Introduction
Grain sorghum was harvested on more than 2 million hectares in the United States in 2019 (NASS 2019a). The challenges of individual management strategies, along with low commodity prices, cause grain sorghum production to widely fluctuate year to year (Espinoza and Kelley Reference Espinoza and Kelley2004). Producers who grow grain sorghum face challenges controlling disease and insects (Moore et al. Reference Moore, Ditmore and TeBeest2009; Singh et al. Reference Singh, Padmaja and Seetharama2004), but perhaps the most troublesome pests in grain sorghum are weeds. When not controlled in a timely manner, weeds compete with grain sorghum, reducing yields and contributing to increased weed seed in the soil seedbank (Espinoza and Kelley Reference Espinoza and Kelley2004). Burnside and Wicks (Reference Burnside and Wicks1969) found sorghum yield may be reduced by 4%, 12%, and 18% when weeding is delayed by 3, 4, and 5 weeks, respectively. Because grain sorghum is a relatively low-input crop, economical and cost-effective approaches to controlling weeds are vital.
Results from a survey conducted by Webster (Reference Webster2012) indicated that the top five most troublesome weeds in Arkansas, Alabama, Florida, Georgia, Mississippi, Missouri, and Texas grain sorghum were barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], Palmer amaranth [Amaranthus palmeri (S.) Watson], morningglories (Ipomoea spp.), broadleaf signalgrass [Urochloa platyphylla (Munro ex C. Wright) R.D. Webster], and johnsongrass [Sorghum halepense (L.) Pers.]. Johnsongrass is especially problematic and difficult to control given genetic similarities between it and grain sorghum and the ineffectiveness of atrazine on this weed (Kegode et al. Reference Kegode, Bishnoi and Mays1994). Atrazine does not control johnsongrass, and other herbicides are needed to control this problematic weed. Although all weeds have potential to result in grain sorghum yield loss, Feltner et al. (Reference Feltner, Hurst and Anderson1969) reported that broadleaf weeds left uncontrolled hinder yield more than weedy grasses do.
Producers of cotton (Gossypium hirsutum L.), corn, soybean [Glycine max (L.) Merr.], and rice (Oryza sativa L.) may be able to cope with weed pressure using new herbicide-resistant crop technologies, but grain sorghum producers are restricted to a narrow selection of labeled herbicides. Herbicides such as 2,4-D, dicamba, mesotrione, prosulfuron, and bromoxynil can be used for effective POST control of many broadleaf weeds, including the ones earlier noted to be most problematic, although timing of application according to weed size is vital for good control (Barber et al. Reference Barber, Butts, Selden, Norsworthy, Burgos and Bertucci2020). However, atrazine is currently the foundational broad-spectrum herbicide used for weed control in grain sorghum, as evidenced by it being applied to more than 60% of the U.S. grain sorghum hectares annually (NASS 2019b). Atrazine controls cocklebur (Xanthium strumarium L.), common ragweed (Ambrosia artemisiifolia L.), morningglories, and Palmer amaranth, as well as an assortment of grassy species when applied PRE or POST (Anonymous 2018; Culpepper and York Reference Culpepper and York1999; Geier et al. Reference Geier, Stahlman, Regehr and Olson2009; Krausz and Kapusta Reference Krausz and Kapusta1998; Sprague et al. Reference Sprague, Kells and Penner1999; Webster et al. Reference Webster, Cardina and Loux1998).
Use of atrazine continues to draw scrutiny because of its persistence in the environment and sometimes contamination of waterbodies surrounding agricultural areas (Barbash et al. Reference Barbash, Thelin, Kolpin and Gilliom2006). One way to reduce the impact of atrazine is to limit the amount of the herbicide applied to agricultural soils, but herbicide options as effective as atrazine are unlikely to be available. Furthermore, there are fewer herbicide options available in grain sorghum than in other agronomic crops. It is well established that weed control is often improved by mixing 4-hydroxylphenylpryuvate dioxygenase inhibitors with PSII-inhibiting herbicides (Kohrt and Sprague Reference Kohrt and Sprague2017). In addition, S-metolachlor provides effective control of many annual grasses and small-seeded broadleaf weeds in grain sorghum (Bararpour et al. Reference Bararpour, Hale, Kaur, Singh, Tseng, Wilkerson and Willett2019) and would likely bring improved weed control to grain sorghum production when used in mixture with a PSII-inhibiting herbicide. For this reason, research was initiated to test grain sorghum tolerance to PSII-inhibiting herbicides alone and in combination with S-metolachlor (PRE or POST) and mesotrione (POST only).
Materials and Methods
Grain Sorghum Trial Common Methodology
Field experiments tested grain sorghum tolerance to PRE and POST applications of PSII-inhibiting herbicides in 2017 and 2018. All grain sorghum experiments were planted in May or early June each year to variety DK553-67 (Dekalb, St Louis, MO), which was fluxofenim-treated (Concep®; Syngenta, Greensboro, NC) and planted at 197,000 seeds ha−1 into conventionally tilled, raised beds at a 2-cm depth (Table 1). Plot size was 3.7 m wide by 6.1 m long and all rows were spaced 91 cm and 97 cm apart in Fayetteville and Marianna experiment locations, respectively. Grain sorghum trials were maintained weed-free with labeled applications of quinclorac (Facet L; BASF Corp., Research Triangle Park, NC) and by hand-weeding as needed. All trials were furrow irrigated as needed to prevent drought stress. Grain sorghum trials were otherwise managed according to the Arkansas Grain Sorghum Production Handbook to prevent nutrient and pest stresses to the crop (Espinoza and Kelley Reference Espinoza and Kelley2004).
Table 1. Planting, herbicide application, and harvest dates for PRE and POST grain sorghum trials in Fayetteville and Marianna, AR, in 2017 and 2018.

PRE and POST Experimental Sites
PRE and POST field experiments were conducted both years on a Captina silt loam (fine-silty, siliceous, active, mesic Typic Fragiudults) at the Arkansas Agricultural Research and Extension Center (36.09oN, -94.17oW in Fayetteville, AR. The soil at Fayetteville consisted of 34% sand, 53% silt, and 13% clay, with an organic matter content of 1.5% and a pH of 6.8. The PRE study was also conducted on a Memphis silt loam (fine-silty, mixed, active, thermic Oxyaquic Fragiudalfs) at the Lon Mann Cotton Research Station (LMCRS) (34.73oN, -90.74oW) near Marianna, AR. The soil at this site consisted of 4% sand, 81% silt, and 15% clay, with an organic matter content of 1.3% and a pH of 6.6. In addition to the Fayetteville location, the POST study was conducted for 2 yr on a Calloway silt loam (fine-silty, mixed, active, thermic Aquic Fraglossudalfs) at the LMCRS (34.73oN, -90.74oW). The soil at this site consisted of 12% sand, 70% silt, and 18% clay, with an organic matter content of 1.25% and a pH of 6.4.
PRE and POST Study Experimental Setup and Data Collection
All experiments were designed as a factorial, randomized complete block; the two factors were (1) PSII herbicide and (2) the herbicide added to create the mixture. The PSII herbicides were prometryn, ametryn, simazine, fluometuron, metribuzin, linuron, diuron, atrazine, and propazine (Table 2). PSII-inhibiting herbicides were applied at the same rate they would be applied in a labeled crop (Anonymous 2020; Barber et al. Reference Barber, Butts, Selden, Norsworthy, Burgos and Bertucci2020). Of these herbicides, propazine is the only one other than atrazine labeled for use in grain sorghum, and its use is restricted to a PRE-only application (Anonymous 2020). The second factor consisted of either no herbicide or S-metolachlor for the PRE study and either no herbicide, mesotrione, or S-metolachlor for the POST study. A nontreated was included for both studies. All treatments were applied at 140 L ha−1 using a CO2-pressurized backpack sprayer fitted with AIXR 110015XR nozzles (TeeJet Technologies, Wheaton, IL) immediately after grain sorghum planting. The experiment consisted of 19 experimental treatments, including the nontreated, with each treatment replicated four times. Visible crop injury was estimated at 14 and 28 d after application (DAA) on a scale from 0 to 100, where 0 represented no crop injury and 100 represented complete crop necrosis. Canopy height of three random plants plot−1 was measured and recorded 28 DAA. Relative height was calculated by dividing the average of each plot by the overall average of the nontreated plots. Heights were not recorded in Marianna during 2017 because of an oversight and timing issue. Yield of the center two rows in each plot was collected with a small-plot combine and recorded (in kg ha−1) after adjusting to 14% moisture, and relative yield was determined by dividing the average of each plot by the overall average of the nontreated plots.
Table 2. Herbicide names, rates, and manufacturers for PRE and POST grain sorghum trials in 2017 and 2018.
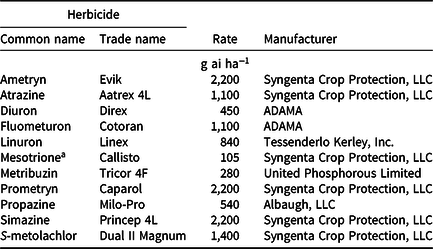
a Mesotrione applied only in POST trial.
Statistical Analyses
Analyses for the two trials were conducted in the same manner. To account for different environments and growing conditions between locations and years, all environments and replications nested within environments were considered random effects to permit inferences to be made over a range of conditions (Blouin et al. Reference Blouin, Webster and Bond2011; Carmer et al. Reference Carmer, Nyquist and Walker1989). Visual estimates of crop injury for the nontreated plots in all site-years were zero and, therefore, were excluded from analysis. Relative height and relative yield for nontreated plots in all site-years were equal to 1 and, therefore, were excluded from analysis. Data were subjected to ANOVA using the GLIMMIX procedure in SAS, version 9.4 (SAS Institute Inc., Cary, NC), assuming a beta distribution for all assessments to see if the main PSII-inhibiting herbicide, the additive herbicide, or the interaction had an effect (Gbur et al. Reference Gbur, Stroup, McCarter, Durham, Young, Christman, West and Kramer2012). Mean separations were analyzed for injury, relative crop height, and relative yield using Fisher protected LSD (α = 0.05) where appropriate.
Results and Discussion
PRE Study
Rainfall
All studies received at least 2 cm of rainfall within 5 d of application (data not shown). Hence, it was assumed all herbicides were properly activated.
Injury
Grain sorghum injury 14 DAA was influenced by both main effects of PSII herbicide and herbicide added (Table 3), with less than 10% injury from all PSII herbicides, averaged over herbicide added (Table 4). When averaged over herbicide added, all injury was comparable to atrazine-containing treatments. When averaged over PSII herbicide, grain sorghum injury from S-metolachlor–containing treatments was more than treatments with PSII herbicide alone (Table 4).
Table 3. Significance of P for interactions and main effects of PSII herbicide and herbicide added on grain sorghum injury, relative height, and relative yield by application timing in grain sorghum trials conducted at Fayetteville and Marianna, AR, in 2017 and 2018. a b c

a Data averaged across site-years within a timing.
b Refer to Table 2 for herbicides and rates.
c The Marianna 2017 site-year was excluded from the relative height analysis.
d Abbreviations: DAA, days after application; PSII, photosystem II.
e Significant at P < 0.05.
Table 4. Grain sorghum injury, relative height, and relative yield as influenced by PSII herbicide and herbicide added in PRE trials, averaged over site-years at Fayetteville and Marianna, AR, in 2017 and 2018.

a Means within a factor followed by the same letter are not significantly different according to Fisher protected LSD (P = 0.05).
b Height of plants in nontreated plots averaged across site-year was 26 cm. The Marianna 2017 site-year was excluded from the analysis.
c Yield of nontreated plots averaged across site-years was 5,180 kg ha−1.
d Abbreviations: DAA, days after application, PSII, photosystem II.
e Injury averaged over herbicide added.
f Injury averaged over PSII herbicide.
Again at 28 DAA, injury was influenced by both main effects (Table 3). Averaged over herbicide added, none of the PSII-inhibiting herbicides differed from atrazine in causing injury to grain sorghum (Table 4). When averaged over PSII herbicide at 28 DAA, S-metolachlor–containing treatments caused statistically greater injury than PSII herbicides alone, but the injury was unlikely of biological significance. Injury observed at 14 and 28 DAA was less than 12% for all treatments.
Relative height
Crop height was influenced by the herbicide added (Table 3). Generally, S-metolachlor–containing treatments, averaged over PSII herbicide, caused a 15% height reduction relative to plants from nontreated plots, which was greater than with PSII herbicide alone (Table 4). Similarly, Geier et al. (Reference Geier, Stahlman, Regehr and Olson2009) found that S-metolachlor at 2.8 kg ha−1, when applied PRE in combination with atrazine at 1.12 kg ha−1, may cause occasional stunting in grain sorghum. Although height was reduced only by a few centimeters, this reduction corroborates the injury that was observed 28 DAA in the present study (Table 4).
Relative yield
Relative yield was influenced only by the main effect of PSII herbicide (Table 3). Although there was minimal injury and height reduction, grain sorghum treated with atrazine had significantly less yield reduction than did plots treated with other PSII treatments (Table 4). The reduction in grain sorghum yield from propazine, a currently labeled PRE option (Anonymous 2020), was surprising, and the cause for this loss is not known. Given the yield loss associated with each PSII herbicide other than atrazine, it appears there was a yield-loss component that went unmeasured. Although not directly measured in this study other than through injury evaluations, one potential reason for the yield loss observed could be attributed to a reduction in crop density caused by other nonatrazine-containing treatments. Another reason may be a hindrance in physiological development. Saeed et al. (Reference Saeed, Francis and Clegg1986) demonstrated that the period from emergence to bloom was vital for number of heads plant−1 and seeds head−1. If the sorghum plants are using more energy for metabolism of herbicides than is needed for atrazine during this time and not toward development, the effects could be observed in the yield. More research is needed to determine the yield loss mechanism(s) caused by these PSII herbicides and any differential effects on physiological development among them.
POST Study
Injury
Injury was influenced by an interaction between PSII herbicide and herbicide added 14 DAA (Table 3). Ametryn- and prometryn-containing treatments caused greater than 28% injury to grain sorghum plants, which was greater than other treatments (Table 5). Injury of the other treatments was less than 20%. Except for ametryn-, diuron-, and linuron-containing treatments, the addition of mesotrione to each PSII herbicide increased injury to grain sorghum (Table 5). The increased injury could be due to the synergy that occurs between some PSII herbicides and mesotrione (Abendroth et al. Reference Abendroth, Martin and Roeth2006). Except for diuron- and propazine-containing treatments, the addition of S-metolachlor did not increase injury from a PSII herbicide. Unlike mesotrione, S-metolachlor rarely induces foliar symptomology but is taken up only through the roots and shoots of plants (Fuerst Reference Fuerst1987).
Table 5. Grain sorghum injury, relative height, and relative yield as influenced by interactions between PSII herbicide and herbicide added in POST trials, averaged over site-years at Fayetteville and Marianna, AR, in 2017 and 2018.

a Abbreviations: DAA, days after application; PSII, photosystem II.
b Means within a column followed by the same letter are not significantly different according to Fisher protected LSD (P = 0.05).
c Height in the nontreated plots averaged across site-years was 72 cm. The Marianna 2017 site-year was excluded from the analysis.
d Yield in the nontreated plots averaged across site-years was 5,448 kg ha−1.
Injury was influenced by the main effects of PSII herbicide and herbicide added 28 DAA (Table 3). Averaged over the herbicide added, ametryn- and prometryn-containing treatments caused 14% and 16% injury, respectively, which was more than other PSII herbicides (Table 6). All other PSII herbicides caused comparable injury to atrazine-containing treatments, excluding linuron-containing treatments, which caused 6% injury. Averaged over PSII herbicides, mesotrione-containing treatments caused greater injury 28 DAA than treatments with no herbicide added or treatments with S-metolachlor (Table 6).
Table 6. Grain sorghum injury as influenced by PSII herbicide and herbicide added in POST trials, averaged over site-years at Fayetteville and Marianna, AR, in 2017 and 2018. a

a Abbreviations: DAA, days after application; PSII, photosystem II.
b Means within a factor followed by the same letter are not significantly different according to Fisher protected LSD (P = 0.05).
c Injury averaged over herbicide added.
d Injury averaged over PSII herbicide.
Relative height
Relative height was influenced by an interaction between PSII herbicide and herbicide added 28 DAA (Table 3). Ametryn- and prometryn-containing treatments, excluding prometryn alone, reduced grain sorghum height compared with atrazine-containing treatments. The only other treatment that was not comparable to any atrazine-containing treatment was linuron alone, with a 13% height reduction relative to the nontreated (Table 5). Generally, treatments that caused injury greater than 25% 14 DAA reduced height by 10% or more. Although height was reduced by certain herbicide combinations when compared with atrazine combinations, most treatments did not cause a biologically meaningful difference in height.
Relative yield
Yield was influenced by an interaction between PSII herbicide and herbicide added (Table 3). Grain sorghum yield for all treatments was comparable to atrazine-containing treatments, except prometryn plus mesotrione, which also had the highest level of grain sorghum injury 14 DAA and the greatest height reduction (Table 5). Overall, yield from 14 of 15 treatments was comparable to atrazine-containing treatments.
Recommended Additional Testing
Recommending which herbicides should undergo additional resting and evaluation relative to atrazine should be based on all response variables. However, yield is likely considered the most important crop response for producers. For PRE-applied PSII herbicides, alternatives to atrazine may be difficult to find, based on grain sorghum yield reductions caused by all herbicides evaluated. One possibility is to reduce the rate of these herbicides or test additional ones other than the few evaluated in this experiment. Similarly, for the POST study, herbicide treatments that resulted in grain sorghum yields significantly less than the atrazine-containing treatments should not undergo additional testing as viable replacements at the rates evaluated. Considering crop injury as a factor that heavily weighs on consideration of acceptable tolerance, a level of 15% injury was chosen to refine the list of herbicides that should be evaluated in future trials. Using this criteria, any ametryn- or prometryn-containing treatments should not be tested further at these rates. On the basis of both visible injury and grain sorghum yield, it is recommended that additional research on weed control and crop tolerance be conducted for POST applications of diuron, fluometuron, linuron, metribuzin, prometryn, propazine, and simazine. Several of these herbicides are labeled in other agronomic crops such as cotton, soybean, or corn (Barber et al. Reference Barber, Butts, Selden, Norsworthy, Burgos and Bertucci2020), but understanding the weed control value at the rates and combinations tested in this experiment would be beneficial.
Acknowledgments
This research was funded by the Arkansas Corn and Grain Sorghum Board. No conflicts of interest are reported.








