Introduction
The round sardinella Sardinella aurita Valenciennes, 1847 is a very common small pelagic fish widely distributed in the Atlantic Ocean. On the east side, it is found from the Strait of Gibraltar to South Africa, and eastward to the Mediterranean Sea and Black Sea (Froese & Pauly, Reference Froese and Pauly2017). During recent decades, this species has become an important fishery resource in north-west Africa, constituting the main small pelagic fish caught in most of the riparian countries of the region (Morocco, Mauritania, Senegal, Gambia and Guinea-Bissau) (Mbaye et al., Reference Mbaye, Brochier, Echevin, Lazar, Lévy, Mason, Gaye and Machu2015; Ba et al., Reference Ba, Schmidt, Dème, Lancker, Chaboud, Cury, Thiao, Diouf and Brehmer2017; Corten et al., Reference Corten, Braham and Sadegh2017). In this area, no conclusive information is available on the population structure of S. aurita and it is assumed that there is a single population which migrates among the different Exclusive Economic Zones (EEZs) shared by these countries (Garcia, Reference Garcia1982; Bacha et al., Reference Bacha, Jeyid, Jaafour, Yahyaoui, Diop and Amara2016; Corten et al., Reference Corten, Braham and Sadegh2017; Ba et al., Reference Ba, Chaboud, Schmidt, Diouf, Fall, Dème and Brehmer2019).
In NW African waters, round sardinella is targeted by industrial and artisanal fleets (FAO, 2018), the former through international access agreements and private arrangements. Since 1996, freezer-pelagic trawlers from the European Union (EU) have exploited small pelagic fish in this region, mainly in the Mauritanian EEZ (ter Hofstede & Dickey-Collas, Reference ter Hofstede and Dickey-Collas2006; Zeeberg et al., Reference Zeeberg, Corten and de Graaf2006). This European fleet targets S. aurita jointly with Sardina pilchardus (Walbaum, 1792), Sardinella maderensis (Lowe, 1838), Trachurus spp. and Scomber colias Gmelin, 1789. In addition, round sardinella demand has increased with the establishment of fishmeal industries, but also with the opening of new export markets in the sub-region, which inevitably contributes to the increase in fishing effort in the area (Corten et al., Reference Corten, Braham and Sadegh2017; Diankha et al., Reference Diankha, Ba, Brehmer, Brochier, Sow, Thiaw, Gaye, Ngom and Demarcq2018).
The overall assessment of the small pelagic fish in the Central-East Atlantic is coordinated by the Food and Agriculture Organization of the United Nations (FAO). Since 2001, a permanent working group on the assessment of small pelagic fish off north-west Africa yearly reports updated information for the north sub-region to the Committee for the Eastern Central Atlantic Fisheries (CECAF), including Atlantic Morocco, the Canary Islands (Spain), Mauritania, Senegal and Gambia (Figure 1). This Regional Fishery Body reports that available data do not allow conclusive results to be determined through a variety of assessment models. In this sense, based on the decreasing trend of the catch per unit effort (CPUE) and the scarce biological and ecological information for the fish species in the area, the status of fish species within NW African waters has been considered to be overexploited as a precautionary approach, and a general reduction of the fishing effort has been recommended for the whole sub-region (FAO, 2019).
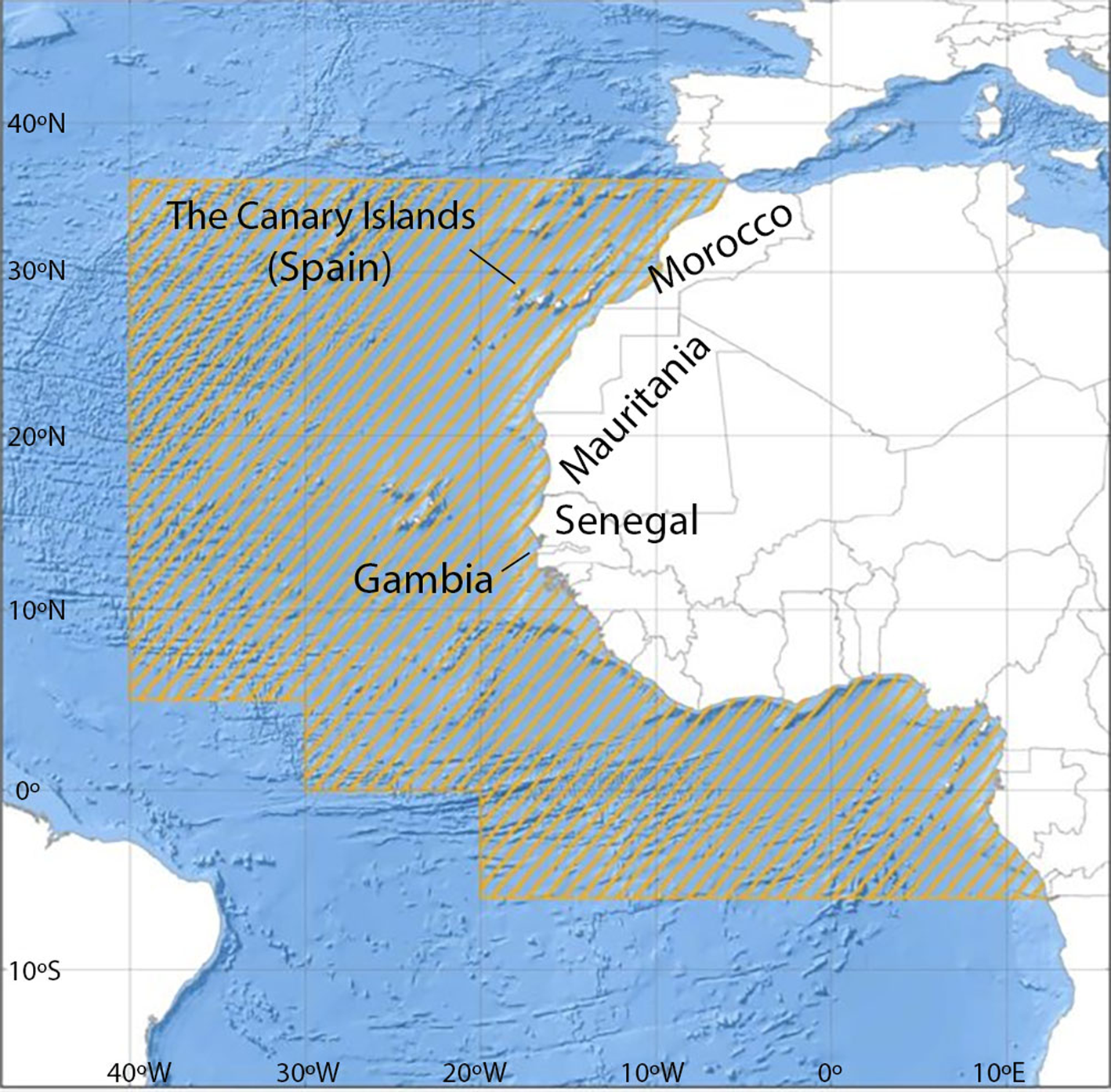
Fig. 1. CECAF Competence area (dashed). The northern sub-region includes the indicated countries (modified from FAO 2016).
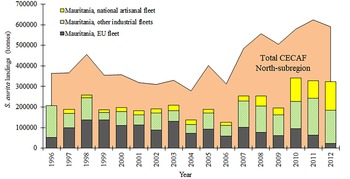
Regarding the round sardinella caught in Mauritanian waters, landings (including industrial and artisanal catches) varied between 126,000 and 342,000 tonnes (for the period 1996–2012), constituting around 52% of the overall landings of S. aurita reported for the northern CECAF sub-region at this time. Among the industrial and national artisanal fleets operating in Mauritania, the EU fleet produced an annual mean value of almost 90,000 tons, that represents more than 40% of the round sardinella landed in the country during the same period (Figure 2) (FAO, 2018).
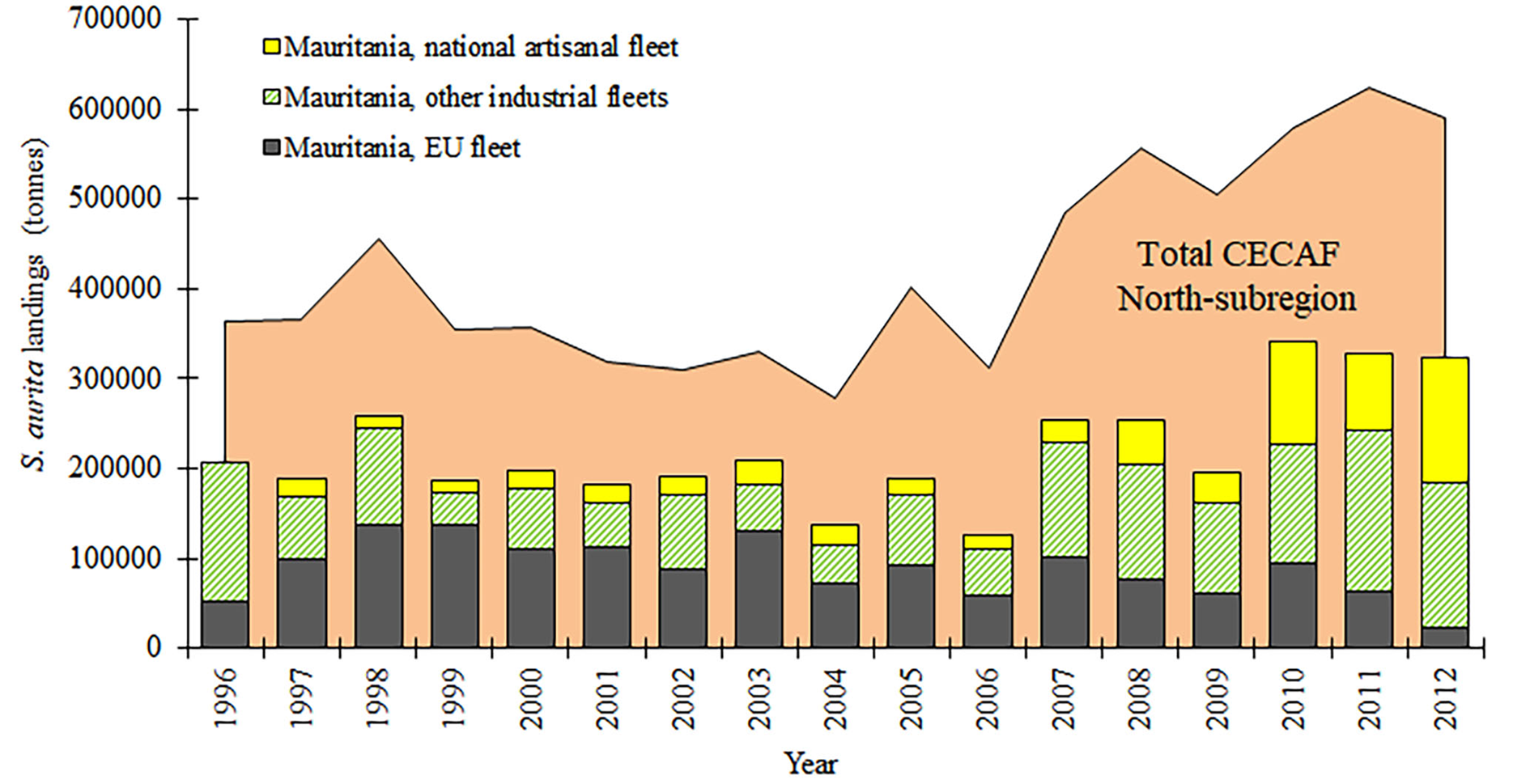
Fig. 2. Total landings of S. aurita in the north CECAF sub-region (1990–2012), and correspondence to the fleets operating in waters of Mauritania (FAO, 2018).
The EU freezer-pelagic trawler fleet operating in Mauritanian waters were landing at the Spanish port of Las Palmas de Gran Canaria (the Canary Islands) from 2004–2012, when the requirements of the fishery agreement changed and the fleet started landing exclusively in Nouadhibou (Mauritania). During this period, the Canary Centre of Oceanography (IEO-Spanish Institute of Oceanography) was responsible for monitoring this fishery and obtaining biological information of the exploited species, under the regulations established by the Data Collection Framework (Official Journal of the European Communities, Council Regulation (EC) No 1543/2000 and Commission Decision 2008/949/EC), the EU framework for the collection, management and use of fishery data.
As indicated, regular international assessments have highlighted the risks of overfishing in this area (FAO, 2018). However, essential biological studies on S. aurita are required to fill the current gaps in knowledge about its life-history in the sub-region in order to implement proper and specific management measures. One key element to manage a fish stock is knowledge of the characteristics of the reproductive cycle, which has been unevenly addressed for S. aurita in the area, including CE Atlantic (Pham-Thouc, Reference Pham-Thouc1973; Garcia, Reference Garcia1982; Chesheva, Reference Chesheva2006), Morocco (Ettahiri et al., Reference Ettahiri, Berraho, Vidy, Ramdani and Do Chi2003; Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015, Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017; Amenzoui & Baali, Reference Amenzoui and Baali2018), Mauritania (Boëly et al., Reference Boëly, Chabanne, Fréon and Stéquert1982a; ter Hofstede et al., Reference ter Hofstede, Dickey-Collas, Mantingh and Wagué2007), Mauritania-Senegal (Mbaye et al., Reference Mbaye, Brochier, Echevin, Lazar, Lévy, Mason, Gaye and Machu2015) and Senegal (Boëly, Reference Boëly1982; Ba et al., Reference Ba, Thiaw, Lazar, Sarr, Brochier, Ndiaye, Faye, Sadio, Panfili, Thiaw and Brehmer2016; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018). Regarding the growth characterization of round sardinella in the area, different methods have been used. Length frequency analyses have been performed for the species inhabiting NW African waters (Boëly et al., Reference Boëly, Fréon and Stequert1982b; Maxim & Maxim, Reference Maxim and Maxim1987–88; Amenzoui & Baali, Reference Amenzoui and Baali2018; Baldé et al., Reference Baldé, Fall, Kantoussan, Sow, Diouf and Brehmer2019a, Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b) and waters of the Gulf of Guinea (Amponsah et al., Reference Amponsah, Danson, Nunoo and Ameyaw2017). In contrast, scales were the main calcified structure used in the first growth studies in the sub-region (Pham-Thouc, Reference Pham-Thouc1973; Krzeptowski, Reference Krzeptowski1981; Boëly et al., Reference Boëly, Fréon and Stequert1982b; Chesheva, Reference Chesheva1998, Reference Chesheva2001) and otoliths have been used in a study in south Morocco (Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015).
The key role of round sardinella in the Canary Current Marine Ecosystem (Cury et al., Reference Cury, Bakun, Crawford, Jarre, Quiñones, Shannon and Verheye2000) and the importance of the species as a source of protein intake in the NW African waters (Corten et al., Reference Corten, Mendy and Diop2012, Reference Corten, Braham and Sadegh2017) underlines the need to expand our knowledge of this species (Mbaye et al., Reference Mbaye, Brochier, Echevin, Lazar, Lévy, Mason, Gaye and Machu2015). In this context, the aim of the present study is to describe the main life-history traits of the species caught off Mauritania, based on high resolution sampling of commercial catches, to contribute to the required information for a scientific-based assessment of the stock status and to enable the formulation of scientific-based proposals to ensure the conservation of the resource and the sustainability of the fisheries involved.
Materials and methods
Data collection
Samples of Sardinella aurita were collected from commercial landings by the European freezer-pelagic trawlers operating in Mauritanian waters, between May 2004 and March 2012, trying to cover all the months of the period (Table S1). Except for winter seasons of 2006 and 2007 (when the fleet landed in other European countries) and autumn 2009, all the quarters were represented in the total sample. Official landing declarations were used to get boxes of all the fish categories in proportional amounts in relation to the total landings. After defrosting, a total of 40,725 specimens were measured for their total length (TL, 0.1 cm) and weighed (TW, 0.1 g). Inter-annual differences in landings considering length frequency distributions (LFDs) were tested by the two-sample Kolmogorov–Smirnov test, using the ‘Fishmethods’ R-package (Nelson, Reference Nelson2019; R Core Team, 2020).
From 2005, additional biological information was registered from 9489 of these fish, analysing the first 100 individuals randomly by month and completing quotas of 10 individuals by length class (L i, rounding to the lowest cm) and sex, when possible. These samples consisted of sex determination, macroscopic maturity stage assignment (based on the maturity scale for partial spawners by Holden & Raitt (Reference Holden and Raitt1975): I, virginal; II, immature or recovering; III, maturing; IV, spawning; V, post-spawning), and gonad weight (GW, 0.1 g). In addition, 2856 sagitta otoliths were extracted, recording biological data and completing quotas to 10 individuals by sex and length class. Due to the loss of the scales during the packaging handling and defrosting, scales were not considered for the study.
Finally, the following oceanographic parameters sea surface temperature –SST- and its anomaly –SSTA- (IGOSS-IRI database; Reynolds et al. Reference Reynolds, Rayner, Smith, Stokes and Wang2002) were explored for the study period (Table S2).
Length-weight relationships (LWR) and Condition factor (Kn)
LWR parameters were estimated for males, females and the total of the analysed individuals. Length and weight data were fitted to the power function TW = a × TLb (where a is the condition factor and b is the allometry coefficient), by linear regression using the minimum method squares, using the log-transformed potential relationship: log(TW) = log(a) × b × log(TL). An analysis of covariance (ANCOVA) was performed in order to find out inter-annual significant differences in the slope (b) and intercept (a) for the whole sample, and between sexes for the individuals biologically analysed, using GraphPad Prism® 8 software. Growth pattern (allometry) was tested using the Student's t-test modified by Pauly (Reference Pauly1984). As an expression of seasonal variation of somatic growth, monthly mean values of the relative condition factor (Kn) were obtained following the index by Le Cren (Reference Le Cren1951): Kn = TWobserved/TWtheoretical, with the theoretical TW being calculated from the TL and the estimated LWR.
Reproductive cycle
Sex-ratio was studied for the whole sample, by quarters and by L i. The χ2 test was applied in order to detect significant differences in the hypothesized 1:1 relationship. Spawning behaviour was followed based on the seasonal changes in gonad development, including both sexes, by correlating two approaches: quantitative (the monthly evolution of the mean gonado-somatic index (GSI = GW × 1000/TW)) and qualitative (the monthly variation of the percentage of mature stages in relation to the total) (Jennings et al., Reference Jennings, Kaiser and Reynolds2001; King, Reference King2007).
The length at first maturity (LFM, length at which 50% of individuals are in mature sexual stage) was estimated from the curves of maturity by L i (or ogives) for males and females by year and for the total. Proportions of mature specimens (Pi; mature sexuality was considered for individuals in stage IV and V) were estimated for each L i for the whole year, due to round sardinella being a partial spawner. Maturity ogives were then estimated using the non-linear regression of the Gompertz model (Pope et al., Reference Pope, Margetts, Hamley and Akyüz1983). The equation for the model is: Pi = exp[−c × exp(d × L i)], pi being the proportion of mature individuals for each L i (P' for moving average was used = (Pi−1 + Pi + Pi+1)/3), c and d are the function parameters. LFM was calculated for Pi = 0.5. A Student's t-test was applied to each pair of maturity ogives (male-female) by year and for the total, in order to find significant differences.
Age and growth
Seasonal changes related to reproduction cycle produce variations in the growth rate, that lead to different deposition rates in calcified structures, such as in otoliths, detectable in the marginal increments (May, Reference May1965). The deposition periodicity assumed of one increment or annulus (1 opaque ring + 1 translucent ring) deposited yearly was assessed by means of marginal increment analysis using a qualitative approach (Campana, Reference Campana2001; Panfili et al., Reference Panfili, de Pontual, Troadec and Wright2002). When possible, otoliths were classified between opaque edge (somatic growth) vs translucent edge (reproductive period).
For the assignment of age, whole otoliths were immersed in distilled water and were interpreted by two readers with no knowledge of fish length, using a compound microscope (10 × magnification) under reflected light with dark background, adopting the age scheme/criteria established by FAO (2007). Growth parameters were estimated for the direct readings (DR) using the von Bertalanffy growth model TL = L∞ × [1-exp(-k × (t−t 0))], where L ∞ is the asymptotic length, k the growth coefficient and t 0 the theoretical age when TL is 0 cm.
To test the coherence of the growth parameters obtained from otolith interpretation and also the representativeness of the landings in respect of the actual population, a length frequency distribution analysis (LFDA) was performed. First, the annual length frequencies were quarterly grouped. Then, the Bhattacharya method, which is incorporated in the FiSAT software (Gayanilo et al., Reference Gayanilo, Sparre and Pauly2006), was used to discriminate the normal distribution assuming that each mode in the overall size-frequency distribution represented a cohort. The separation index among different cohorts was taken into account and values <2 indicated a large overlap between cohorts, which was considered unacceptable. Finally, statistically significant differences between the VBGF growth curves obtained by sexes and methods (otolith reading vs LFDA) were analysed using the Chen-test, based on the analysis of the residuals sum of squares (Chen et al., Reference Chen, Jackson and Harvey1992).
Natural mortality
The natural mortality rate (M, year–1) was considered to be a constant value for the exploitable fraction of the stock, and was estimated by empirical methods (Rikhter & Efanov, Reference Rikhter and Efanov1976; Hoenig, Reference Hoenig1983; Jensen, Reference Jensen1996; Hewitt & Hoenig, Reference Hewitt and Hoenig2005).
Results
Length distributions
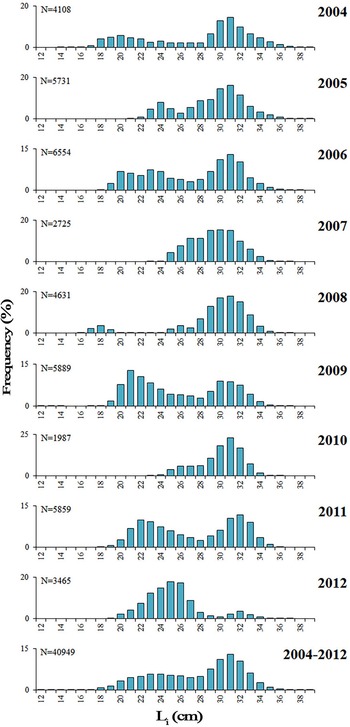
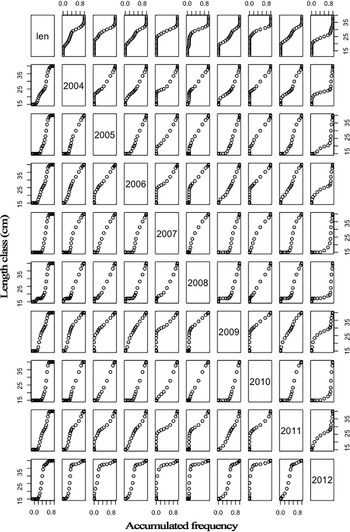
The TLs of the 40,725 Sardinella aurita collected ranged between 12.8–39.8 cm. The maximum size in females (16.2–39.5 cm TL) was greater than in males (16.4–37.3 cm TL). Overall, two modes seemed appear in landings every year (Figure 3), with the main mode around 30–32 cm and, the secondary one, in smaller sizes (20–25 cm). However, additional mode(s) seemed more or less apparent in some years (2005, 2006 and 2008), groups were closer in 2007, and the smaller group outnumbered the bigger mode in 2009 and 2012. Inter-annual statistical comparisons (Figure 4) identified 2009 with the most different landing in terms of length frequencies, with significant differences compared with 2005, 2007, 2008 and 2010; and also the pair 2008–2012 (P < 0.05; Table S3).
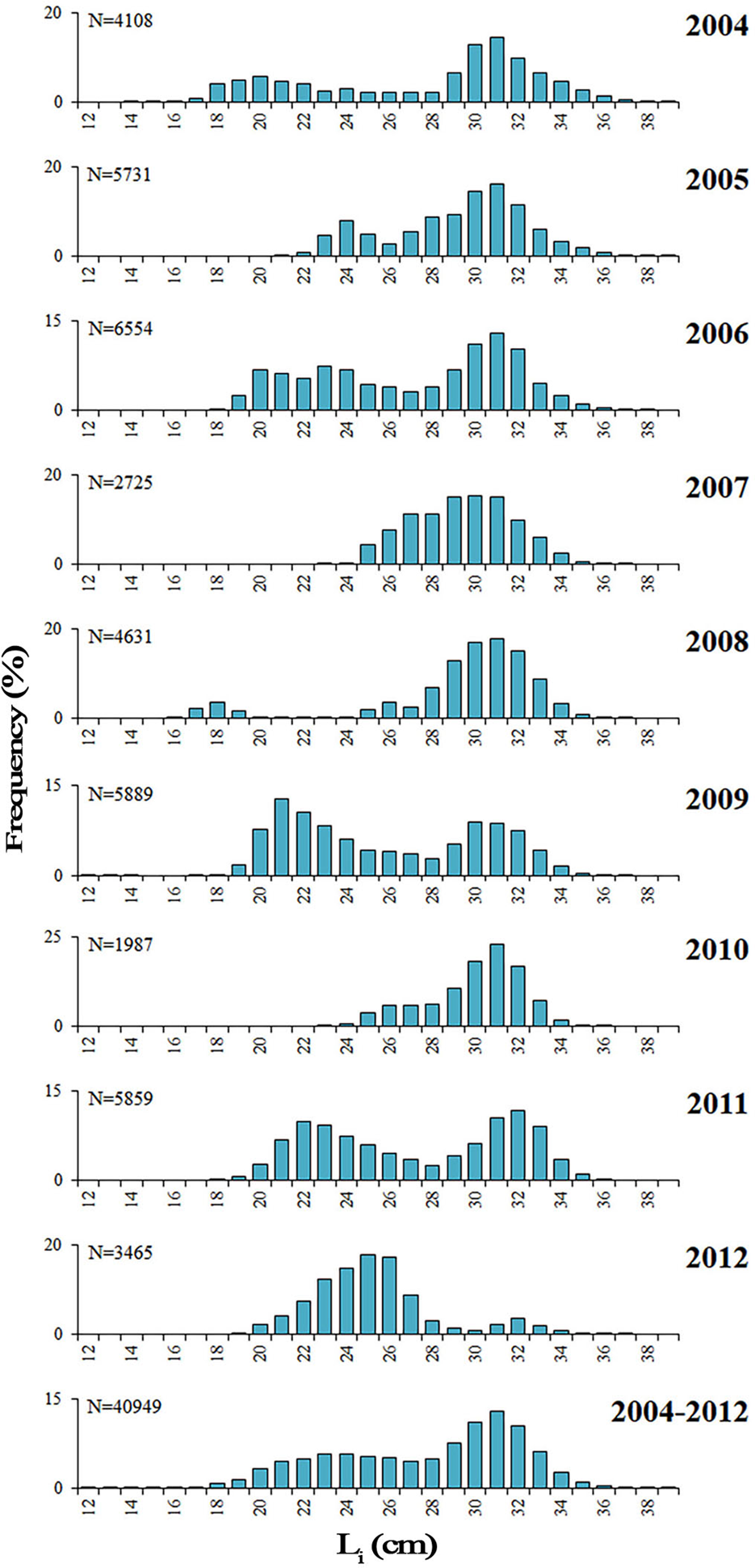
Fig. 3. Overall and annual length frequencies distributions (LFD, %) for the S. aurita commercial landings from Mauritania. N, number of individuals; L i, length class.

Fig. 4. Accumulated length frequencies of the Mauritanian S. aurita landings, totals on the top and yearly compared.
Length-weight relationships (LWR) and growth pattern
Descriptive statistics and LWR parameters by sexes and for the total (including undetermined juveniles) are presented in Table 1. In the linear regression comparison, a statistically significant difference between sexes was found for the intercept (F = 16.89, P < 0.0001), but not for the slopes (F = 2.08, P = 0.149). Therefore, it does not seem problematic to use the LWR for the total. In all the cases, the growth pattern was allometric positive (t statistic > 22, P < 0.0005).
Table 1. Descriptive statistics summary for the length-weight samplings and LWR parameters of S. aurita from Mauritania, by year, by sex and for the total (including undetermined juveniles)
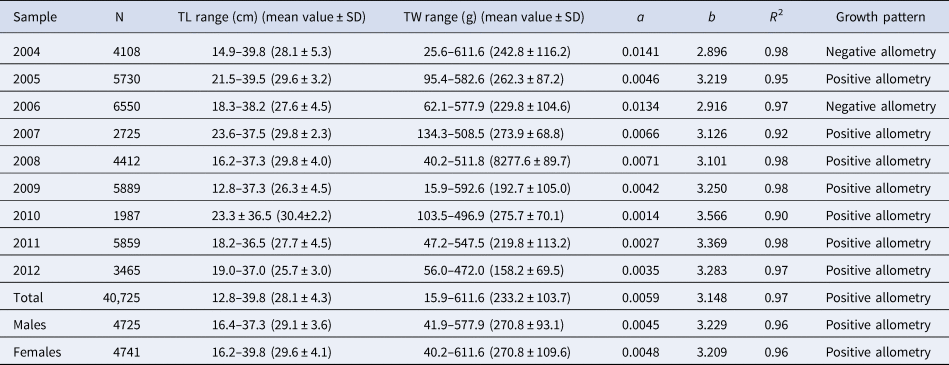
N, number of individuals; TL, total length; TW, total weight; SD, standard deviation; a and b, estimated function parameters; R 2, coefficient of determination.
Regarding the inter-annual LWRs comparisons (Table S4), only the b values obtained for 2007–2008 could be considered statistically equal, but differences between their a values were extremely significant. Therefore, none of the pairs can be considered statistically similar.
Reproduction cycle
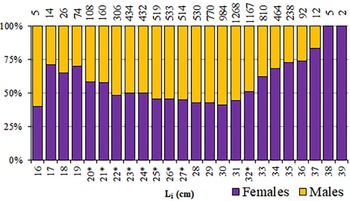
Among the 9495 round sardinellas analysed, 4725 were males, 4741 females and sex was undetermined in 29 individuals (TL 12.8–24.0 cm). The overall sex-ratio showed a non-significant predominance of females (50.1%) (χ2 = 0.027, P = 0.869), although seasonal significant differences were found in winter, spring and summer (χ2 > 5.9; P < 0.05), with females significantly outnumbering males (autumn: χ2 = 2.868; P = 0.09). However, a noticeable variability was found in the sex-ratio among length classes (L i), mainly in the smallest and the biggest size classes of the range (<22 and >32 cm) (Figure 5), where females were generally predominant, in some cases represented by a small number of individuals. Therefore, in length classes from 22–32 cm, a balance between sexes was observed, with no statistical difference from unity for length classes from 20–27 cm.
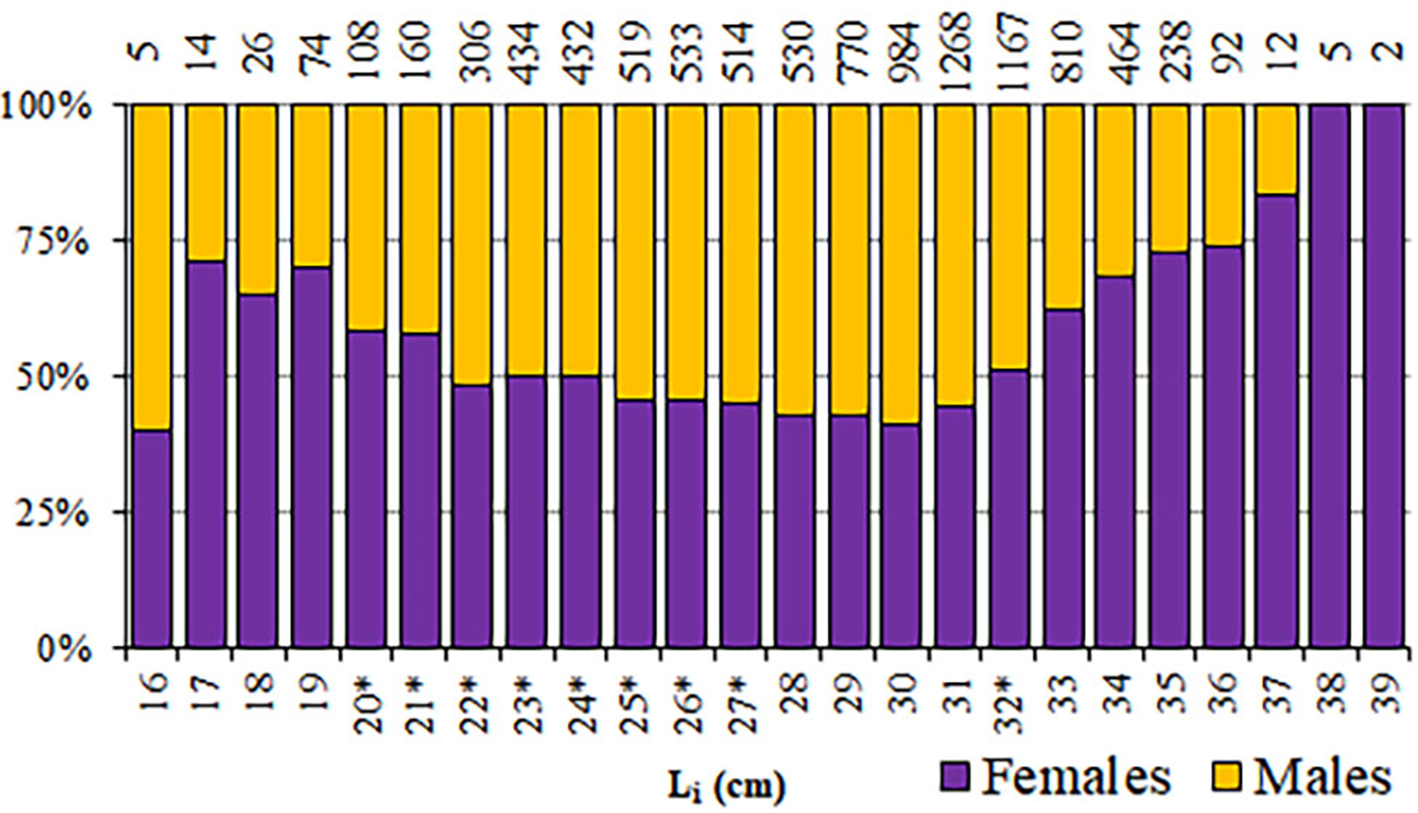
Fig. 5. Proportion of females/males of S. aurita from Mauritania, by length class (L i). Numbers of individuals are presented above the columns. (*) indicates balanced sex-ratios (1:1; P > 0.01).
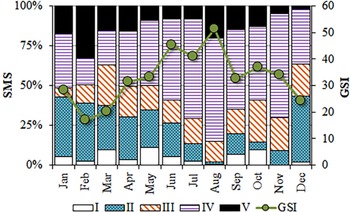
The smallest reproductively active individuals corresponded to a male with 22.7 cm TL, and a female of 19.8 cm. Although breeding round sardinellas were found in all months, from June to November, more than 50% of the analysed individuals were in spawning or post-spawning stages, peaking between June and August (Figure 6).
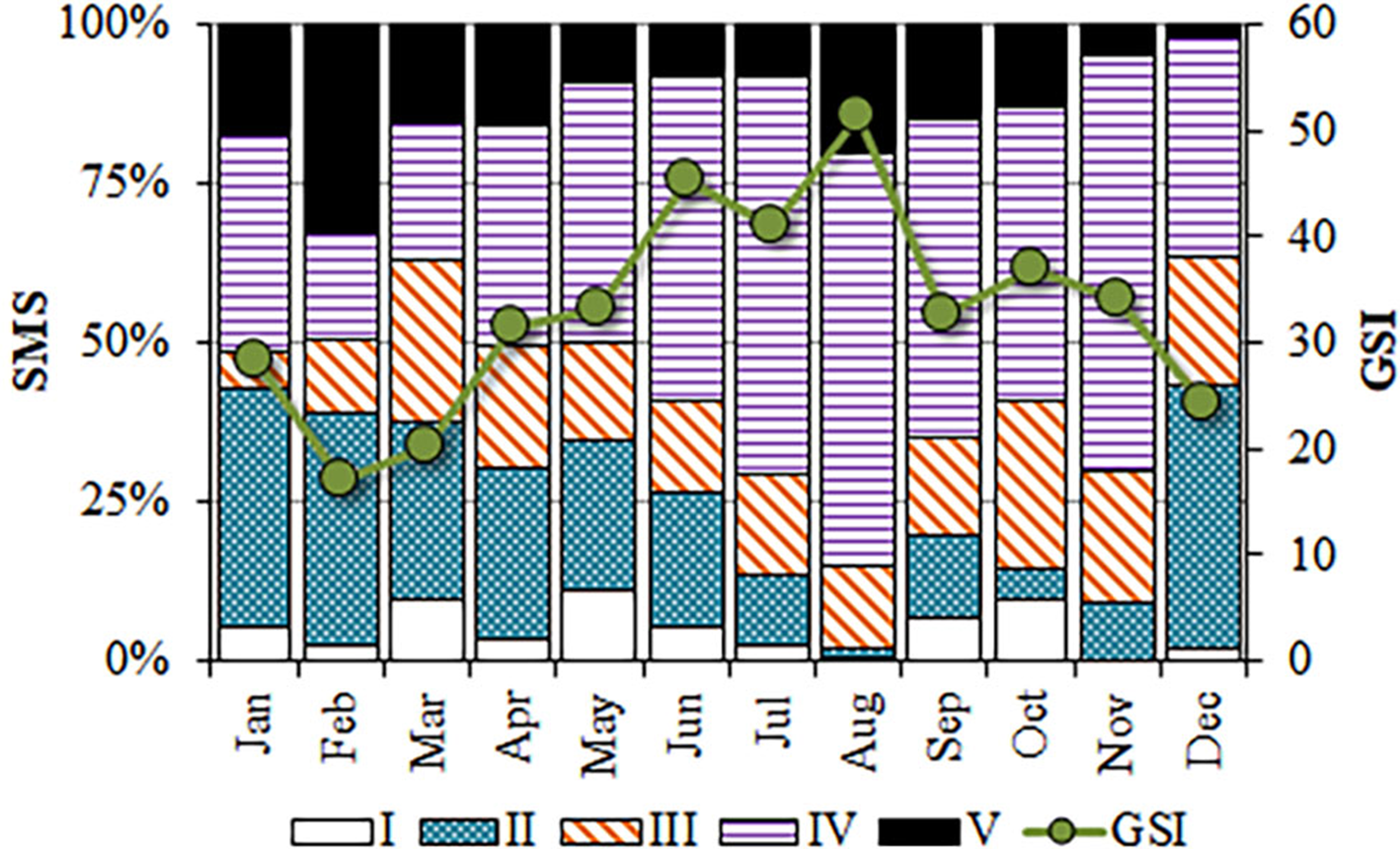
Fig. 6. Monthly evolution of the gonadosomatic index (GSI) and proportions of the sexual maturity stages (SMS) identified in S. aurita from Mauritania.
The estimated LFM was 24.2 cm for males, 24.4 cm for females and 24.3 cm for both sexes pooled (Figure 7, Table 2). From comparisons for the total of the analysed individuals and yearly splits, significant differences in LFMs were only found between males and females in 2012 (t = 2.78; P = 0.01).
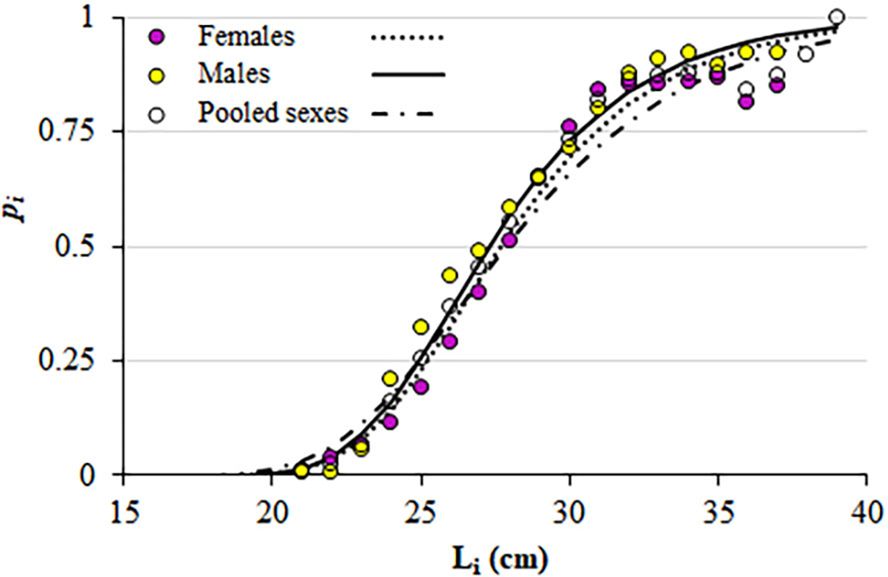
Fig. 7. Maturity ogives for S. aurita caught off Mauritania. Dots indicate the proportion of mature individuals (P i) by length class (L i) and lines, the fitted maturity ogives.
Table 2. Maturity ogives parameters and length at first maturity (LFM) for S. aurita from Mauritania by sex and the total (including undetermined, if any), and yearly estimated
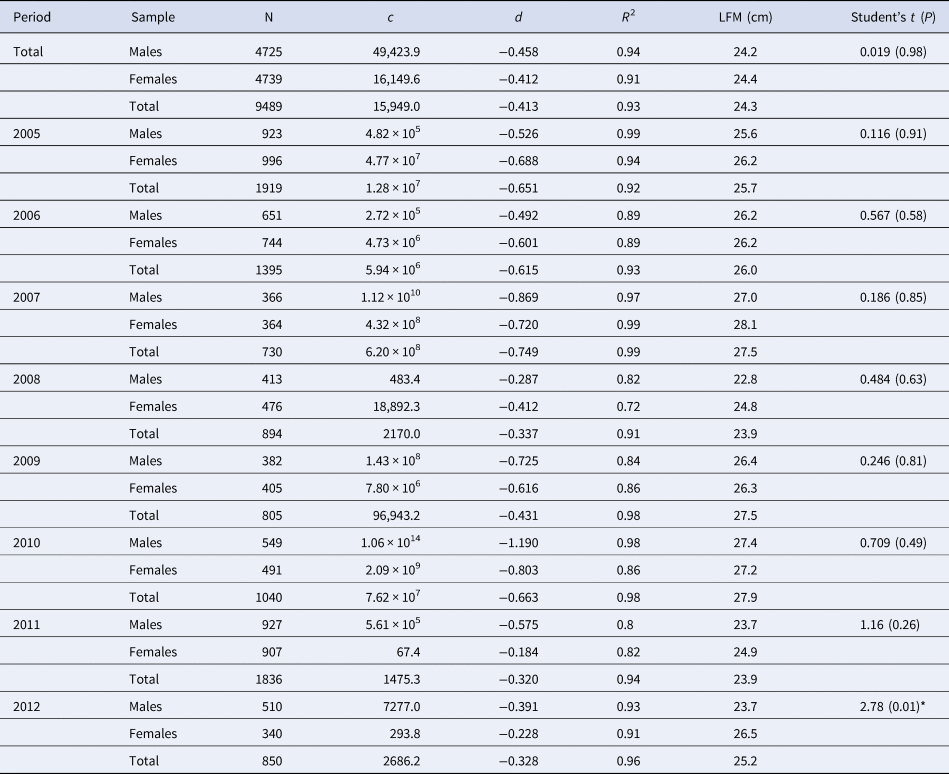
N, number of individuals; c and d, estimated function parameters; R 2, coefficient of determination. * indicate significant differences between sexes (P < 0.05).
Student's t-test differences between males/female ogives.
Age and growth
Among the 2856 pairs of otoliths analysed, 1127 were rejected because they were in a bad state or were assigned low reliability of interpretation by the reader. Finally, 1732 readings were accepted (i.e. 61% coincidence between readers). The assigned age classes ranged from 0–7 years, with age-class 3 being the most represented (Table 3). The Bhattacharya's method results are summarized in Table 4.
Table 3. Age-length key for S. aurita from Mauritania
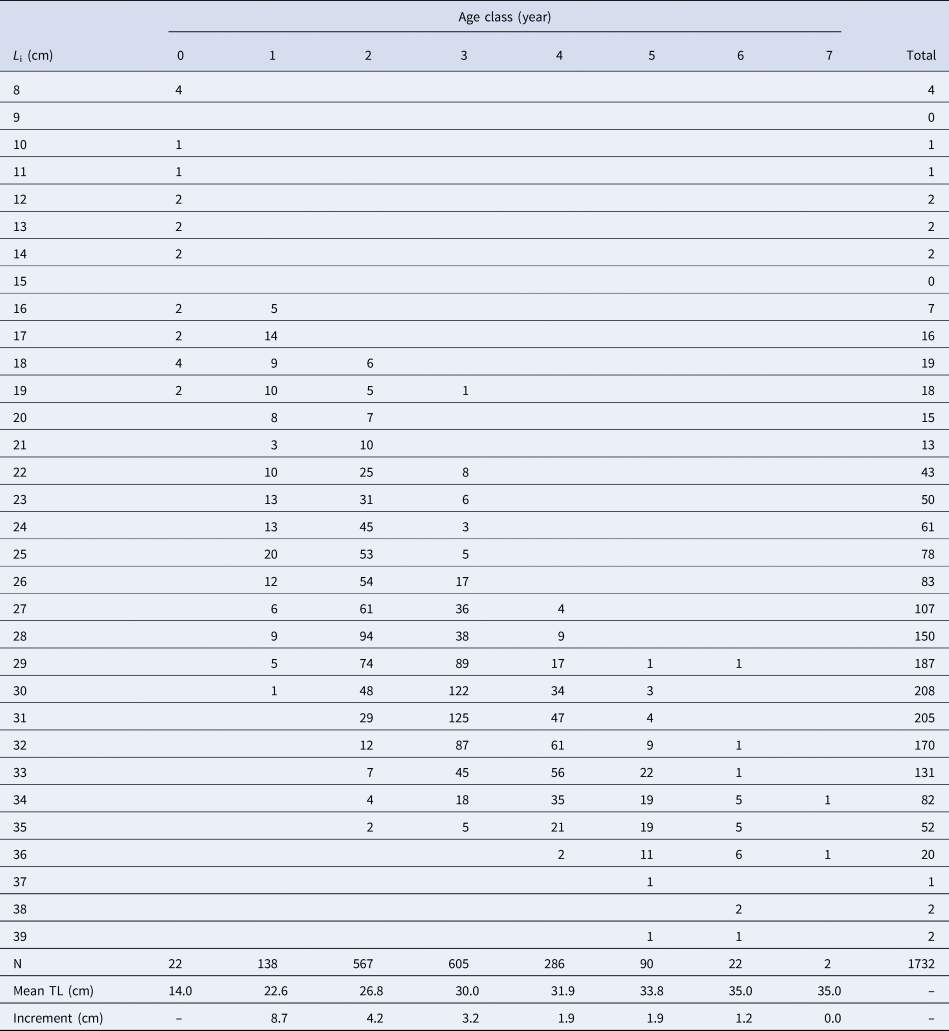
L i, length-class (to the lower cm); N, number of individuals; TL, total length.
Table 4. Mean total length (TL) by age of the Mauritanian S. aurita obtained from decomposing biannual length frequencies (time period: 2004–2012) using the Bhattacharya method
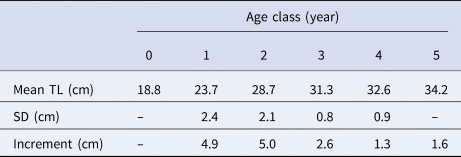
SD, standard deviation.
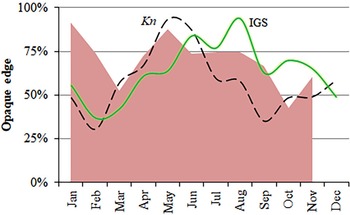
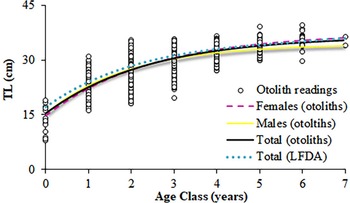
Nature of the otoliths' edges was possible to identify in 1664 otoliths from January–November (Figure 8). Although none were clearly identifiable in December, opaque edges were more identifiable than translucent ones throughout the year. However, minimum percentages of opaque edges (March and October) happened one month later than the minimum values of Kn and GSI (February and September) (Figure 8) and, hence, the percentage of translucent edges increased before and at the end of the main spawning period. Almost 40% of the maximum length is reached during the first year of life, and more than 60% during the second year of life (Figure 9). The statistical comparison (Chen-test) of the two pairs of growth curves (sexes and methods; Table 5), did not show statistically significant differences (F obs > F crit in both cases).
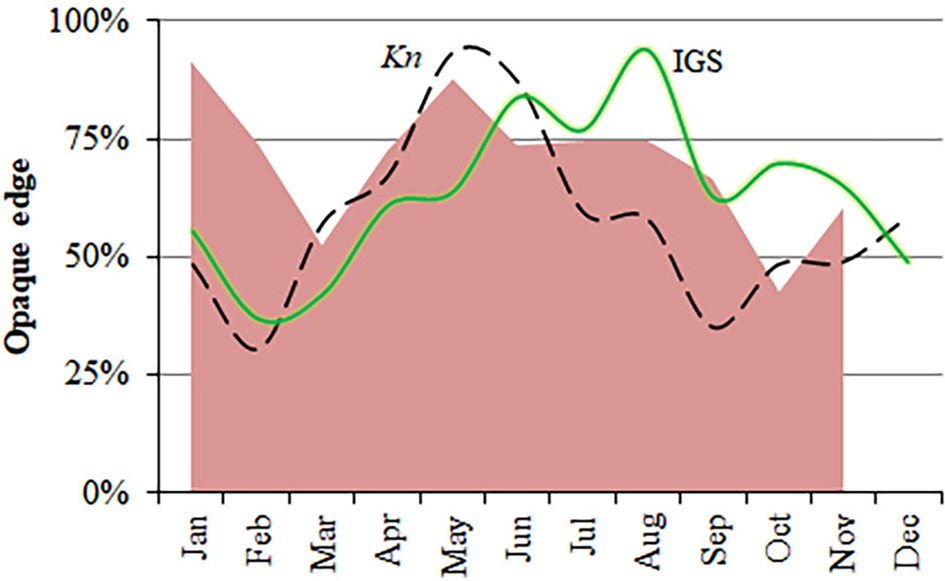
Fig. 8. Monthly proportion of opaque-edges in otoliths of S. aurita from Mauritania (shaded area), mean values of the gonadosomatic index (GSI, continuous line) and the condition factor (Kn, dashed line).
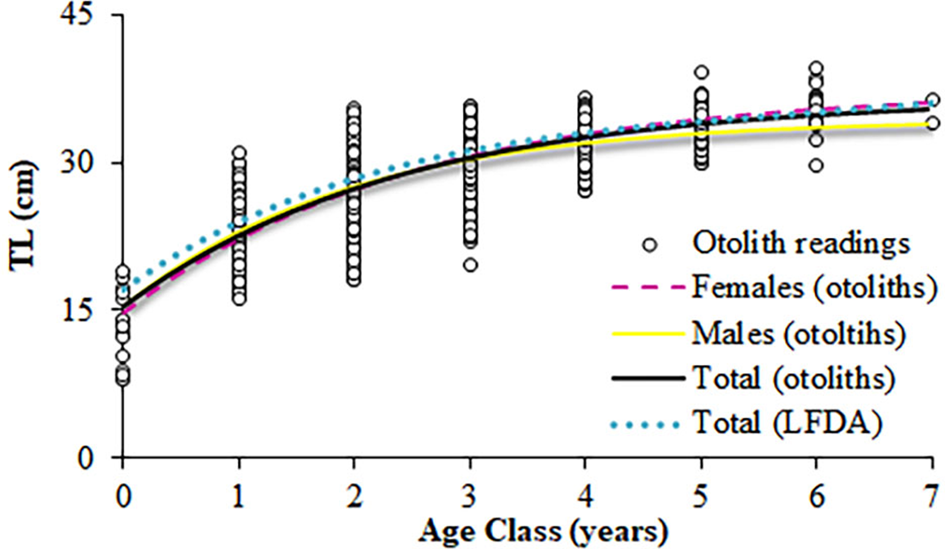
Fig. 9. Von Bertalanffy growth curves for S. aurita from Mauritania, fitted from the direct otolith-readings and the length frequency distributions analysis (LFDA).
Table 5. Estimated von Bertalanffy growth parameters (standard errors in parentheses and 95% of confidence intervals) of S. aurita from Mauritania, both from otolith age interpretation (for males, females and the total, including indeterminate juveniles) and from length frequencies distributions analysis (LFDA)
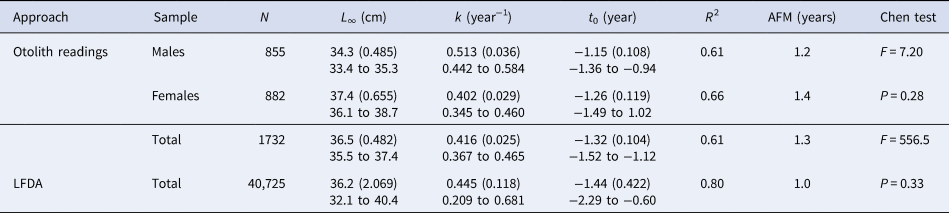
N, number of individuals; L ∞, the asymptotic length, k, growth coefficient; t 0, theoretical age when TL = 0 cm; R 2: coefficient of determination. AFM, estimated age at first maturity, based on the length at first maturity.
Natural mortality
Estimated natural mortality values (Ms) by method are presented in Table 6. Values varied between 0.17 and 1.10 year–1, with an average value of ~0.63 year–1.
Table 6. Natural mortality rates (M) estimates for S. aurita from Mauritania

t mas, massive maturation age (95% if individuals in mature stage); AFM, Age at First Maturity; Linf, k and t 0, growth parameters; t max, theoretical maximum age of the population (t max = t 0 + (3/k)) (Taylor, Reference Taylor1960).
a. Rikhter & Efanov (Reference Rikhter and Efanov1976); b. Hoenig (Reference Hoenig1983), Hewitt & Hoenig (Reference Hewitt and Hoenig2005); c. Jensen (Reference Jensen1996).
Discussion
In an overall scenario of data limited-fisheries worldwide (Costello et al., Reference Costello, Ovando, Hilborn, Gaines, Deschenes and Lester2012), a first step to address the status assessment of any exploited species should be to improve the available data, including the time-series, not only of catches, discards and fishing effort, but also the biological characteristics of the species in a particular area (Bentley, Reference Bentley2015; Dowling et al., Reference Dowling, Smith, Smith, Parma, Dichmont, Sainsbury, Wilson, Dougherty and Cope2019). In this sense, although many efforts are being carried out to fill knowledge gaps for this important fishery resource, collecting data and obtaining accurate and representative biological parameters may be expensive and time-consuming. This work presents the life-history of the Sardinella aurita from Mauritanian waters, where most of the NW African catches come from. It is agreed that one stock of round sardinella (whose catches are not restricted by quotas) is present along the NW African coast; therefore, although the analyses presented in this study are based on fish caught in a partial zone of the whole distribution of the stock, the central part of the stock's range occurs in Mauritanian waters, where the time biomass trends are representative of the overall stock-biomass (Samb & Pauly, Reference Samb and Pauly2000).
The main modes in the length frequency distributions (LFDs) found for the Mauritanian round sardinellas coincide with those identified off Senegal (Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). With the exception of 2009 and the pair 2008/2012, the analysis of the landings' length frequencies resulted in similar patterns for all the years, with an overall predominance of greater sizes. When paying attention to the annual LFDs, the smaller mode was more important than the larger mode only in 2009 and 2012. The importance of smaller modes are probably related to the specific fishing area (close to coastal waters where smaller sizes are more abundant (Garcia, Reference Garcia1982)) and, obviously, to the recruitment success of particular spawning events. Regarding the oceanographic parameters explored, 2009 and 2012 were notably different to other years, with a cooler first semester (from January to June) compared with the other years (with negative anomalies of the sea surface temperature from the last quarter of 2008 to the second quarter of 2009, and for the first quarter of 2012; Table S2). It is known that oceanographic conditions promote variations in the abundance and distribution of fish, particularly in small pelagic species (Alheit et al., Reference Alheit, Lorenzo, Rykaczewski and Sundby2019), and expansion/contraction of geographic distribution of breeding and/or nursery areas may have made the smaller fractions of the stock available to the monitored industrial fleet momentarily.
Due to there being a loss of weight and length in thawed samples (Anderson & Gutreuter, Reference Anderson, Gutreuter, Nielsen and Johnson1983), when analysing the length-weight relationships (LWR) and growth pattern, previous conversion from ‘frozen’ to ‘fresh’ of measures should be done, as the fish was provided frozen at the landing sites. However, the conversion factors available for S. colias are calculated for furcal length (Ajah & Nunoo, Reference Ajah and Nunoo2003) and the current authors considered them unsuitable, and all the calculations are based on defrost measurements. It is important to clarify this point as some studies performed on this species have been also based on fish preserved in different ways and data were not transformed to ‘fresh’ (Tsikliras & Antonopoulou, Reference Tsikliras and Antonopoulou2006; ter Hofstede et al., Reference ter Hofstede, Dickey-Collas, Mantingh and Wagué2007).
Regarding the morphometric relationships obtained, it is well known that fishing gear selectivity places limits on their use with regard to the size ranges used for the estimation of linear regression parameters (Petrakis & Stergiou, Reference Petrakis and Stergiou1995), and extrapolation of data to fish larvae or immature individuals must be avoided (Bagenal & Tesch, Reference Bagenal, Tesch and Bagenal1978; Pepin, Reference Pepin1995). Based on the classification by Boëly et al. (Reference Boëly, Chabanne, Fréon and Stéquert1982b), only immature juveniles (TL < 12 cm) were absent in the present analysis, where length classes from 12–39 cm were available. This is because the industrial fleet monitored does not have access to coastal areas functioning as nurseries (Garcia, Reference Garcia1982). In this sense, Mustac & Sinovcic (Reference Mustac and Sinovcic2012) found significant differences in the length distributions and growth patterns between offshore and inshore individuals of round sardinella in the Adriatic Sea. With regard to the LWR obtained for the round sardinella from Mauritania, the estimated parameters and the allometrically positive growth models obtained were similar to other studies in the region (Camarena, Reference Camarena1986; Maxim & Maxim, Reference Maxim and Maxim1987–88; Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). This contrasts with the isometric growth pattern of S. aurita obtained in Senegal (Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b) and the Gulf of Guinea (Castro et al., Reference Carbonara, Intini, Kolitari, Joksimović, Milone, Lembo, Casciaro, Bitetto, Zupa, Spedicato and Sion2017). Regarding the LWR estimations by year, S. aurita showed positive allometry in most of the years in the present work, except in 2004 and 2006, with negative allometry. Interannual growth pattern variations have been described for the species by other authors both in Mauritania (Chesheva, Reference Chesheva2006) and close areas, where even seasonal variations have been found (Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Bartolomé, Carrasco and Duque-Nogal2016). Although no direct relation to the variations in oceanographic parameters has been found, these results highlight the importance of obtaining yearly updated information for assessment purposes. This includes, among others, that small changes in LWR parameters can cause great differences in total catch estimations (Froese, Reference Froese2006).
As in the present study, other authors had observed that females of S. aurita slightly outnumber males or are approximately equal to males in Mauritanian waters (Wagué & M'Bodj, Reference Wagué and M'Bodj2002; Chesheva, Reference Chesheva2006). A slight predominance of females has also been observed in South Morocco (Baali et al., Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017), Senegal (Boëly, Reference Boëly1982; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b) and in the Mediterranean Sea (Tsikliras & Antonopoulou, Reference Tsikliras and Antonopoulou2006; Mustac & Sinovcic, Reference Mustac and Sinovcic2012). Nevertheless, a significant unbalanced sex-ratio has been described in the Mediterranean Sea (Navarro, Reference Navarro1932; Dahel et al., Reference Dahel, Tahri, Bensouilah, Amara and Djebar2016) and in the SW Atlantic (Gassman et al., Reference Gassman, Eslava and González2008; Tagliafico et al., Reference Tagliafico, Walter González and Eslava2008). Regarding the sex-ratio variation according to the length, the predominance of females in the smallest and the biggest sizes, as well as a balance for the middle length classes in the range, had been observed both for Atlantic (Wagué & M'Bodj, Reference Wagué and M'Bodj2002; Baali et al., Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017; Amenzoui & Baali, Reference Amenzoui and Baali2018) and Mediterranean stocks (Tsikliras & Antonopoulou, Reference Tsikliras and Antonopoulou2006).
In NW Africa, round sardinella spawns year round with a maximum period from June to September–December (depending on the area included) (Boëly et al., Reference Boëly, Chabanne, Fréon and Stéquert1982a; Chesheva, Reference Chesheva2006; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). Therefore, the major spawning period observed in the present study (from June to November) matches with the migrations related to the sexual cycle of the species described in the CECAF region (Boëly et al., Reference Boëly, Chabanne, Fréon and Stéquert1982a; Garcia, Reference Garcia1982). Some studies have pointed that round sardinella's breeding is synchronized with the major upwelling events in nearby areas off South Morocco (Amenzoui & Baali, Reference Amenzoui and Baali2018), Gulf of Guinea (Castro et al., Reference Castro, Skrobe, Asare and Kankam2017), or with the period when the sea surface temperature (SST) reaches the annual minimum values (Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). In contrast, in our study, the stronger spawning period observed in Mauritanian waters matches with the annual maximum SST. The upwelling processes occurring in Mauritanian waters cause a crucial effect on the life cycle of S. aurita (Mbaye et al., Reference Mbaye, Brochier, Echevin, Lazar, Lévy, Mason, Gaye and Machu2015; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). In this sense, round sardinella increases spawning effort when the coastal retention index is higher in the area (Mbaye et al., Reference Mbaye, Brochier, Echevin, Lazar, Lévy, Mason, Gaye and Machu2015) and is also detectable in the time-lag of three months observed by Demarcq & Faure (Reference Demarcq and Faure2000) between the maximum upwelling and the beginning of the July-spawning peak in the South Moroccan (Ettahiri et al., Reference Ettahiri, Berraho, Vidy, Ramdani and Do Chi2003) and the Mauritanian waters (Wagué & M'Bodj, Reference Wagué and M'Bodj2002; ter Hofstede et al., Reference ter Hofstede, Dickey-Collas, Mantingh and Wagué2007). All these processes start at the end of spring, when the main part of the population starts the migration from Senegal and countries further south to feed and spawn off Mauritania and Morocco (Boëly et al., Reference Boëly, Chabanne, Fréon and Stéquert1982a; Garcia, Reference Garcia1982; Zeeberg et al., Reference Zeeberg, Corten and de Graaf2008).
The lengths at first maturity (LFMs) estimated in the present study (~24 cm TL) are similar to results obtained by other authors for the Mauritanian round sardinella (ter Hofstede et al., Reference ter Hofstede, Dickey-Collas, Mantingh and Wagué2007; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018). The species reaches its maturity at bigger sizes both in NW Africa (including South Morocco) (Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015, Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017; Amenzoui & Baali, Reference Amenzoui and Baali2018), compared with the LFM estimated southwards (Castro et al., Reference Castro, Skrobe, Asare and Kankam2017), and also in the Mediterranean Sea (Tsikliras & Antonopoulou, Reference Tsikliras and Antonopoulou2006) and in SW Atlantic waters (Gassman et al., Reference Gassman, Eslava and González2008; Tagliafico et al., Reference Tagliafico, Walter González and Eslava2008), probably due to the influence of the powerful upwelling in the area. This process could increase the growth rate, leading to the achievement of greater sizes than in other less resource-rich areas, at the same ontogenetic state. In addition, both the spawning peak timing-occurrence and the increase of the LFM northwards, match with the reproduction-related migration described in the CECAF area (Boëly et al., Reference Boëly, Chabanne, Fréon and Stéquert1982a; Garcia, Reference Garcia1982), due to the year cycle beginning in the southern limit of the sub-region, allowing S. aurita to attain bigger sizes when it reaches northern breeding areas. As for the spawning seasonality, LFM depends on environmental and genetic factors (Wootton, Reference Wootton1998), but may also be influenced by anthropic factors, such as fishing pressure (Samb & Pauly, Reference Samb and Pauly2000; Pauly et al., Reference Pauly, Christensen, Guénette, Pitcher, Sumaila, Walters, Watson and Zeller2002; Ernande et al., Reference Ernande, Dieckmann and Heino2004; Thiaw et al., Reference ter Hofstede, Dickey-Collas, Mantingh and Wagué2017). As occurs with S. maderensis (Ba et al., Reference Ba, Thiaw, Lazar, Sarr, Brochier, Ndiaye, Faye, Sadio, Panfili, Thiaw and Brehmer2016), round sardinella seems to display a noticeable adaptive phenotypic plasticity, which permits them to react quickly to environmental changes and ensure their survival (Via & Lande, Reference Via and Lande1985).
Although age interpretation based on otoliths of this species was difficult, the absence of significant differences found between the growth curves obtained from direct otolith readings and the LFDA either corroborated or indirectly validated the growth pattern assumed. The otolith criteria interpretation of S. aurita, was useful when the direct validation methods (e.g. mark-recapture, captivity, radiochemical) are difficult to implement, as is the case for this species (Carbonara et al., Reference Carbonara, Intini, Kolitari, Joksimović, Milone, Lembo, Casciaro, Bitetto, Zupa, Spedicato and Sion2018). Moreover, the mean total lengths by year-class obtained from the age-length key resulted in coherent data and was in accordance with the previous studies based on length frequencies and scales in the area (Pham-Thouc, Reference Pham-Thouc1973; Krzeptowski, Reference Krzeptowski1981; Boëly et al., Reference Boëly, Fréon and Stequert1982b; Maxim & Maxim, Reference Maxim and Maxim1987–88; Chesheva, Reference Chesheva2001; Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015; Amenzoui & Baali, Reference Amenzoui and Baali2018; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). The overlap of length-classes (L i) among age groups in the age–length key reflects the variability of sizes of individuals at the same age (and vice versa), which is usually related to high individual growth variability (Panfili et al., Reference Panfili, de Pontual, Troadec and Wright2002). Based on the ageing results, catches were mainly represented by individuals that were 3–4 years old and, secondarily, by 1-year-old fish. Therefore, as in the Senegalese waters, S. aurita is recruited to the fishery at an age of 1–2 years (Boëly et al., Reference Boëly, Fréon and Stequert1982b), when reaching its sexual maturation. Pre-spawner individuals comprised more than 30% of the catches in 2004 and 2006, and represented 40–50% of the catches in 2009 and 2012. However, the industrial fishery mainly exploited adult fish that have spawned more than once: >50% for all the years monitored, and exceeding 85% in 2005, 2007–2008 and 2010–2011.
Validation of growth increment formation in calcified structures of S. aurita has only been carried out using scales of fish from the Mediterranean Sea (Tsikliras et al., Reference Tsikliras, Koutrakis and Stergiou2005). The qualitative approach seems to support the annual deposition of one annulus. However, the high difficulty of identifying the edge-nature of the otoliths (opaque/translucent) is probably related to thickness and due to it being a partial spawner species, with no abrupt change in the energy flux between somatic growth vs reproduction effort. The monthly values of Kn for S. aurita off Mauritania did reflect an increase in somatic growth in April–May (previous to the rise in spawning) and a minimum in September (previous to the end of the main spawning period). Ter Hofstede et al. (Reference ter Hofstede, Dickey-Collas, Mantingh and Wagué2007) analysed these trends by sex for the period 2000–2003 and observed a similar pattern for both males and females, with the maximum condition factor occurring in May, and the minimum, in September. Baldé et al. (Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b) found the same results in Senegalese waters, where these cycles match with the upwelling intensity and the SST changes. Conversely, slight differences in the duration and seasonal changes of these cycles have been described in Moroccan waters (Baali et al., Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017), which is probably due not only to environmental influence and food availability (Roy et al., Reference Roy, Cury, Fontana and Belvèze1989), but also to the latitudinal migration of the stock along the NW African coast (Boëly et al., Reference Boëly, Chabanne, Fréon and Stéquert1982a; Garcia, Reference Garcia1982).
Although age and growth studies are very influenced by the methodology employed, within the East Atlantic distribution of the species, round sardinella reaches greater longevity and greater L ∞ in the north CECAF sub-region (Navarro, Reference Navarro1932; Krzeptowski, Reference Krzeptowski1981; Boëly et al., Reference Boëly, Fréon and Stequert1982b; Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015; Amenzoui & Baali, Reference Amenzoui and Baali2018; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b), especially in the central area including Mauritania and Senegalese-Mauritanian waters (Pham-Thouc, Reference Pham-Thouc1973; Maxim & Maxim, Reference Maxim and Maxim1987–88; Chesheva, Reference Chesheva1998, Reference Chesheva2001). Conversely, both longevity and L ∞ decrease southwards in the Gulf of Guinea (6 years) (Rossignol, Reference Rossignol1955; Castro et al., Reference Castro, Skrobe, Asare and Kankam2017) and in the Mediterranean Sea (Navarro, Reference Navarro1932; Tsikliras et al., Reference Tsikliras, Koutrakis and Stergiou2005; Salem et al., Reference Salem, El_Aiatt and Ameran2010; Dahel et al., Reference Dahel, Tahri, Bensouilah, Amara and Djebar2016). This could be due to the influence of the NW African upwelling system, which promotes really high productive periods when food availability drastically increases (Barton et al., Reference Barton, Arístegui, Tett, Cantón, García-Braun, Hernández-León, Nykjaer, Almeida, Almunia, Ballesteros, Basterretxea, Escánez, García-Weill, Hernández-Guerra, López-Laatzen, Molina, Montero, Navarro-Pérez, Rodríguez, van Lenning, Vélez and Wild1998; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b). In fact, most of the species landed in Morocco correspond to fisheries around Dakhla, under the influence of this system (Baali et al., Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017; Amenzoui & Baali, Reference Amenzoui and Baali2018). Although they did not result in statistical significance for the Mauritanian round sardinella, differences in the growth parameters between sexes have been described for populations from Morocco (Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015) and from the Mediterranean Sea (Navarro, Reference Navarro1932; Dahel et al., Reference Dahel, Tahri, Bensouilah, Amara and Djebar2016). In all these analyses, females are described to achieve greater asymptotic lengths, while males present quicker growth rates, as demonstrated in the present work.
Baldé et al. (Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019b) found that S. aurita reaches greater L ∞ in the eastern Atlantic Ocean than in the western Atlantic and in the Mediterranean Sea. These authors observed that growth parameters remained constant in the Mediterranean Sea from 1932–2012, supporting the balanced effect between the environmental conditions and the level of fishing pressure on the growth parameters of small pelagic fish species, at least for S. aurita, and thus should be considered as phenotypic parameters (understood as the expression of the ability/plasticity of an organism to phenotypically respond to variation in ecological pressures, for survival/adaptation to the changing conditions (Via and Lande, Reference Via and Lande1985)), as described for S. maderensis off Senegal (Ba et al., Reference Ba, Thiaw, Lazar, Sarr, Brochier, Ndiaye, Faye, Sadio, Panfili, Thiaw and Brehmer2016).
A great variability has been found in literature regarding the natural mortality estimations (M) for S. aurita. A value of 0.52 year−1 was estimated for Senegalese-Mauritanian waters (Maxim & Maxim, Reference Maxim and Maxim1987–88) and values from 0.61–1.24 year−1 were gathered by Lévénez (Reference Lévénez, Barry-Gérard, Diouf and Fontenau1994) for Senegalese waters, all based on studies developed during the 1980s. For the Mediterranean Sea a similar value of 0.73 year−1 was estimated by Salem et al. (Reference Salem, El_Aiatt and Ameran2010). In all the estimations mentioned, empirical methods were used. In situations where identification of the most appropriate value of M is not possible, it is considered acceptable to calculate several estimators (Brodziak et al., Reference Brodziak, Ianelli, Lorenzen and Methot2011; Kenchington, Reference Kenchington2014). Identifying the true value of M for fishery management purposes was not possible with the current information. However, the abovementioned estimates of M from other populations of S. aurita are similar to the mean estimate of M obtained (~0.63 year–1) for the population from Mauritania.
In conclusion, it is important to note that the phenotypic plasticity and adaptability of S. aurita to the fluctuations of the environment indicate an advantage for this warm water species in the current climate change scenario (Amenzoui & Baali, Reference Amenzoui and Baali2018), where the geographic expansion of tropical species such as S. aurita has been found to be a new fishing opportunity to replace cooler resources (Blanchet et al., Reference Blanchet, Primicerio, Smalås, Arias-Hansen and Aschan2019). At the same time, these expansion processes obviously challenge effective management of fisheries (Baudron et al., Reference Baudron, Brunel, Blanchet, Hidalgo, Chust, Brown, Kleisner, Millar, Mackenzie, Nikolioudakis, Fernandes and Fernandes2020), and will in addition need a monitoring system able to describe the population structure in the East Atlantic and the more than probable variations. Nevertheless, based on the assumed overexploitation situation of its NW African stock and the ignorance of the biological characteristics of the species in Atlantic waters (FAO, 2019), it is mandatory to use reliable biological information to be able to recommend and implement accurate management measures to ensure: first, the survival and recovery of the population; second, the sustainability of the fishing activities, which constitute an invaluable source of food and economic resource for the riparian countries.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S002531542000079X
Acknowledgements
Sincere thanks to the anonymous reviewers for their insightful comments, enriching the analyses and the text.
Financial support
This project was partially funded by the EU through the European Maritime and Fisheries Fund (EMFF) within the Spanish National Program of collection, management and use of data in the fisheries sector and support for scientific advice regarding the Common Fisheries Policy.