Mitochondria have become a primary focus for the regulation of viability and death of neurons. As a major source of reactive oxygen species and oxidative stress, mitochondrial dysfunction has been linked to numerous diseases including those of the nervous system(Reference Simpkins, Yi and Yang1).
The key contributors to the neurodegenerative disorders include superoxide dismutase (SOD), monoamine oxidases (MAO) and malondialdehyde (MDA). SOD dismutates superoxide to hydrogen peroxide, and overexpression of SOD leads to an increased production of hydrogen peroxide and may lead to brain injury. Large amounts of unsaturated lipids are also present in the brain which are easy targets for free radicals. The resulting lipid peroxidation initiates a series of complex, autocatalytic propagation reactions that generate a variety of thiobarbituric acid-reactive substances such as the MDA, indicating the oxidative damage in the brain(Reference Jayakumar, Thomas and Geraldine2). MAO are mitochondria-bound isoenzymes that catalyse the oxidative deamination. The byproducts of MAO-mediated reactions include several chemical species with neurotoxic potential. So, the prolonged excessive activity of these enzymes may be conducive to mitochondrial damage and neurodegenerative disturbances(Reference Bortolato, Chen and Shih3).
It is intriguing that the vulnerable component of mitochondrial function is ovarian controlled in the central nervous system(Reference Borras, Sastre and Garcia-Sala4). It was reported that oestrogen could increase the activity, but not the levels, of MnSOD in the brain. Oxidative stress damage caused by chronic exposure to ozone could be prevented by oestradiol treatment(Reference Guevara-Guzman, Arriaga and Kendrick5). Thus, oestrogen suppression of mitochondrial oxidative stress may influence neurodegenerative disorders in ageing women(Reference Razmara, Duckles and Krause6). As such, oestrogens and novel non-hormonal analogues have come to figure prominently in the protection against neurodegeneration contributed by reduced oestrogen levels after menopause(Reference Simpkins, Yi and Yang7).
Genistein (Gen) has been shown to have the therapeutic potential to reduce cognitive decline and neurodegenerative disease associated with menopause either alone(Reference Xu, Zhu and Shi8)or in combination with other phyto-oestrogens(Reference Zhao, Mao and Brinton9). However, the mechanism of its neuroprotective effect remains unclear. Recently, it was found that Gen could diminish oxidative damage in the brain. In vitro, Gen at 0·5 μm could mimic the protective effect of oestradiol by decreasing the rate of reactive oxygen species formation and preventing the release of cytochrome c from the mitochondria(Reference Borras, Gambini and Lopez-Grueso10). In vivo, soya consumption could reduce plasma MDA and increase plasma total antioxidant capacity in postmenopausal women(Reference Azadbakht, Kimiagar and Mehrabi11). A pioneer study specified that Gen (2·5 mg/rat) that is injected subcutaneously every other day for 30 d could increase serum SOD and reduce MDA levels in rats(Reference Xu, Zhu and Shi8). Although serum parameters could reflect the overall oxidative status to some extent, the direct effect of Gen on the brain remains to be explored.
Our purpose was to investigate the antioxidative mechanism underlying the neuroprotective effects of Gen. Consequences on learning and memory were subsequently investigated using Morris water maze test. The acetylcholinesterase (AchE) activity, which is essential for the normal functioning of brain, was also examined. The oxidative stress status was revealed by detecting SOD and MDA contents and MAO activity in the brain. Moreover, neural apoptosis was revealed by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay and caspase-3 activity.
Experimental methods
Experimental animals and drug administration
Sixty-five female Sprague–Dawley rats (180–220 g, 3–4 months old), provided by the Animal Facility of 4th Military Medical University, were housed in a temperature-controlled room (22–24°C) with water and food freely available. The study followed the national guidelines for care and use of animals, and all the procedures were approved by the Ethics Committee for the Use of Experimental Animals. The rats were randomly divided into five groups, i.e. sham-operated (sham), ovariectomised (Ovx)-only, Ovx with 17β-oestradiol (E2), Ovx with low and high doses of Gen (Gen-L and Gen-H). All the rats were anesthetised with sodium pentobarbital for surgery.
One week after the surgery, the rats were orally administered daily with drugs or vehicles for six consecutive weeks. Gen (Sigma, St Louis, MO, USA) was dissolved in sesame oil and injected at 15 mg/kg for the Gen-L group and at 30 mg/kg for the Gen-H group, respectively, which was documented to confer significant neuroprotection(Reference Xu, Zhu and Shi8). E2 (Sigma) was chosen at the dose of 100 μg/kg as reported(Reference Irwin, Yao and Hamilton12). The sham and Ovx rats received sesame oil as the vehicle control. Eight rats from each group were used for behavioural test and histological study, and another five rats from each group were used for the biochemical measurement.
Behavioural test
Behavioural test began on day 43 after surgery and lasted for 5 d. The process included two tests. First, place navigation was performed. Daily sessions consisted of two trials separated by a 90 min interval. Trials began with the rat placed in the pool facing the side wall at the start position and ended once the rat had found the platform. If the rat had not found the platform within 120 s, it was guided there by hand. After a period of 30 s on the platform, the rat was immediately replaced in the pool at a different start position for the next trial. The escape latency, i.e. the time of locating the hidden platform, was automatically calculated by a commercial video/computer system (Jieri'ou Limited, Beijing, China). Secondly, a spatial probe trial was performed during the afternoon of the last day. For this, the platform was removed, and the time spent in the region that previously contained the platform, called ‘frequency of memory’, and the time taken to reach the platform location, called ‘swimming time’, were recorded over 120 s.
Preparation of tissue extracts
The extracts of brain cortex and hippocampus of five rats were prepared by the following procedure. Tissues were weighed and homogenised in saline on ice at a concentration of 10 g/l. Then, the homogenates were centrifuged at 4000 rpm for 20 min at 4°C, and the supernatants were collected and stored at − 70°C.
Biochemical assay
The biochemical analysis was performed as per instructions (Jian Cheng Limited, NanJing, China). AchE activity was determined colorimetrically by a microtiter plate-adapted modification of the Ellman method. The initial absorbance and the absorbance after 4 min were read at 412 nm. Brain SOD was measured by the xanthine oxidase–nitroblue tetrazolium assay. One unit of SOD is defined as the amount required to inhibit the reduction rate of nitroblue tetrazolium by 50 %. The results were expressed as specific activity of the enzyme in units per mg protein (U/mg). MDA was measured by thiobarbituric acid test. Briefly, an aliquot (100 μl) of the supernatant was added to the reaction mixture containing 100 μl of 8·1 % SDS, 750 μl of 20 % acetic acid (pH 3·5), 750 μl of 0·8 % thiobarbituric acid and 300 μl of distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 4000 g for 10 min. The absorbance of the supernatant was measured spectrophotometrically at 532 nm, and the results were expressed as nmol per mg of protein. The level of MAO activity, as measured by ammonia production, was detected as per instructions, and was expressed as nmol per mg of protein.
The amount of soluble protein was determined as described by the method of Bradford.
Apoptosis examination
Measurement of caspase-3 activity (Clontech, Mountain View, CA, USA) was done as described by Enari et al. (Reference Enari, Sakahira and Yokoyama13). Briefly, cytosolic extract (100 μg protein) was incubated for 1 h at 37 °C with the reaction buffer (25 mm-HEPES, pH 7·5, 10 % sucrose, 0·1 % 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid (CHAPS), 5 mm-dithiothreitol and 5 mm-EDTA) in a total volume of 150 μl containing 25 μM acetyl-asp–glu–val–asp-p-nitroanilide (Sigma). Enzyme-catalysed release of p-nitroanilide was measured at 405 nm using a microtiter plate reader.
TUNEL staining was performed using in situ cell death detection kit (Promega, Beijing, China) on the cryostat coronal sections as described. The number of TUNEL-positive cells was counted by an investigator who was blinded to the experimental conditions. Three fields (1000 μm2/field) in one section were randomly chosen, and five sections were counted in one group. The apoptotic cells were expressed as the average number per square micrometre.
HPLC determination of genistein
Gen was measured using HPLC (Type 168; Beckman, Miami, FL, USA) assay. Approximately, 1 g of brain tissue from different regions was weighed and homogenised in 5 ml of saline. Then, the homogenates from different brain regions were pooled by treatment. The samples (1 ml for brain tissue and 0·5 ml for serum) were added to 0·2 ml of an internal standard solution and 1·5 ml of an extraction solution (methyl-tert-butyl ether–normal pentane, 8:2 v/v) before being vortexed for 2 min. After centrifugation (3000 g, 5 min), each supernatant was transferred to a clean tube. The supernatant was extracted twice, and the two collections were pooled for drying under vacuum at ambient temperature overnight. The residue was reconstituted in 50 μl of methanol, and a total of 20 μl of each sample was added to the column for HPLC analysis.
Primary standards (100 μg/ml) were prepared in methanol. Working standards (0·5, 1, 5, 10, 20 and 30 μg/ml) were prepared in 0·1 ml methanol–water (80:20, v/v) before being dried under vacuum. Samples and standards were loaded onto a C8 column (5 μm, 250 × 4·6 mm; Phenomenex, Torrance, CA, USA) with a mobile phase of sodium citrate buffer (pH 4·1, 50 mmol/l)–acetonitrile (6:4, v/v) at a flow rate of 1 ml/min. The limit of quantitation for Gen was 12 ng/ml.
The data are presented as the mean of separate determinations from two different experiments.
Statistical analysis
The effect of diet treatment was assessed by ANOVA with Statistical Package for the Social Sciences software (IBM, Beijing, People's Republic of China). Values are presented as means and standard deviations. If significant differences were observed, the least significant difference t test was applied to the multiple comparisons of means between each group.
Results
Effects of genistein on cognitive abilities of ovariectomised rats
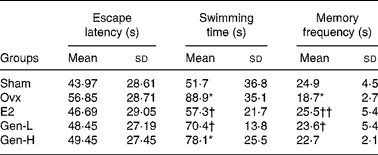
In the visual spatial learning and memory test, the average escape latency declined rapidly during the five consecutive days. Although there was a trend of decline of the escape latency in the E2 and Gen-L groups on days 2, 3 and 5 than in the Ovx group, there were still no significant differences among the population means of the five groups at any time point (Table 1).
Table 1 Average escape latency in the spatial learning and memory test and swimming and memory frequency in the spatial probe trial test of rats under different treatments (n 13)
(Mean values and standard deviations)
Ovx, ovariectomised; E2, 17β-oestradiol; Gen-L, genistein-low; Gen-H, genistein-high.
Mean values were significantly different from the sham group: *P < 0·05.
Mean values were significantly different from the Ovx group: †P < 0·05, ††P < 0·01.
In the spatial probe trial test, the memory frequency on the original platform quadrant and the swimming time taken to find the original position of the platform were determined. The escape latency was enhanced, while the memory frequency was reduced in the Ovx group than in the sham group. The declined cognitive abilities after ovariectomy were significantly improved in the E2 and Gen-L groups as exhibited by reduced escape latency and increased memory frequency. Neither of the two parameters was improved in the Gen-H group (Table 1).
Acetylcholinesterase activity
AchE activity was significantly higher in the Ovx group than in the sham group (P = 0·007 in the hippocampus and P = 0·043 in the cortex), and was remarkably suppressed by either E2 or Gen treatment. To our surprise, AchE activity was suppressed by Gen so profoundly (P = 0·006 in the hippocampus, P = 0·008 in the cortex v. Ovx) that the magnitude of suppression was even higher than that observed in the E2 group (Fig. 1).

Fig. 1 Acetylcholinesterase (AchE) activities in the frontal cortex and hippocampus of rats orally administered with 17β-oestradiol (E2), 15 mg/kg genistein (Gen-low (L)) and 30 mg/kg (Gen-high (H)) for 6 weeks (n 5). Values are means and standard deviations. Mean values were significantly different when compared with the sham group: *P < 0·05, **P < 0·01. Mean values were significantly different when compared with the ovariectomised (Ovx) group: †P < 0·05, ††P < 0·01. □, Sham; ▨, Ovx; ![]() , E2; ▧, Gen-L;
, E2; ▧, Gen-L; ![]() , Gen-H.
, Gen-H.
Effects of genistein on brain superoxide dismutase, malondialdehyde and monoamine oxidases
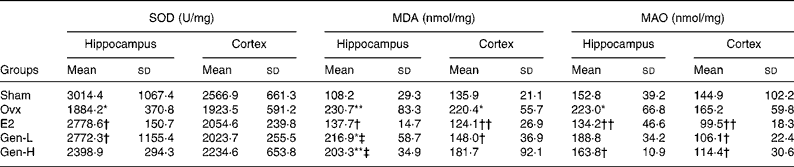
To test the degree of oxidative damages, brain SOD, MDA and MAO were measured (Table 2). In the hippocampus, there were significant differences of SOD level among the five groups (P = 0·043), while in the cortex, no differences were found. It was further demonstrated by least significant difference t test that there was a sharp decline of hippocampus SOD in the Ovx group than in the sham controls, which could be recovered by E2 or Gen-L treatment (Table 2). It was noteworthy that 30 mg/kg Gen could not exert the same effect as 15 mg/kg Gen, suggesting a dose-dependent regulation of SOD by Gen.
Table 2 Mitochondria-generated superoxide dismutase (SOD), monoamine oxidases (MAO) and malondialdehyde (MDA) in the frontal cortex and hippocampus of rats under different treatments (n 5)
(Mean values and standard deviations)
Ovx, ovariectomised; E2, 17β-oestradiol; Gen-L, genistein-low; Gen-H, genistein-high.
Mean values were significantly different from the sham group: *P < 0·05, **P < 0·01.
Mean values were significantly different from the Ovx group: †P < 0·05, ††P < 0·01.
‡ Mean values were significantly different from the E2 group (P < 0·05).
In both the hippocampus and cortex, MDA was remarkably higher in the Ovx group than in the sham controls (P = 0·004 in the hippocampus and P = 0·031 in the cortex). E2 could attenuate the elevated MDA levels in both of the regions (P = 0·008 in the hippocampus and P = 0·007 in the cortex v. Ovx). On the other hand, neither dose of Gen could affect the elevated MDA levels in the hippocampus, but in the cortex, MDA level was reduced by the low dosage of Gen (P = 0·005) (Table 2).
MAO was elevated after ovariectomy in the hippocampus, and it was recovered at a level that was similar to that observed in the sham controls by both E2 and high dose of Gen (Table 2). In the cortex, although MAO was not altered by ovariectomy, it could be suppressed by both E2 and Gen.
Effects of genistein on the apoptosis of the brain
Caspase-3 activity in the hippocampus region was increased in the Ovx group (247·24 (sd 27·50) pmol/mg) than in the sham group (102·53 (sd 39·86) pmol/mg, P = 0·003). Treatment with low dose and high dose of Gen could inhibit caspase-3 activity to 157·36 (sd 29·97) and 144·99 (sd 27·91) pmol/mg, respectively (P = 0·036). Supplementation with E2 could decrease caspase-3 activity to even lower levels (93·87 (sd 16·78) pmol/mg, P = 0·002).
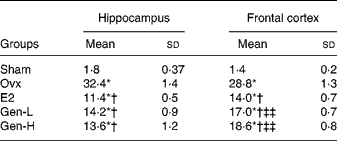
In line with caspase-3, in both the hippocampus and cortex, the apoptotic cells as detected by TUNEL staining were notably enhanced in the Ovx group than in the sham controls (Fig. 2(a) and (b)). Replacement of Gen and E2 could reduce the number of the apoptotic cells prominently in both the hippocampus and cortex to almost a similar extent (Fig. 2 and Table 3).

Fig. 2 Representative micrographs of TUNEL-positive cells in the frontal cortex (a) and hippocampal CA1 region (b) from negative control (A), sham (B), ovariectomised (C), 17β-oestradiol (D), genistein-low (E) and genistein-high (F) rats (n 8).
Table 3 The number of terminal deoxynucleotidyl transferase dUTP nick end labelling-positive cells per square micrometre in the brain (n 8)
(Mean values and standard deviations)
Ovx, ovariectomised; E2, 17β-oestradiol; Gen-L, genistein-low; Gen-H, genistein-high.
* Mean values were significantly different from the sham group (P < 0·05).
† Mean values were significantly different from the Ovx group (P < 0·05).
‡‡ Mean values were significantly different from the E2 group (P < 0·01).
Genistein concentrations
Gen concentrations in both sham and Ovx groups were too low to be detected, while the levels of Gen in both the brain and serum were still at the physiological range (Table 4). Gen levels in the Gen-H group that were even lower than those in the Gen-L group and serum concentration that was almost three times of that in the tissue were of particular interest.
Table 4 Genistein concentrations in the brain and serum determined by HPLC in ovariectomised rats after oral administration with genistein for 6 weeks (pooled by treatment, n 5 per group)
(Mean values and standard deviations)

Gen-L, genistein-low; Gen-H, genistein-high.
Discussion
In the present study, we explored the novel antioxidant mechanism of Gen on neuroprotection in Ovx rats. Our data revealed that Gen could inhibit the neurodegeneration induced by ovariectomy via modulation of mitochondrial oxidative stress.
Gen that was orally administered at 15 mg/kg instead of at 30 mg/kg for 6 weeks was able to reduce the escape latency and to increase the memory frequency, which were compromised by ovariectomy. In the visual spatial learning and memory test, although the escape latency showed no difference among the five groups, there was still a trend of decline in the E2 and Gen-L groups. It was not surprising that in Xu et al. study(Reference Xu, Zhu and Shi8), the average escape latency was significantly reduced by Gen that was injected subcutaneously at about 10 mg/kg every other day for 5 weeks. This discrepancy might be due to Gen being purchased from different companies or the disparate injection routes used, subcutaneously in Xu et al. study and through oral administration in our rats, both of which might result in differential Gen levels. The absence of improvement in the learning abilities in the Gen-H group was not unexpected. Considering the non-linear pharmacokinetics of Gen in the gastrointestinal tract due to its low water solubility and transportation saturability, the concentrations of Gen in both the serum and brain of the Gen-H rats were even lower than those in the Gen-L rats in the present study, which thus might be less biologically effective.
It was reported previously that the rats fed on soya isoflavone diet took significantly shorter time for swimming than the group fed on the control diet in spatial delayed matching-to-place performance(Reference Lee, Lee and Won14). Moreover, isoflavone supplementation in healthy males may also enhance cognitive processes(Reference Thorp, Sinn and Buckley15). Gen is only one of the six constituents of isoflavone, thus it may be less effective than that given in combination(Reference Zhao, Mao and Brinton9). In a double-blind randomised trial, the use of soya protein containing isoflavones did not improve cognitive function in healthy women when it was administered at the age of 60 years or later(Reference Kreijkamp-Kaspers, Kok and Grobbee16). This implies that the effect of Gen may be influenced by the timing of administration. In the present study, Gen was given 1 week after the surgery, while in Xu's study(Reference Xu, Zhu and Shi8), it was given from the second day after the surgery. The later the administration of the Gen is done, the less is the effect it exerts.
In the spatial probe trial test, Gen did exert a beneficial effect. The memory frequency was reduced and the swimming time was prolonged in the Ovx group, both of which were reversed by Gen-L treatment. The present study along with Xu's study(Reference Xu, Zhu and Shi8) suggested that Gen at a certain dose was able to improve the memory abilities in rats.
It is intriguing that the memory abilities were only improved in the Gen-L group but not in the Gen-H group. The effect of phyto-oestrogen was reported to differ depending on the dose and the sex. In the delayed matching-to-place water maze task, the swimming time was shortened by use of low levels of isoflavones (0·3 g/kg), but no change was found when higher levels of isoflavones were used (1·2 g/kg)(Reference Lee, Lee and Won14). This may be explained by the fact that Gen at a high dose may induce cytotoxicity and apoptosis in the brain(Reference Choi and Lee17, Reference Jones, Barnes and Kirkby18), and could increase the levels of cleaved caspase-3 in vivo and in primary neuronal culture(Reference Choi and Lee17).
Besides the lower concentrations of Gen in the Gen-H group accounting for the less efficiency of higher dose Gen, there might be other possibilities. In our previous study, the percentages of serum Gen v. total Gen (Gen plus glucuronidated Gen) after intake of different doses of Gen were 18·68 % for 12·5 mg/kg and 4·16 % for 50 mg/kg, respectively. Despite the total Gen levels being still higher at 50 mg/kg dose than at 12·5 mg/kg dose, the conjugated form might not be an effective antioxidant as Gen.
It was reported that isoflavones are ingested mainly in the glycoside form, and undergo extensive hydrolysis by intestinal and bacterial β-glucosidases that release the principal aglycones, Gen and daidzein. Following absorption in the gastrointestinal tract, isoflavones are conjugated with glucuronic acid or sulphuric acid by the enzymes in the intestinal epithelium and the liver(Reference Rimbach, Weinberg and de Pascual-Teresa19). The formation of conjugates converts isoflavones to more water-soluble products, and may affect their chemical and biological activities. For example, the gut and liver metabolite equol can be a more potent antioxidant than its parent isoflavone daidzein. However, sulphation, which masks the important hydroxyl groups of the isoflavone molecules, could decrease the antioxidant potential of these compounds(Reference Turner, Baron and Wolffram20). Since most of the Gen circulates as a conjugate at a high-dose intake, the glucuronidated Gen might not be an effective antioxidant in vivo.
To further investigate the neurotransmitters that account for the cognitive improvement by Gen, AchE activity was examined in the brain. Cholinergic parameters decline continuously during ageing in the hippocampus, cortex and other areas related to recognition(Reference Jones, Barnes and Kirkby18). In the present study, the rats subjected to ovariectomy presented a significant increase in AchE activity, which was similar to that reported previously(Reference Monteiro, Matte and Delwing21). For the first time, we have also observed the in vivo inhibitory effect of Gen on AchE activity, which was consistent with that found in soya isoflavones(Reference Lee, Lee and Won14), suggesting that Gen could ameliorate deficits in memory tasks resulting from the loss of cholinergic input or cholinergic degeneration in Ovx rats.
Recent evidence has indicated that phyto-oestrogen may protect against the neurodegeneration via maintenance of mitochondrial structure and functions(Reference Xu, Shi and He22). This prompted us to examine the key parameters of mitochondrial oxidative stress after Gen treatment.
First, there were significant differences in SOD content in the hippocampus, but not in the cortex, among the five groups. This suggests that modulation of brain SOD by Gen may be region dependent. E2 or Gen-L treatment, but not Gen-H treatment, could recover the decline of SOD by Ovx (Table 2), suggesting that modulation of brain SOD by Gen was also dose dependent. It was reported previously that Gen could result in a relatively higher SOD level in cultured cortical neurons(Reference Ho, Li and Zhao23) and reverse the decreased serum SOD in Ovx rats(Reference Xu, Zhu and Shi8), both of which reflect the effect of Gen on brain SOD indirectly. For the first time, we have also reported SOD content in the tissue from two regions of the brain, which could reveal the neuroprotective effect of Gen more precisely.
Secondly, MDA and MAO levels could be enhanced after ovariectomy, which were further suppressed by E2. The increased MDA level was only inhibited in the cortex in the Gen-L group, suggesting a region- and dose-dependent effect of Gen. In the cerebral injury model of ischaemia/reperfusion, Gen was shown to decrease reactive oxygen species generation, MDA concentration and the apoptotic indices in rat hippocampus(Reference Liang, Qiu and Shen24). The oxidative damage in this model was more severe than that in the Ovx rats, and the hippocampus was more inclined to be insulted. Our findings of MDA modulation in the brain tissue were supportive of those of Xu's study(Reference Xu, Zhu and Shi8), in which Gen could reduce the serum MDA distinctly.
Lastly, we found that Gen could suppress MAO activity in the brain. In the cortex, MAO was remarkably reduced by Gen at both doses, but in the hippocampus, it was only decreased in the Gen-H group but not in the Gen-L group. The dose independency of MAO inhibition added more complexity to the action of Gen in the brain. Monoaminergic signalling is regarded as one of the key mechanisms for the modulation of mood and emotion, as well as for the control of motor, perceptual and cognitive functions(Reference Bortolato, Chen and Shih3). Attenuation of the oxidative stress by the inactivation of MAO may contribute to enhanced cognitive ability by Gen.
Significant attenuation of mitochondrial stress by Gen may result in less apoptosis being induced by ovariectomy. In cultured cortical neurons, Gen was observed to down-regulate caspase-3 expression and lead to fewer apoptotic cells(Reference Yu, Li and Zhang25). We found that Gen treatment not only decreased the number of TUNEL-positive cells, but also reduced the level of caspase-3 activity in the brain, indicating that the neuroprotection of Gen is through the inhibition of the mitochondria-mediated apoptotic pathway.
Other mechanisms may also account for the enhanced memory induced by Gen. The activation of cyclic AMP-response element-binding protein-dependent gene expression is thought to be critical for the formation of different types of long-term memory(Reference Ho, Li and Zhao23). As Gen was reported to induce cyclic AMP-response element-binding protein phosphorylation in the medial preoptic area and anteroventral periventricular nucleus(Reference Liang, Qiu and Shen24), it is possible that Gen also exerts its effect via the activation of cyclic AMP-response element-binding protein in the present study. Besides the activation of transcription factors described above, phyto-oestrogen could also bind to oestrogen receptor-β to exhibit its effect on neuronal survival against toxic insults and promote neural proactive defence mechanisms against neurodegeneration(Reference Yu, Li and Zhang25). In summary, Gen exerted its neuroprotective effect via multiple pathways, one of which was by modulation of the mitochondrial oxidative stress, a newly emerging concept that may lead to in-depth understanding of its biological characteristics.
The present study systemically demonstrated the antioxidant activities of Gen in different brain regions and its ultimate consequence on neurodegeneration in rats, which could be extrapolated to women after menopause; nevertheless, the prescription of Gen for long-term use should be carefully considered(Reference Xu, Zhu and Shi8).
Acknowledgements
This work was supported by Chinese National Natural Science Foundation (no. 30973120). The authors have no conflict of interest to declare. Y.-H. H. designed and conducted the study. Q.-H. Z. initiated the experiments and wrote the manuscript.








