To the Editor—Klebsiella pneumoniae often causes severe nosocomial infections against which carbapenem agents are frequently used when isolates produce broad-spectrum β-lactamase enzymes (ie, extended-spectrum β-lactamase [ESBL] or ampC type). In the last decade, carbapenem-resistant K. pneumoniae has been increasing worldwide, mainly due to K. pneumoniae carbapenemase (KPC) enzymes. Bla KPC-2 is especially common in Brazil, conferring resistance to almost all available antibiotics in association with high morbidity and mortality rates.Reference Rodrigues Perez1, Reference Rodrigues Perez and Dias2
Biofilm formation is a well-recognized pathway by which pathogenic bacteria evade adverse conditions (oxygen and nutrient deprivation) as well as bactericidal antimicrobial agents. Matrix-enclosed biofilms are formed preferentially on a variety of surfaces inside the body and on invasive devices.Reference Williams and Costerton3
Recently, we evaluated the ability to produce biofilms among Pseudomonas aeruginosa clinical isolates and their impact on antimicrobial susceptibility profiles; there was an alarming impact when overlapping resistance mechanisms (biofilm plus enzymatic production) were present.Reference Carvalho and Perez4 These results prompted a study evaluating these overlapping mechanisms because few reports are available regarding biofilm production among KPC-2–producing K. pneumoniae (KPC-2-Kp) isolates.Reference Naparstek, Carmeli, Navon-Venezia and Banin5,Reference Lee, Ko, Song and Peck6
In the present report, we describe for the first time the ability to produce biofilms among KPC-2-Kp clinical isolates from extensive clonal spread in patients in an intensive care unit. This is a substudy of the evaluation of the impact of biofilms on the potential development of antimicrobial resistance.Reference Perez7
We analyzed 10 pairs of KPC-2-Kp isolates from rectal swabs and clinical isolates obtained from 10 unique patients as previously described.Reference Rodrigues Perez and Dias2 These isolates were subjected to microtiter plate assays performed in triplicate, biofilm status characterization, and determination of both polymyxin B minimum inhibitory concentrations (MIC) and minimum biofilm eradication concentrations (MBEC) as described elsewhere.Reference Carvalho and Perez4
All KPC-2-Kp isolates were identical by molecular typing,Reference Rodrigues Perez and Dias2 but most of them had different biofilm profiles (Table 1). Four pairs of KPC-2-Kp had the same biofilm formation status: 3 of these isolates were recovered from blood and 1 was recovered from cerebrospinal fluid. However, for the remaining 6 pairs, an increase in biofilm production was observed in the clinical isolates compared to their colonizing counterparts (Table 1). A worrisome consequence of these results was the association with increasing resistance levels or bacterial tolerance in the face of higher polymyxin B concentrations. Moreover, polymyxin B MBEC results revealed that 90% of clinical isolates showed a 2–16-fold increase in resistance in biofilms (Table 1). Similar to our results, Naparstek et al.Reference Naparstek, Carmeli, Navon-Venezia and Banin5 found a dramatic increase in polymyxin resistance with K. pneumoniae isolates in biofilms. Although K. pneumoniae are not as notorious for biofilm formation as Pseudomonas aeruginosa, we did find that K. pneumoniae clinical isolates indeed produce robust biofilms compared with the same bacterial clone previously recovered from surveillance cultures reflecting gut colonization.
Table 1. Biofilm Status for KPC-2-Klebsiella pneumoniae Colonizing and Infecting Isolates and Polymyxin B MBEC/MIC Determination for Infecting Isolates
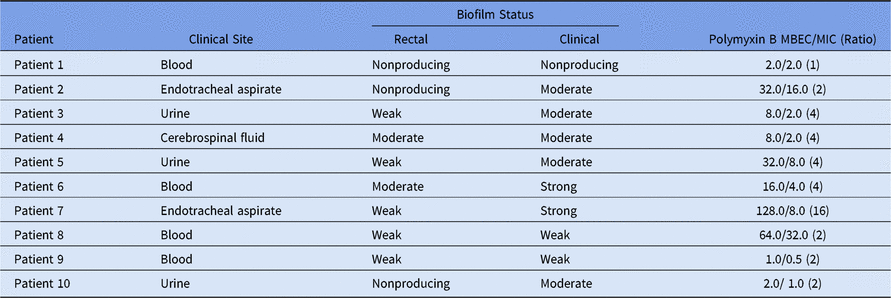
Note. MBEC, minimum biofilm eradication concentration; MIC, minimum inhibitory concentration.
Many bacteria grow on surfaces forming biofilms, but high dosages of antimicrobial often cannot clear infectious biofilms. Most of our isolates were recovered from patients with some invasive medical device (eg, bladder catheter, central venous catheter, or cerebrospinal fluid diversion), which is a well-characterized risk factor for both an infection process and biofilm production.Reference Stewart8
A potential limitation of this study was the lack of information about the genetic lineage of our KPC-2-Kp isolates. Some evidence shows that ST258 are widely disseminated in many Brazilian hospitalsReference Andrade, Curiao and Ferreira9 and that this ST258 lineage has a lower biofilm biomass than other lineages.Reference Naparstek, Carmeli, Navon-Venezia and Banin5 Little is known about the ability to produce biofilms in sets of the same KPC-2-Kp clonal isolates when recovered from distinct clinical specimens in the same patient.
In conclusion, a KPC-2-Kp predominant clone exhibits exuberant biofilm formation and high polymyxin B resistance levels, as noted with other species.Reference Carvalho and Perez4, Reference Lee, Ko, Song and Peck6 Although our results should be validated by further studies involving other clonal lineages, the ability to produce biofilm may be responsible for maintaining refractory infections due to KPC-2-Kp isolates, with an impact on polymyxin B resistance development.
Author ORCIDs
Leandro Perez, 0000-0002-6662-6503
Acknowledgments
The author would like to thank Sophia Perez for technical support.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



