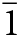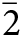Introduction
The Vesuvius volcano; in Italy is one of the most scientifically studied volcanoes in the world. Despite the fact that there have been no new eruptions since 1944, its list of minerals continues to be regularly updated, both with mineral species new to the locality and those completely new to science in general (Campostrini and Gramaccioli, Reference Campostrini and Gramaccioli2005; Russo and Campostrini, Reference Russo and Campostrini2011; Campostrini et al., Reference Campostrini, Demartin and Russo2019; Demartin et al., Reference Demartin, Campostrini, Castellano and Russo2014; Russo et al., Reference Russo, Campostrini, Demartin, Cesare, Erba, Carmina, Fascio, Petti and Zuccari2014). This happens primarily as a result of the re-examination of old samples by modern analytical techniques. The most recent example is the discovery and characterisation of the new mineral napoliite, Pb2OFCl, in a sample collected more than a decade ago from one of the fumaroles of the 1944 eruption. Two other minerals, hephaistosite and susannite, were found in the same association for the first time at Vesuvius (Kasatkin et al., Reference Kasatkin, Siidra, Nestola, Pekov, Agakhanov, Koshlyakova, Chukanov, Nazarchuk, Molinari and Rossi2023).
Manuelarossiite, described in this article, represents the 68th mineral species for which the Somma–Vesuvius volcanic complex is the type locality. The new mineral honours Dr. Manuela Rossi (born 28.11.1977), a researcher at the Dipartimento di Scienze della Terra, dell’Ambiente e delle Risorse, Università degli Studi di Napoli Federico II (Naples, Italy), for her contribution to the study of the minerals from the Vesuvius volcano (e.g. Rossi, Reference Rossi2010; Rossi et al., Reference Rossi, Ghiara, Chita and Capitelli2011, Reference Rossi, Nestola, Zorzi, Lanza, Peruzzo, Guastoni and Kasatkin2014, Reference Rossi, Nestola, Ghiara and Capitelli2016; Malcherek et al., Reference Malcherek, Bindi, Dini, Ghiara, Molina Donoso, Nestola, Rossi and Schluter2014). She also collected and provided to the authors the sample where the new mineral was found. The mineral, its name and symbol (Mnrs) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA No. 2022-097, Nestola et al., Reference Nestola, Kasatkin, Biagioni, Škoda, Santello and Agakhanov2023). The holotype specimen is deposited in the collections of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia with catalogue number 98145.
In this paper the description of this new mineral species is reported.

Figure 1. (a) Manuelarossiite tabular crystal (grey in the centre) associated with cerussite (white) in a vug of the volcanic scoria; (b) group of manuelarossiite crystals in a vug of the volcanic scoria. Back-scattered electron images. Specimen number 98145.
Occurrence and physical properties
A complete description of the environment where manuelarossiite was discovered is extensively provided in the type description of napoliite, a new species found in the same scoria as manuelarossiite by Kasatkin et al. (Reference Kasatkin, Siidra, Nestola, Pekov, Agakhanov, Koshlyakova, Chukanov, Nazarchuk, Molinari and Rossi2023). In 2020, our research team started the investigation of a sample of lava scoria collected by Dr. Manuela Rossi in the early 2010s from a large fragment located at the eastern rim of the ‘Gran Cono’ crater. This research project not only resulted in the discovery of both napoliite and manuelarossiite, but the occurrence of further new minerals within the same sample cannot be excluded.
Manuelarossiite overgrows volcanic scoria and is intimately associated with cerussite. In the same scoria where manuelarossiite was found, anglesite, artroeite, atacamite, calcioaravaipaite, cerussite, challacolloite, cotunnite, hephaistosite, matlockite, napoliite and susannite were identified.
Manuelarossiite occurs as very rare tabular crystals, up to 0.06 × 0.04 × 0.015 mm (but usually much smaller), in the vugs of volcanic scoria. Crystals are individual or grouped in clusters (Fig. 1a, b). Manuelarossiite crystals are flattened on {001}; other forms are {100}, {101}, {110}, {111}, {0![]() $\bar 1$0}, {0
$\bar 1$0}, {0![]() $\bar 1$1}, {1
$\bar 1$1}, {1![]() $\bar 1$0}, {1
$\bar 1$0}, {1![]() $\bar 1$1} and {
$\bar 1$1} and {![]() $\bar 1\bar 1$1} (Fig. 2). Twinning along {001} is observed. The new mineral is colourless and transparent with a white streak and adamantine lustre. It is brittle and has a laminated fracture. No fluorescence is observed under long- or short-wave ultraviolet light. The hardness could not be measured because of the tiny crystal size but is estimated at 2–3 on the Mohs scale by analogy with other lead aluminofluorides (e.g. aravaipaite, artroeite and calcioaravaipaite). Cleavage is perfect on {001}. The density calculated using the empirical formula and unit-cell volume obtained from single-crystal X-ray diffraction data is 5.095 g cm–3. The optical properties of manuelarossiite cannot be measured due to the extremely small size and thinness of its crystals. A mean index of refraction calculated on the basis of Gladstone-Dale equation is 1.625.
$\bar 1\bar 1$1} (Fig. 2). Twinning along {001} is observed. The new mineral is colourless and transparent with a white streak and adamantine lustre. It is brittle and has a laminated fracture. No fluorescence is observed under long- or short-wave ultraviolet light. The hardness could not be measured because of the tiny crystal size but is estimated at 2–3 on the Mohs scale by analogy with other lead aluminofluorides (e.g. aravaipaite, artroeite and calcioaravaipaite). Cleavage is perfect on {001}. The density calculated using the empirical formula and unit-cell volume obtained from single-crystal X-ray diffraction data is 5.095 g cm–3. The optical properties of manuelarossiite cannot be measured due to the extremely small size and thinness of its crystals. A mean index of refraction calculated on the basis of Gladstone-Dale equation is 1.625.

Figure 2. Crystal drawing of manuelarossiite. Drawn using JCrystal (©JCrystalSoft, 2018).
Chemical and spectroscopic data
Quantitative chemical analyses were carried out using a Cameca SX 100 electron microprobe (WDS mode, 15 kV, 4 nA and 2 μm beam diameter) at the Department of Geological Sciences, Faculty of Science, Masaryk University, Brno, Czech Republic. Results (average of 5 spot analyses) are given in Table 1. Determination of the F content was done using PC1 monochromator (2d = 60 Å) and special care (peak maxima position and background selection) was taken prior the analysis. Both the crystal structure data and Raman spectroscopy (see below) confirm the presence of small amount of (OH)– groups in the mineral and the absence of H2O, borate and carbonate groups. The contents of other elements with atomic numbers higher than that of C are below detection limits. X-phi matrix correction (Merlet, Reference Merlet1994) was applied to the data. The empirical formula (calculated on the basis of 7 anions) is: Ca1.00Pb1.00Al1.00[F6.25(OH)0.75]Σ7.00. The ideal formula is CaPbAlF7, which requires CaO 13.77, PbO 54.80, Al2O3 12.52, F 32.66, –O=F –13.75, total 100.00 wt.%
Table 1. Chemical composition of manuelarossiite

* calculated by stoichiometry; S.D. – Standard deviation
The Raman spectrum of manuelarossiite (Fig. 3) was collected in the range 100–4000 cm−1 using a WITec alpha300 R Raman Imaging Microscope equipped with a green laser (532 nm, grating 300 gr/mm) and a 50× LWD (long working distance) objective at the Department of Geosciences of the University of Padova, Italy. The data were collected using a laser power of 6 mW for 30 seconds of integration time and 4 accumulations.

Figure 3. Raman spectrum of manuelarossiite.
The Raman spectrum of manuelarossiite shows the most intense band at 560 cm−1 accompanied by an intense shoulder at 540 cm−1, whereas lower intensity bands (at least 10) are located in the region between ∼120 and 425 cm−1. A significant band is also located at 650 cm−1. In the O–H stretching region (see inset in Fig. 3), one single band at 3604 cm−1 is present with an intensity about eight times lower than that of the most intense band at 560 cm−1. No bands are observed in the range 700–3500 cm−1.
The Raman spectrum of manuelarossiite can be directly compared with the spectrum published for calcioaravaipaite PbCa2AlF9 (Kampf et al., Reference Kampf, Yang, Downs and Pinch2011). For manuelarossiite, the same band assignments done for calcioaravaipaite can be adopted, with the Raman bands between 100 and 200 cm−1 that could be assigned to complex vibration modes of Ca–O and Pb–O, those between 200 and 400 cm−1 assigned to the F–Al–F bending vibrations, and with the main two bands at 560 and 540 cm−1 probably related to the stretching vibrations of the AlF6 octahedra. The band at 650 cm−1 could be assigned to the (Ca,Pb)–OH stretching vibrations. The low intensity of the 3604 cm−1 band is consistent with a limited presence of the (OH)– anion. The O–H stretching position in manuelarossiite is consistent with the position at 3582 cm−1 for calcioaravaipaite (Kampf et al., Reference Kampf, Yang, Downs and Pinch2011).
X-ray crystallography
Powder X-ray diffraction (XRD) data were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with a cylindrical image plate detector using the Debye-Sсherrer geometry (d = 127.4 mm), CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV and 15 mA. The angular resolution of the detector is 0.045°2θ (pixel size 0.1 mm). The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). The powder XRD data of manuelarossiite are given in Table 2 in comparison to that calculated from single-crystal XRD data using the Vesta program (Momma and Izumi, Reference Momma and Izumi2011). Unit-cell parameters from the powder data were calculated from the observed d spacing data using UnitCell software (Holland and Redfern, Reference Holland and Redfern1997) and are as follows: a = 7.682(2), b = 7.454(2), c = 9.284(2) Å, β = 93.93(3)°, V = 530.4(2) Å3 and Z = 4.
Table 2. Powder X-ray diffraction data (d in Å) of manuelarossiite compared to the calculated pattern from single-crystal data

* The calculated pattern was obtained using the crystal structure data in Table 4 and the software Vesta (Momma and Izumi, Reference Momma and Izumi2011). Only reflections with intensities > 5 and up to 1.656 Å are reported.
To obtain single-crystal XRD data, a small tabular crystal of manuelarossiite was mounted on a thin glass fibre and examined using a Supernova Rigaku-Oxford Diffraction single-crystal diffractometer (MoKα radiation, 50 kV and 0.12 mA working conditions) equipped with a Pilatus 200 K Dectris detector. The detector-to-crystal distance was 69 mm. Data were collected by 1707 frames over 30 runs, in 1° slices, with an exposure time of 45 s per frame and a total time of ∼20 hours. The data were corrected for Lorentz and polarisation factors and absorption using the software package Crysalis Pro (version 41.64.113a). The refined unit-cell parameters of manuelarossiite are the following: a = 7.6754(3), b = 7.4443(4), c = 9.2870(5) Å, β = 93.928(5)° and V = 529.39(5) Å3.
The crystal structure of manuelarossiite was solved through direct methods using Shelxs-97 (Sheldrick, Reference Sheldrick2015) and refined through Shelxl-2018 (Sheldrick, Reference Sheldrick2015). After having located the heavier atoms, the positions of the remaining atoms were identified through successive difference-Fourier maps. Three cation and five anion positions were found. Neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992), were used. Twinning on {001} was modelled, giving a ratio between the two individuals of 0.044(2). During the final stages of the refinement, the Pb site was found to be split into two sub-positions, Pb(1a) and Pb(1b). Moreover, relatively high residuals in the difference-Fourier maps (up to ca. 7.5 e/Å3) were observed. These structural features can be due to some difficulties in the appropriate modelling of the absorption using the multi-scan technique or to the possible order–disorder (OD) nature of manuelarossiite, in accord with the OD nature of the related mineral calcioaravaipaite (Kampf et al., Reference Kampf, Merlino and Pasero2003). However, notwithstanding this shortcoming, the crystal-chemical features are sound (see below) and the anisotropic structural model of manuelarossiite converged to R 1 = 0.0561 for 849 reflections with F o > 4σ(F o) and 58 refined parameters. Details of data collection and refinement are given in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4, whereas Table 5 reports selected bond distances. Table 6 shows bond-valence sums calculated according to the bond-parameters of Brese and O’Keeffe (Reference Brese and O’Keeffe1991). The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Summary of crystal data and parameters describing data collection and refinement for manuelarossiite

* w = 1/[σ2(F o2)+0.0455P 2+103.7547P], where P = (F o2+2F c2)/3
Table 4. Sites, Wyckoff positions, site occupancy factors (s.o.f.), fractional atomic coordinates and equivalent isotropic displacement parameters (Å2) for manuelarossiite

Table 5. Selected bond distances (Å) for manuelarossiite

Table 6. Weighted bond-valence sums (in valence units) for manuelarossiite

Crystal structure description
The crystal structure of manuelarossiite (Fig 4a, b) is characterised by {001} layers formed by CaF8 polyhedra decorated on both sides by AlF6 octahedra, connected to the {001} layer through edge-sharing. The average <Ca–F> distance is 2.360 Å, to be compared with the values observed at the two independent Ca sites in calcioaravaipaite, i.e. 2.360 and 2.361 Å, respectively (Kampf et al., Reference Kampf, Yang, Downs and Pinch2011). The bond-valence sum at the Ca site is 1.98 valence units (vu). The Al-centred octahedron has an average <Al–F> distance of 1.807 Å, in agreement with the values observed by Kampf et al. (Reference Kampf, Yang, Downs and Pinch2011) in aravaipaite and calcioaravaipaite, i.e. 1.806 and 1.808 Å, respectively. The bond-valence sum at the Al site of manuelarossiite is 2.97 vu.

Figure 4. Crystal structure of manuelarossiite as seen down a and b (in Figure 4a and b, respectively). Lead and F atoms are shown as dark grey and green circles, respectively, whereas Ca- and Al-centred polyhedra are shown as blue and light blue polyhedra, respectively.
Successive {001} layers are bonded through Pb atoms. Lead atoms are mainly hosted at the Pb(1a) [site occupancy factor = 0.963(3)], whereas only minor Pb occurs at the Pb(1b) position [site occupancy factor = 0.037(3)]. Lead atoms at Pb(1a) have an asymmetric bonding environment, with five bond distances shorter than 2.70 Å and seven longer ones, ranging between 2.96 and 3.33 Å. This asymmetry is probably related to the stereoactive nature of the 6s 2 lone-electron-pair of Pb2+. The average <Pb–F> of the five shortest distances is 2.504 Å, to be compared to 2.511 Å given by Kampf et al. (Reference Kampf, Yang, Downs and Pinch2011) for calcioaravaipaite. The bond-valence sum at Pb(1a), assuming its full-occupancy, is 1.86 vu. Whereas Pb(1a) has a twelve-fold coordination, Pb(1b) has an eleven-fold coordination, as the PbF11 polyhedra reported by Kampf et al. (Reference Kampf2001) in aravaipaite; also in this case, the coordination environment is asymmetric, with three distances shorter than 2.70 Å. Assuming its full-occupancy by Pb, the bond-valence sum at Pb(1b) is 2.18 vu.
Five independent F sites occur in manuelarossiite. According to electron microprobe data, 0.75 (OH) groups per formula unit occur. The location of the H atom was not possible and only some hypotheses about the actual position of (OH) groups can be put forward. All F sites show bond-valence sums ranging between 0.87 and 1.10 vu. Three of them, i.e. F(3), F(4) and F(5) have a distorted tetrahedral coordination and the occurrence of an H atom seems unlikely; F(1) and F(2) show a triangular coordination. This situation is similar to what was found by Kampf et al. (Reference Kampf, Merlino and Pasero2003) in calcioaravaipaite, where no conclusive evidence for assigning O to any F site was found. Taking into account the results of crystal structure refinement, coupled with electron microprobe data, the crystal chemical formula of manuelarossiite is proposed as CaPbAl(F,OH)7 (Z = 4).
Relationship with other Pb–Al fluorides
Lead–Al fluoride are currently represented by very few phases, i.e. aravaipaite, Pb3AlF9·H2O (Kampf et al., Reference Kampf, Dunn and Foord1989; Kampf, Reference Kampf2001; Kampf et al., Reference Kampf, Yang, Downs and Pinch2011), artroeite, PbAlF3(OH)2 (Kampf and Foord, Reference Kampf and Foord1995), and calcioaravaipaite, Ca2PbAlF9 (Kampf and Foord, Reference Kampf and Foord1996; Kampf et al., Reference Kampf, Yang, Downs and Pinch2011). Manuelarossiite is the fourth mineral showing Pb, Al and F as essential chemical constituents (Table 7).
Table 7. Comparison between currently known Pb–Al fluorides

Aravaipaite, artroeite and calcioaravaipaite were first identified on specimens from the Grand Reef mine, Aravaipa Mining District, Graham County, Arizona, USA, at the end of the 1980s and in the mid-1990s, respectively (Kampf et al., Reference Kampf, Dunn and Foord1989; Kampf and Foord, Reference Kampf and Foord1995, Reference Kampf and Foord1996). Calcioaravaipaite was named for its apparent relationship with aravaipaite. Actually, these two species show some differences, discussed by Kampf et al. (Reference Kampf, Yang, Downs and Pinch2011). Moreover, both of them display an OD nature (Kampf et al., Reference Kampf, Merlino and Pasero2003; Kampf et al., Reference Kampf, Yang, Downs and Pinch2011). Since the first American findings, these two species have been reported from the fumaroles of Vesuvius, along with artroeite (Campostrini and Gramaccioli, Reference Campostrini and Gramaccioli2005; Russo and Campostrini, Reference Russo and Campostrini2022).
Manuelarossiite has a structural relationship with calcioaravaipaite (Fig. 5). These species belong to a homologous series of layered (Ca/Pb)–Al compounds with structural formula M 2+NPbAlF5+2N, where M 2+ = Ca. Manuelarossiite is the N = 1 homologue, whereas calcioaravaipaite is the N = 2 homologue. Artroeite is chemically identical with the N = 0 homologue of this series, with some (OH) replacing F; however, it shows a different crystal structure, with dimers formed by edge-sharing Al-centred octahedra (Kampf and Foord, Reference Kampf and Foord1995).

Figure 5. Structural relationship between manuelarossiite, aravaipaite and calcioaravaipaite. Same symbols as in Figure 4.
Geological environment of formation
In the sample studied, manuelarossiite along with artroeite, atacamite, calcioaravaipaite and susannite are the latest minerals occurring in the vugs of volcanic scoria altered by fumarolic gas. All of them contain (OH)− groups either as a species-defining component (artroeite, atacamite and susannite) or a small admixture partly substituting F (manuelarossiite and calcioaravaipaite). We infer therefore that manuelarossiite was probably formed, not as a result of direct crystallisation from gaseous phases, but as a product of the interactions between earlier-formed high-temperature sublimate Pb-bearing minerals (anglesite, cerussite, cotunnite, matlockite and napoliite), HF-containing fumarolic gas and atmospheric water vapour at relatively low temperatures, presumably not higher than 150°C. Similar trends have been noted previously in the literature for OH-bearing minerals found in the active fumaroles of the Tolbachik volcano at Kamchatka Peninsula in Russia (e.g. Pekov et al., Reference Pekov, Zubkova, Zolotarev, Yapaskurt, Krivovichev, Belakovskiy, Lykova, Vigasina, Kasatkin, Sidorov and Pushcharovsky2021).
Conclusion
Manuelarossiite is a new addition to the long list of mineral species discovered in fumaroles of active volcanoes, one of the most important kinds of occurrence of new minerals according to Pekov and Pushcharovsky (Reference Pekov, Pushcharovsky, Bindi and Cruciani2023). This mineral is also an interesting improvement of our knowledge about the mineral systematics and crystal-chemistry of Pb–Al fluorides, showing the possible existence of a homologous series involving some of these compounds.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.59.
Acknowledgements
M. Rossi is acknowledged for providing us with the studied specimen.
Competing interests
The authors declare none.