liu, Rusch and Lombard 17 – Reference Zhu, Huang and Lombard 21 ). Similarly, human studies demonstrate that Na restriction (≤1·5 g/d) reverses age-associated endothelial dysfunction by increasing NO-dependent vasodilation( Reference Jablonski, Racine and Geolfos 22 ). Similarly, non-invasive measures of conduit artery endothelial function show that low dietary Na intake is associated with enhanced flow-mediated vasodilation in middle-aged and older adults( Reference Jablonski, Gates and Pierce 23 ). In contrast to Na restriction, even short-term increases in dietary Na (7 d) impair flow-mediated vasodilation in conduit arteries of otherwise healthy young adults( Reference Tzemos, Lim and Wong 24 – Reference Eisenach, Gullixson and Kost 26 ), and even a single high-salt meal can significantly suppress brachial artery flow-mediated dilation within 30 min in healthy young adults( Reference Dickinson, Clifton and Keogh 27 ).
The human cutaneous circulation is an accessible vascular bed for examining mechanisms of microvascular dysfunction in vivo ( Reference Holowatz, Thompson-Torgerson and Kenney 28 ). There is a significant relationship between microvascular dysfunction measured in the skin and that measured invasively in the coronary and renal circulations, and intervention-induced improvements in vascular function are detectible in the cutaneous circulation before improvements in clinical outcomes( Reference Holowatz, Thompson-Torgerson and Kenney 28 – Reference Abularrage, Sidawy and Aidinian 30 ). Importantly, dietary Na-induced impairments in endothelial function are detectable in the cutaneous microvasculature of otherwise healthy adults, independent of changes in blood pressure or blood chemistry( Reference DuPont, Greaney and Wenner 25 , Reference Greaney, DuPont and Lennon-Edwards 31 , Reference DuPont, Farquhar and Edwards 32 ). These mechanistic in vivo human studies further demonstrate that even short-term (7 d) increases in dietary Na impair endothelial function and reduce NO bioavailability via an increase in oxidative stress( Reference Jablonski, Gates and Pierce 23 , Reference DuPont, Greaney and Wenner 25 , Reference Greaney, DuPont and Lennon-Edwards 31 ).
Increased consumption of dairy products in the form of natural cheese may inadvertently increase dietary Na intake. Consequently, increasing dairy product consumption, particularly in the form of cheese, may paradoxically hinder adherence to dietary Na recommendations, while still mitigating CVD risk. It is currently unknown whether vasoprotective activities of dairy products, provided as natural cheeses, protect against Na-induced impairments in the vasculature. Therefore, we sought to examine the protective role of macronutrients in natural dairy cheese against acute dietary Na-induced microvascular dysfunction. We hypothesised that acute natural dairy cheese ingestion would improve NO-dependent vasodilation compared with an equal dietary Na intake from non-dairy sources. Further, we hypothesised that this effect would be mediated by a reduction in ascorbate-sensitive oxidants.
Methods
Subjects
All protocols were approved by the Institutional Review Board at The Pennsylvania State University and complied with the guidelines in the Declaration of Helsinki. All participants voluntarily provided written and verbal consent before the experiment. In all, fourteen subjects (61 (sem 2) years; eight men, six women) participated in the study. Before participation, subjects underwent a medical screening that included a twelve-lead electrocardiogram, fasting blood chemistry and physical examination. Subjects also completed a 24-h ambulatory blood pressure monitoring while enrolled in the study. Inclusion criteria required a daily dairy product intake of less than two servings. Daily dairy product intake was assessed using a modified FFQ specific to dairy product consumption. Subjects had a 2-d wash-in period where they did not consume any dairy products. Experimental visits were separated by at least 3 d, to ensure the 2 d, low-dairy product wash-in before each visit. There were no recommendations regarding Na intake during the wash-in period. Subjects abstained from alcoholic and caffeinated beverages for 12 h, vigorous physical activity for 24 h and food for 8 h before each experiment. All subjects were non-smokers, non-diabetic, non-obese (BMI<30 kg/m2) and were not taking prescription medications that may alter vascular function (e.g. statins, antidepressants, antihypertensives, dietary supplements, aspirin, etc.). Women taking any form of hormone-replacement therapy were excluded from the study. Subject characteristics are presented in Table 1.
Table 1 Human subject characteristics (Mean values with their standard errors)
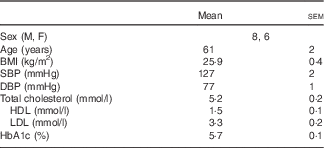
M, male; F, female; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated Hb.
Experimental protocol
Fig. 1 presents a schematic representation of the experimental protocol. On five separate visits, subjects arrived at the laboratory following an overnight (≥8 h) fast. Each experimental visit spanned approximately 4 h. For the local delivery of pharmacological agents, two intradermal microdialysis fibres (10-mm, 20-kDa cut-off membrane, MD 2000; Bioanalytical Systems) were placed into the dermal layer of the ventral left forearm( Reference Bruning, Santhanam and Stanhewicz 33 ). Pharmacological agents were mixed just before use, dissolved in lactated Ringer’s solution, sterilised using syringe microfiltres (Acrodisc; Pall) and wrapped in foil to prevent degradation due to light exposure. Microdialysis sites were randomly assigned to receive either 10 mm-ascorbic acid (Sigma) for local delivery of the non-specific antioxidant( Reference Greaney, DuPont and Lennon-Edwards 31 , Reference Holowatz and Kenney 34 ) or lactated Ringer’s solution to serve as control. Site-specific pharmacological solutions were perfused through the microdialysis fibres at a rate of 2 µl/min( Reference Bruning, Santhanam and Stanhewicz 33 , Reference Holowatz, Thompson and Kenney 35 ) (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems).

Fig. 1 Schematic representation of the protocol. Subjects entered the laboratory after fasting, had two intradermal microdialysis fibres placed, a fasted blood draw and then ingested the treatment diet. Following the resolution of hyperaemia, subjects were instrumented and skin blood flow data were collected at baseline, throughout local heating and during maximal vasodilation. The entire protocol lasted approximately 4 h. Each arrow represents a microdialysis site. l-NAME, N G -nitro-l-arginine; SNP, sodium nitroprusside; VD, vasodilation.
Following microdialysis fibre placement, subjects were instrumented with an intravenous catheter for blood collection. A fasted sample was collected before dietary treatment administration. Blood samples were then collected every 30 min after treatment until completion of the study. Whole blood samples were collected in EDTA-treated tubes containing o-phenanthroline, p-hydroxymercuribenzoic acid and pepstatin (Wake Forest University). Whole blood samples were centrifuged, and plasma samples were frozen and stored at −80°C until future use. Plasma Na was measured at baseline and at 90 min after ingestion using an electrolyte analyzer (ProLyte; Diamond Diagnostics). Plasma angiotensin II concentrations were measured at baseline and at 90 min after ingestion using a commercially available ELISA kit (Abcam) according to the manufacturer’s instructions. Samples were analysed in duplicate with an average CV <10 %.
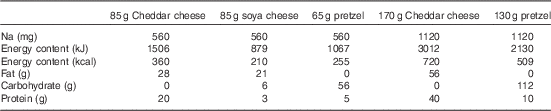
Subjects consumed either 85 g Cheddar dairy cheese (560 mg Na), 85 g soya cheese (560 mg Na), 65 g pretzels (560 mg Na), 170 g dairy cheese (1120 mg Na) or 130 g pretzels (1120 mg Na) in a randomised order 30 min after microdialysis fibres were placed. The treatment order was randomly assigned for each subject using a random number generator and was administered by the investigators; one subject did not consume soya cheese because of palatability issues. Our initial study design included a 170-g soya cheese treatment (1120 mg Na); however, several subjects refused this treatment because of palatability and we excluded it from further testing. The energy, macronutrient and Na contents of each dietary treatment are displayed in Table 2. The 30-min time point for consumption was chosen such that the local heating plateau for our endothelial nitric oxide synthase (eNOS)-dependent vascular stimulus( Reference Bruning, Santhanam and Stanhewicz 33 ) would occur 60–90 min after treatment, corresponding with the time period after milk peptide ingestion when peak intestinal concentrations of bioactive peptides are recovered( Reference Boutrou, Gaudichon and Dupont 36 ).
Table 2 Sodium, energy and macronutrient contents of dietary treatments
We allowed 60–90 min for hyperaemia associated with fibre placement to resolve before baseline data were collected, followed by a standard local heating protocol to induce eNOS-dependent vasodilation as previously described( Reference Bruning, Santhanam and Stanhewicz 33 , Reference Minson, Berry and Joyner 37 ). After approximately 30–40 min of local heating, when skin blood flow reached an established plateau, 15 mm-N G -nitro-l-arginine methyl ester (l-NAME; Calbiochem) was perfused at a rate of 4 µl/min to quantify NO-dependent vasodilation at all sites( Reference Alexander, Kutz and Kenney 38 , Reference Stanhewicz, Greaney and Larry Kenney 39 ). After infusion of l-NAME and subsequent stabilisation of a post-l-NAME plateau in skin blood flow, 28 mm-sodium nitroprusside (SNP) (Nitropress; Abbott Laboratories) was perfused and local temperature was increased to 43°C to elicit maximal dilation (CVCmax)( Reference Minson, Berry and Joyner 37 , Reference Johnson, O’Leary and Taylor 40 ). Studies in our laboratory and others have demonstrated that this protocol is highly specific to eNOS production of NO and allows the direct quantification of functional NO-dependent vasodilation in the cutaneous microcirculation( Reference Alexander, Kutz and Kenney 38 , Reference Bruning, Santhanam and Stanhewicz 41 , Reference Kellogg, Zhao and Wu 42 ).
Cutaneous erythrocyte flux was continually measured directly over each microdialysis site with an integrated laser-Doppler flowmetry probe placed in a local heating unit (Moor Instruments SHO2). Mean arterial pressure (MAP) was measured at the brachial artery throughout the protocol using an automated blood pressure monitor (CardioCap; GE Healthcare). Cutaneous vascular conductance (CVC) was calculated as erythrocyte flux divided by MAP and expressed as a per cent of site-specific maximal vasodilation (%CVCmax)( Reference Minson, Berry and Joyner 37 , Reference Minson 43 ).
Data acquisition and statistical analysis
Sample size was determined a priori by power analysis (P=0·8, α=0·05). Data were collected using Windaq (Windaq; DATAQ Instruments) at a frequency of 40 Hz. A three-way (dietary treatment×local treatment×subject) repeated-measures, mixed-model ANOVA was used to detect dietary treatment and local treatment differences in local heating plateau, NO-dependent vasodilation and maximal CVC (version 9.1.3; SAS). A two-way (dietary treatment×subject), repeated-measures ANOVA was used to detect dietary treatment differences in plasma Na and angiotensin II. Bonferroni post hoc corrections were performed to account for multiple comparisons when necessary. Significance was accepted at α=0·05. All values are presented as means with their standard errors.
Results
There was no difference in baseline or maximal (28 mm-SNP, 43°C) CVC between microdialysis sites or across treatments.
Fig. 2 shows original data records of the skin blood flow response normalised to maximal CVC (%CVCmax) during local heating in the control and ascorbate-treated microdialysis sites of one subject following non-dairy dietary Na (pretzel, 1120 mg Na) and dairy cheese Na (Cheddar cheese, 1120 mg Na) intakes. The per cent decrease with nitric oxide synthase inhibition (l-NAME) following each treatment is indicated.

Fig. 2 Representative tracing of skin blood flow (%CVCmax) during local heating in the control and ascorbate-treated microdialysis sites of one subject following non-dairy dietary sodium (a; pretzel, 1120 mg Na) and dairy cheese sodium (b; Cheddar cheese, 1120 mg Na) consumption. The difference between the local heating plateau and the post-N G -nitro-l-arginine methyl ester (l-NAME) plateau indicates the vasodilation attributed to the production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS) (%NO-dependent dilation). CVC, cutaneous vascular conductance.
Fig. 3 illustrates the total vasodilatory response to local heating (local heating plateau, %CVCmax) in Ringer’s (control) and ascorbate-perfused microdialysis sites following each dietary treatment. There was no difference in the local heating plateau between sites or among dietary treatments (all P>0·05).

Fig. 3 Values are means (n 14), with their standard errors of vasodilation response (%CVCmax) to local heating in Ringer’s solution (![]() , control) and ascorbate-perfused (
, control) and ascorbate-perfused (![]() , antioxidant) microdialysis sites following each dietary treatment. CVC, cutaneous vascular conductance.
, antioxidant) microdialysis sites following each dietary treatment. CVC, cutaneous vascular conductance.
Fig. 4 shows the percent NO-dependent vasodilation during local heating in Ringer’s (control) and ascorbate-perfused microdialysis sites following each dietary treatment. NO-dependent vasodilation was greater following 560 mg Na contained in dairy cheese (57 (sem 3) %) compared with 560 mg Na in soya cheese (42 (sem 3) %; P=0·002) or 560 mg Na in pretzels (43 (sem 4) %; P=0·004). NO-dependent vasodilation was also greater following 1120 mg Na contained in dairy cheese (55 (sem 5) %) compared with 1120 mg Na in pretzels (46 (sem 5) %; P=0·04). Local ascorbate perfusion augmented NO-dependent vasodilation compared with the control microdialysis site following Na ingestion from soya cheese (control: 42 (sem 3) v. ascorbate: 54 (sem 3) %) (P=0·01) and pretzel treatments with 560 mg Na (control: 43 (sem 4) v. ascorbate: 56 (sem 5) %; P=0·006) and 1120 mg Na (control: 46 (sem 5) v. ascorbate: 56 (sem 3) %; P=0·02). Local ascorbate perfusion did not augment NO-dependent vasodilation compared with the control microdialysis site following 560 mg Na ingestion (control: 57 (sem 3) v. ascorbate: 60 (sem 3) %; P=0·6) and 1120 mg Na ingestion (control: 55 (sem 5) v. ascorbate: 54 (sem 3) %; P=0·7) from dairy cheese.

Fig. 4 Values are means (n 14), with their standard errors of %NO-dependent vasodilation response to local heating in Ringer’s solution (![]() , control) and ascorbate-perfused (
, control) and ascorbate-perfused (![]() , antioxidant) microdialysis sites following each dietary treatment. *P<0·05 compared with dairy products control within sodium ingestion. †P<0·05 compared with Ringer’s site within dietary treatment. NO, nitric oxide.
, antioxidant) microdialysis sites following each dietary treatment. *P<0·05 compared with dairy products control within sodium ingestion. †P<0·05 compared with Ringer’s site within dietary treatment. NO, nitric oxide.
There were no differences in plasma Na or angiotensin II concentrations at baseline, or 90 min after ingestion, among any of the dietary treatments (Na: P=0·1 main effect of treatment, P=0·2 main effect of time) (angiotensin II: P=0·6 main effect of treatment, P=0·8 main effect of time).
Discussion
To our knowledge, this is the first study to directly examine the potential protective effect of Cheddar cheese on dietary Na-induced microvascular endothelial dysfunction. Our data demonstrate that acute (single-meal) dairy cheese consumption is protective against Na-induced impairments in NO-dependent vasodilation in the microcirculation. Further, acute localised administration of the non-specific antioxidant ascorbate normalised NO-dependent vasodilation following non-dairy Na ingestion, but had no effect on NO-dependent vasodilation after ingesting natural cheese, suggesting that non-Na components of natural cheese protect against acute Na-induced endothelial dysfunction in the microvasculature of healthy, older adults. The primary finding of this study is that the presence of bioactive proteins in dairy product-based natural cheese ameliorates acute Na-induced reductions in NO-dependent vasodilation by reducing ascorbate-sensitive oxidants. These data suggest that paradoxically increasing dietary Na by increasing cheese consumption may not confer the same CVD risk as dietary Na consumption in the absence of dairy products.
High dietary Na consumption is independently associated with elevated arterial blood pressure( Reference Elliott, Stamler and Nichols 15 ) as well as increased cardiovascular morbidity and mortality( Reference O’Donnell, Mente and Yusuf 16 ). Individual Na excretion resulting from increased dietary intake of Na >5·8 g/d is strongly associated with increased systolic and diastolic pressures of 10–11 and 6 mmHg, respectively. High-quality meta-analyses indicate that reducing Na intake reduces blood pressure and the risk of stroke and fatal CHD( Reference Aburto, Ziolkovska and Hooper 44 ).
Animal studies of the vascular effects of high dietary Na implicate endothelium-derived oxidative stress, particularly the production of superoxide, in reduced NO bioavailability and endothelial dysfunction( Reference Liu, Rusch and Lombard 17 – Reference Zhu, Huang and Lombard 21 ). Similarly, human studies demonstrate that Na restriction (≤1·5 g/d) reverses age-associated endothelial dysfunction by increasing NO-dependent vasodilation and by decreasing superoxide dismutase expression( Reference Jablonski, Racine and Geolfos 22 ). Even short-term increases in dietary Na (7 d) induce microvascular dysfunction measured in the cutaneous circulation of healthy young adults( Reference DuPont, Greaney and Wenner 25 ) – an impairment that is mediated by increases in oxidant stress and occurs independent of changes in blood pressure( Reference Greaney, DuPont and Lennon-Edwards 31 ). Similar to our current findings, Dickinson et al.( Reference Dickinson, Clifton and Keogh 27 ) demonstrated that a single high-salt meal significantly suppresses brachial flow-mediated dilation 30 and 60 min after ingestion in healthy adults. Collectively, the animal and human literature agree that elevated dietary Na consumption impairs endothelial function and reduces NO bioavailability via increased oxidant stress mechanisms, even in instances where patients do not have overt CVD. Our data add to this body of literature, suggesting that a single high-Na meal/snack from non-dairy sources acutely reduces NO-dependent vasodilation in healthy, older adults.
The positive impact of chronic dairy product consumption on cardiovascular health has been demonstrated in many population-based studies( Reference Moore, Bradlee and Singer 45 , Reference Livingstone, Lovegrove and Cockcroft 46 ). Increased total dairy product intake is associated with improvements in global measures of vascular health and function including blood pressure( Reference Yoshizawa, Maeda and Miyaki 47 ), pulse wave velocity( Reference Crichton, Elias and Dore 48 ), arterial compliance( Reference Yoshizawa, Maeda and Miyaki 47 ) and arterial stiffness( Reference Livingstone, Lovegrove and Cockcroft 46 ). The precise mechanisms by which dairy products may confer cardiovascular benefits are currently unclear but include angiotensin-converting enzyme inhibition( Reference Xu, Qin and Wang 6 – Reference van der Zander, Jakel and Bianco 8 ), protection and enhancement of bioavailable NO( Reference Xia, Liu and Wu 9 – Reference Turpeinen, Jarvenpaa and Kautiainen 11 ), and anti-inflammatory and antioxidant properties( Reference Xia, Liu and Wu 9 , Reference Jain, Velusamy and Croad 12 – Reference De Mattia, Bravi and Laurenti 14 ) of dairy proteins. As no single and specific mechanism has been definitively shown to account for the beneficial effects of increased dairy product intake on vascular function, it is likely that lifetime increases in dietary dairy product consumption confer cardiovascular benefits through several of these putative mechanisms. However, given the specific role of oxidant stress in dietary Na-induced vessel dysfunction, we focused our investigation on the antioxidant properties of dairy proteins. In support of this global hypothesis, our data suggest that the antioxidant properties of dairy proteins may play a primary role in the protection against dietary Na-induced reductions in NO bioavailability. Furthermore, we did not observe changes in circulating angiotensin II in response to our acute dietary treatments.
In the present study, we did not observe a reduction in the total vasodilator response to local heating of the skin, but rather we found attenuation in the direct functional quantification of NO-mediated vasodilation with non-dairy Na ingestion. These findings agree with earlier studies performed in our laboratory, which suggests that middle-aged adults maintain total vasodilator responsiveness to local heating but have reduced eNOS-mediated vasodilation compared with young adults( Reference Bruning, Santhanam and Stanhewicz 33 ). The current data suggest that a secondary NO-independent pathway is up-regulated to compensate for the decrease in eNOS function following acute Na consumption. However, endothelial-derived NO is synthesised ubiquitously throughout the vasculature and plays a crucial anti-atherogenic vasoprotective role. Given the putative role of NO in vascular health and vessel function, as well as possible age-associated reductions in other NO-independent endothelial pathways (PG, endothelium-derived hyperpolarising factors, etc.)( Reference Holowatz, Thompson-Torgerson and Kenney 49 , Reference Minson, Holowatz and Wong 50 ), interventions that target the production and protection of NO at the endothelium are clinically relevant strategies to preserve vascular health and reduce CVD risk across the lifespan.
This study examined acute (single meal) vascular responses to dietary Na with and without dairy products. As expected, we did not observe a time- or treatment-dependent change in plasma Na concentrations. Plasma Na concentration is tightly regulated, and our findings are consistent with other dietary studies in which high dietary Na is associated with vessel dysfunction independent of changes in plasma Na concentrations( Reference Greaney, DuPont and Lennon-Edwards 31 ). We selected our two Na doses on the basis of two and four servings of natural Cheddar cheese. Interestingly, we did not observe a dose–response relationship between dietary Na from non-dairy sources and attenuated NO-dependent dilation. This may have occurred because our doses were relatively close together or because our doses were high enough that we were at or near a ceiling effect. The dose–response relationship between acute dietary Na and endothelial dysfunction was not an experimental end point in this study; however, further research in this area is warranted.
Our test foods were specifically matched for dietary Na, with the soya cheese comparison included to account for potential differences with fat content and gastric emptying. There was a difference in protein content between Cheddar cheese and soya cheese meals in this study. As such, we cannot rule out the possibility that protein from non-dairy sources may similarly affect NO-dependent dilation. Furthermore, our study design did not account for differences in fat or carbohydrate composition between treatments – a factor that may influence the inflammatory milieu of the vascular endothelium( Reference Botham and Wheeler-Jones 51 , Reference Mah, Noh and Ballard 52 ). In addition, we did not match our test foods for Ca content. Ca itself may have a beneficial effect on the vascular endothelium and may also enhance renal Na excretion. However, the literature on the efficacy of Ca supplementation for improved cardiovascular outcomes is equivocal( Reference Reid, Bolland and Sambrook 53 , Reference Reid, Bolland and Grey 54 ). Despite these limitations, given the documented antioxidant properties of dairy peptide hydrolysates( Reference Zhang, Jin and Zhang 55 ) and the beneficial effect of dairy peptide ingestion on vascular endothelial function in humans( Reference Ballard and Bruno 5 , Reference Ballard, Mah and Guo 56 ), our findings fit within the broader hypothesis that specific antioxidant properties of hydrolysed dairy proteins play a role in preserved or maintained vascular function. Collectively, our data strongly suggest that dairy product ingestion protects against acute Na-induced endothelial dysfunction and that this protective effect is mediated by the antioxidant properties of milk proteins. It is still unknown whether this vasoprotection is maintained over longer periods of elevated dietary Na intake. Furthermore, it is unclear whether this effect is dependent on Na being directly incorporated in the dairy products (e.g. natural cheese) or whether the same benefits would occur if the diet contained high amounts of dairy products and high Na separately (e.g. pretzels and milk). Further studies of the protective antioxidant mechanisms of dairy proteins as well as their chronic role in vasoprotection against high dietary Na are warranted.
Summary
Overall, the present study suggests that the macronutrients in natural cheeses ameliorate Na-induced vessel dysfunction following a high-Na meal through antioxidant mechanisms. Increased dairy product consumption is associated with a decreased risk of cardiovascular morbidity and mortality, and our data suggest that the antioxidant properties of dairy proteins likely contribute to this association by protecting against dietary Na-induced impairments in vascular function. Consequently, increasing dietary dairy product intake may represent a modifiable and non-pharmacological lifestyle factor that can increase vascular health and function through the production and protection of bioavailable NO.
Acknowledgements
The authors are grateful to the subjects for their time and effort and to Susan Slimak, RN, and Jane Pierzga, MS, for their assistance.
This research was supported by Dairy Management Inc.
A. E. S. designed the study, conducted the study, analysed the data and performed statistical analysis, wrote the paper and had primary responsibility for the final content; B. K. A. conducted the study, analysed the data and wrote the paper; W. L. K. designed the study and wrote the paper; L. M. A. designed the study, analysed the data, wrote the paper and had primary responsibility for the final content. All the authors read and approved the final version of the manuscript. All laboratory analyses were conducted at Pennsylvania State University.
There are no conflicts of interest.









