Low exercise capacity during an exercise test has been established as an independent predictor of risk for total mortality and CVD( Reference Kokkinos, Myers and Kokkinos 1 , Reference Morris, Ueshima and Kawaguchi 2 ). Exercise cardiac power (ECP), which is defined as a ratio of directly measured VO2max:peak systolic blood pressure (SBP) during exercise test, is an accurate measure for exercise capacity and it is known to be an independent predictor of CVD( Reference Kurl, Laukkanen and Niskanen 3 ). The advantage of ECP compared with other exercise capacity measurements is that ECP provides information not only about cardiorespiratory fitness but also considers the differences in cardiovascular resistance and cardiac afterload( Reference Kurl, Laukkanen and Niskanen 3 , Reference Kurl, Jae and Kauhanen 4 ).
Although little is known about ECP and risk of CVD, previously in the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study cohort, lower ECP was associated with increased risk of sudden cardiac death and stroke in men( Reference Kurl, Laukkanen and Niskanen 3 , Reference Kurl, Jae and Kauhanen 4 ). In addition, low cardiorespiratory fitness (VO2max) and increased SBP during exercise were associated with higher risk of cardiovascular events and CVD-related mortality in the KIHD cohort( Reference Laukkanen, Lakka and Rauramaa 5 – Reference Laukkanen, Mäkikallio and Rauramaa 8 ).
Substantial evidence from epidemiological studies, including KIHD study, demonstrates that long-chain n-3 PUFA may reduce the risk of CVD( Reference Mozaffarian and Wu 9 – Reference Virtanen, Laukkanen and Mursu 11 ). To the best of our knowledge, no previous studies have been conducted to evaluate the association of these fatty acids with ECP. However, a few small supplementation studies have assessed the efficacy of long-chain n-3 PUFA on VO2max ( Reference Guezennec, Nadaud and Satabin 12 – Reference Leaf and Rauch 20 ) and SBP during exercise( Reference Peoples, McLennan and Howe 19 , Reference Rontoyanni, Hall and Pombo-Rodrigues 21 , Reference O’Keefe, Abuissa and Sastre 22 ), but the findings are inconsistent.
We evaluated the association of serum long-chain n-3 PUFA concentrations with ECP, VO2max and maximal SBP during an exercise test among middle-aged and older men from the KIHD cohort. We also evaluated whether high hair Hg concentration, a biomarker for long-term Hg exposure( Reference Roman, Walsh and Coull 23 ), is associated with ECP and whether it could modify the associations with long-chain n-3 PUFA, as it has been shown to do with the risk of CVD in the KIHD population( Reference Virtanen, Voutilainen and Rissanen 10 , Reference Virtanen, Laukkanen and Mursu 11 ).
Methods
Study population
Subjects were participants of the KIHD, which is a prospective, population-based study designed to investigate risk factors for CVD, carotid atherosclerosis and related outcomes in a randomly selected sample of men from eastern Finland( Reference Salonen 24 ). The baseline examinations were carried out in 1984–1989. Of the 3235 eligible men aged 42, 48, 54 or 60 years who lived in the city of Kuopio or its surrounding areas, 2682 men (82·9 %) were recruited to the baseline study. The baseline characteristics of the entire study population have been described previously( Reference Salonen 24 ). The KIHD study protocol was approved by the Research Ethics Committee of the University of Kuopio. All subjects gave their written informed consent for participation. From the analyses, we excluded participants with missing data on ECP measurements (n 207), a history of CVD (n 677) or those with missing data on serum long-chain n-3 PUFA (n 113) or hair Hg (n 13). After exclusions, 1672 men were included in the final analysis.
Measurements
Subjects provided their hair and venous blood samples between 08.00 and 10.00 hours at baseline. Repeat hair samples were collected from twenty-one subjects 4–9 years (mean, 6 years) after baseline examinations to survey the tracking of hair Hg values over time. The subjects were instructed to abstain from alcohol for 3 d and from smoking and eating for 12 h before providing samples. Comprehensive description of the determination of serum lipids and lipoproteins( Reference Salonen, Nyyssonen and Korpela 25 ), assessment of medical history and use of medications( Reference Salonen, Nyyssonen and Korpela 25 ), smoking status( Reference Salonen, Nyyssonen and Korpela 25 ), alcohol consumption( Reference Salonen, Nyyssonen and Korpela 25 ), resting blood pressure( Reference Salonen, Nyyssonen and Korpela 25 ) and physical activity( Reference Lakka, Venalainen and Rauramaa 26 ) have been reported previously. Hypertension diagnosis was defined as SBP/diastolic blood pressure >140/90 mmHg at study visit, clinical diagnosis of hypertension or use of hypertensive medication. Serum C-reactive protein (CRP) was measured using an immunometric assay (Immulite High Sensitivity CRP Assay; DPC). Dietary intakes were assessed using 4-d food recording at the time of blood sampling( Reference Voutilainen, Rissanen and Virtanen 27 ). Educational status was assessed in years using self-administered questionnaires( Reference Voutilainen, Rissanen and Virtanen 27 ).
Serum fatty acid and mercury measurements
Serum fatty acids were determined in a single gas chromatographic run without pre-separation as described previously( Reference Laaksonen, Lakka and Lakka 28 ). Serum fatty acids were extracted using chloroform–methanol solution. The chloroform phase was evaporated and treated with sodium methoxide, which methylated esterified fatty acids. Quantification was carried out with reference standards purchased from Nu-Check Prep Inc. Each analyte had an individual reference standard, and the internal standard was eicosane. Fatty acids were chromatographed in an NB-351 capillary column (HNU-Nordion) by a Hewlett-Packard 5890 Series II gas chromatograph with a flame ionisation detector (Hewlett-Packard Company, since 1999 Agilent Technologies Inc.). Results for fatty acids were obtained in µmol/l, and in the data analyses proportion of fatty acids from total fatty acids was used. The CV% was 9·4 % for EPA (20 : 5n-3), 12·7 % for docosapentaenoic acid (DPA, 22 : 5n-3) and 11·9 % for DHA (22 : 5n-3). For the serum total long-chain n-3 PUFA, we used the sum of EPA, DPA and DHA.
Hair Hg was detected by flow injection analysis-cold vapour atomic absorption spectrometry and amalgamation( Reference Salonen, Seppanen and Nyyssonen 29 ). The Pearson’s correlation coefficient between the original and the repeat measurement collected after 4–9 years was 0·91.
Assessment of exercise cardiac power
A maximal symptom-limited exercise tolerance test was performed between 08.00 and 10.00 hours using an electrically braked cycle ergometer (Medical Fitness Equipment 400 L bicycle ergometer)( Reference Lakka, Laukkanen and Rauramaa 30 ). The standardised testing protocol comprised of an increase in the workload of 20 W/min with the direct analyses of respiratory gases (Medical Graphics). ECP was measured by the ratio of measured VO2max:peak SBP( Reference Kurl, Laukkanen and Niskanen 3 ). VO2max was defined as the highest value for or the plateau on VO2. Blood pressure was measured every 2 min both manually and automatically during exercise until the test was stopped and every 2 min after exercise. In the present study, we used only manually measured blood pressure values. The highest SBP achieved during the exercise test was defined as the maximum exercise SBP. For safety reasons, all tests were supervised by an experienced physician with assistance from an experienced nurse. Electrocardiography was recorded continuously with the Kone 620 electrocardiograph (Kone)( Reference Kurl, Laukkanen and Rauramaa 7 , Reference Laukkanen, Mäkikallio and Rauramaa 8 ).
Statistical analysis
The univariate associations between serum EPA+DPA+DHA concentrations and demographic, lifestyle and clinical characteristics at baseline were assessed by means and linear regressions for continuous variables and by the χ 2-test for categorical variables. Correlations between individual long-chain n-3 PUFA were evaluated by calculating Spearman’s correlation coefficients. Linear regression models were used to determine the association of serum long-chain n-3 PUFA with ECP, VO2max and maximum SBP during exercise. The mean values of ECP, VO2max and maximum SBP during exercise in the exposure quartiles were analysed using ANCOVA.
In addition, two models were run to adjust for potential cofounders. Model 1 was adjusted for age (years) and examination year, and model 2 included the variables in the model 1+BMI (kg/m2), smoking status (non-smoker, previous smoker, current smoker <20 cigarettes/d and ≥20 cigarettes per d), leisure-time physical activity (kJ/d (kcal/d)), use of drugs for hypertension (yes/no), bronchial asthma (yes/no), LDL-cholesterol and HDL-cholesterol (mmol/l), CRP levels (mg/l) and intakes of energy (kJ/d (kcal/d)), carbohydrates (g/d) and alcohol (g/week). The cohort mean was used to replace missing values in covariates (<0·5 %). Test for linear trend across quartiles was assessed using the median value in each quartile as the continuous variable in the linear regression model. Statistical significance of the interactions on a multiplicative scale was assessed by stratified analysis with hair Hg divided by the median and likelihood ratio tests with a cross-product term. For assessing the clinical significance, we calculated effect sizes based on Cohen’s d index (the difference between the group means divided by the standard deviation of the comparison category)( Reference Ferguson 31 ). All P values were two-sided (α=0·05). Data were analysed using SPSS software version 21 for windows (IBM Corp.).
Results
Baseline characteristics
Baseline characteristics of the participants are presented in Table 1. Men with higher serum total long-chain n-3 PUFA concentrations were more likely to be older (P=0·05) and have higher education (P=0·001), BMI (P=0·05), leisure-time physical activity (P=0·03), serum HDL- and LDL-cholesterol concentrations (P<0·001), hair Hg concentration (P=0·05) and alcohol intake (P<0·001). They also had lower carbohydrate intake (P<0·001), lower total energy intake (P<0·001), lower serum TAG levels (P<0·001) and they were less likely to use anti-hypertensive drugs (P=0·001).
Table 1 Baseline characteristics according to total serum long-chain n-3 PUFA (Mean values and standard deviations; percentages)
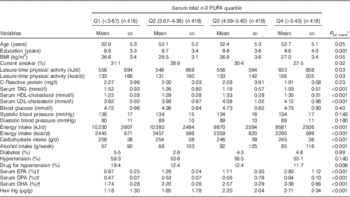
Q, quartiles; DPA, docosapentaenoic acid.
* Participant characteristics at baseline were assessed by means and linear regressions for continuous variables and the χ 2-test for categorical variables.
† Proportion of all serum fatty acids.
The mean serum concentrations, as a percentage of all serum fatty acids, were 4·72 (sd 1·60) % for total long-chain n-3 PUFA, 1·69 (sd 0·92) % for EPA, 0·55 (sd 0·10) % for DPA and 2·48 (sd 0·73) % for DHA. The correlations between the individual long-chain n-3 PUFA were 0·70 for EPA and DHA, 0·56 for EPA and DPA and 0·41 for DHA and DPA. The mean hair content of Hg was 1·94 μg/g and ranged from 0 to 15·67 μg/g.
Serum long-chain n-3 PUFA, hair mercury and exercise cardiac power
The mean ECP was 12·46 (sd 3·07) ml/mmHg. After adjustment for age and examination year (model 1), higher serum total long-chain n-3 PUFA concentration was associated with higher ECP (the mean difference between extreme quartiles was 0·42 ml/mmHg (95 % CI 0·03, 0·81, P trend across quartiles=0·04)). Further multivariate adjustments had little impact on the association (model 2, Table 2). When the fatty acids were investigated individually, generally similar direct associations were observed with EPA, DPA and DHA (Table 2). The effect sizes, based on Cohen’s d index, were 0·10 for total serum long-chain n-3 PUFA, 0·05 for EPA, 0·15 for DPA, 0·20 for DHA and 0·21 for Hg.
Table 2 Exercise cardiac power (ml/mmHg) in quartiles of serum long-chain n-3 PUFA and hair mercuryFootnote * (Mean values and 95 % confidence intervals)

DPA, docosapentaenoic acid.
* The mean values were analysed using ANCOVA.
† Mean difference between the extreme quartiles.
‡ Model 1: adjusted for age and examination year.
§ Model 2: adjusted for model 1+BMI, current smoking status, leisure-time physical activity, energy intake, carbohydrate intake, alcohol intake, use of drugs for hypertension and C-reactive protein, LDL- and HDL-cholesterol concentrations.
Hair Hg concentration was not associated with ECP (Table 2). Although we could not find statistically significant interactions between the long-chain n-3 PUFA and hair Hg for ECP (P for interaction=0·15 for the total long-chain n-3 PUFA, P=0·08 for EPA, P=0·47 for DPA and P=0·50 for DHA), we observed statistically significant associations between the long-chain n-3 PUFA and higher ECP only in participants with lower hair Hg content (<median 1·30 μg/g) (online Supplementary Table S1).
Serum long-chain n-3 PUFA, hair mercury and VO2max
The mean VO2max was 2545 (sd 559) ml/min. Higher serum total long-chain n-3 PUFA concentration was associated with higher VO2max (Table 3). The extreme-quartile difference in the multivariate-adjusted model was 83 ml/min (95 % CI 15, 152, P trend across quartiles=0·02). The associations were again generally similar with EPA, DPA and DHA (Table 3). The effect sizes were 0·13 for total serum long-chain n-3 PUFA, 0·05 for EPA, 0·28 for DPA, 0·15 for DHA and 0·19 for Hg.
Table 3 Maximum VO2 (ml/min) in quartiles of serum long-chain n-3 PUFA and hair mercuryFootnote * (Mean values and 95 % confidence intervals)

DPA, docosapentaenoic acid.
* The mean values in the exposure quartiles were analysed using ANCOVA.
† Mean difference between the extreme quartiles.
‡ Model 1: adjusted for age and examination year.
§ Model 2: adjusted for model 1+BMI, current smoking status, leisure-time physical activity, energy intake, carbohydrate intake, alcohol intake, use of drugs for hypertension and C-reactive protein, LDL- and HDL-cholesterol concentrations.
We did not find a statistically significant association between hair Hg content and VO2max (Table 3). However, adjusting for hair Hg content modestly attenuated the associations between the long-chain n-3 PUFA and VO2max (Table 3). Furthermore, the associations were stronger among those with hair Hg below the median of 1·30 μg/g, although the P for interaction was statistically significant only for total n-3 PUFA (P=0·03) and EPA (P=0·02), but not for DPA (P=0·14) or DHA (P=0·18) (online Supplementary Table S2).
Serum long-chain n-3 PUFA, hair mercury and maximal systolic blood pressure during exercise
The mean maximal SBP during exercise was 206·6 (sd 26·5) mmHg. Serum long-chain n-3 PUFA were not associated with maximal SBP during exercise (Table 4). Although hair Hg was associated with a trend towards higher maximal SBP after adjustment for age and examination year, further adjustments attenuated the association and it was no longer statistically significant (Table 4). Hair Hg did not modify the associations between the long-chain n-3 PUFA and maximal SBP during exercise as well (P for interaction=0·23 for total long-chain n-3 PUFA, P=0·39 for EPA, P=0·13 for DPA and P=0·26 for DHA) (online Supplementary Table S3).
Table 4 Maximum systolic blood pressure (mmHg) in quartiles of serum long-chain n-3 PUFA and hair mercuryFootnote * (Mean values and 95 % confidence intervals)

DPA, docosapentaenoic acid.
* The mean values in the exposure quartiles were analysed using ANCOVA.
† Mean difference between the extreme quartiles.
‡ Model 1: adjusted for age and examination year.
§ Model 2: adjusted for model 1+BMI, current smoking status, leisure-time physical activity, energy intake, carbohydrate intake, alcohol intake, use of drugs for hypertension and C-reactive protein, LDL- and HDL-cholesterol concentrations.
Sensitivity analyses
The associations were generally similar when we excluded participants using hypertension medication (n 209) from the analyses. For example, the mean ECP in quartiles of serum total long-chain n-3 PUFA after excluding those using hypertension medication was 12·20, 12·61, 12·84 and 12·62 ml/mmHg (model 2, P trend=0·05), the mean VO2max 2505, 2582, 2638 and 2585 ml/min (P trend=0·05) and the mean maximal SBP during exercise 207·1, 207·6, 207·1 and 207·7 mmHg (P trend=0·83) (other data not shown).
Discussion
In this cross-sectional study among 1627 middle-aged and older men from eastern Finland, the serum long-chain n-3 PUFA were associated with higher ECP and VO2max, but not with maximal SBP during exercise. However, the clinical significance of the associations was quite modest, but this can be expected because exercise capacity is to a large extent determined by genetics and physical activity( Reference Bouchard, Boulay and Simoneau 32 – Reference Dionne, Turcotte and Thibault 34 ). Furthermore, although hair Hg concentration was not associated with ECP, higher hair Hg concentration modestly attenuated the associations of the long-chain n-3 PUFA with VO2max and ECP.
There is little evidence regarding the association between long-chain n-3 PUFA and ECP and its components; three small, randomised trials found that VO2max was increased dose-dependently by fish oil supplementation( Reference Guezennec, Nadaud and Satabin 12 , Reference Żebrowska, Mizia-Stec and Mizia 18 , Reference Leaf and Rauch 20 ), but this has not been observed in all supplementation studies( Reference Boss, Lecoultre and Ruffieux 13 – Reference Kawabata, Neya and Hamazaki 17 , Reference Peoples, McLennan and Howe 19 ). Moreover, in one study, a DHA-rich meal led to lower systemic vascular resistance and to a smaller increase in SBP during exercise compared with a control meal( Reference Rontoyanni, Hall and Pombo-Rodrigues 21 ), whereas two other studies did not find any effect of fish oil supplementation on exercise-induced blood pressure( Reference Peoples, McLennan and Howe 19 , Reference O’Keefe, Abuissa and Sastre 22 ). It has been reported that intake of long-chain n-3 PUFA is associated with lower resting blood pressure( Reference Miller, Van Elswyk and Alexander 35 ). We have previously reported a modest, inverse association between long-chain n-3 PUFA and resting SBP in the 11-year examination of the KIHD cohort( Reference Virtanen, Nyantika and Kauhanen 36 , Reference Nyantika, Tuomainen and Kauhanen 37 ). However, in the current study, we could not find such an association. The lack of association might be due to haemodynamic response to exercise, which is not taken into account for SBP at rest( Reference McHam, Marwick and Pashkow 38 ).
A possible mechanism underlying the beneficial impact of the serum long-chain n-3 PUFA on exercise capacity during an exercise test might be explained by the effect of the long-chain n-3 PUFA on the vascular endothelial functions( Reference Khan, Elherik and Bolton-Smith 39 ), such as improvement in vascular reactivity( Reference Wu and Meininger 40 , Reference Mickleborough 41 ), increased production of endogenous antioxidant enzymes and decreased inflammatory cytokines( Reference Wu and Meininger 40 , Reference Mickleborough 41 ) and bioavailability of endothelial nitric oxide( Reference Wu and Meininger 40 , Reference Juturu 42 ).
We have previously found that higher hair Hg concentration attenuated the inverse associations of the long-chain n-3 PUFA with CVD outcomes in the KIHD cohort( Reference Virtanen, Voutilainen and Rissanen 10 , Reference Virtanen, Nyantika and Kauhanen 36 ). In the current study, hair Hg attenuated the associations of the long-chain n-3 PUFA with VO2max and ECP, although the interaction was statistically significant only for VO2max. This attenuation may be at least partially explained by the role of Hg on endothelial dysfunction by reduction in nitric oxide bioavailability and nitric oxide synthase expression( Reference Furieri, Galán and Avendaño 43 ).
The strengths of our study include the use of serum long-chain n-3 PUFA and hair Hg as exposures instead of dietary intakes. As serum fatty acids and hair Hg are objective biomarkers for exposure( Reference Roman, Walsh and Coull 23 , Reference Hodson, Skeaff and Fielding 44 ), their use reduced the bias by misclassification, which would attenuate the associations towards the null. Other strengths include the extensive examination of potential confounders and the large number of participants with the assessment of VO2max, which is considered to be the ‘gold standard’ for measuring cardiorespiratory fitness( Reference Laukkanen, Kurl and Salonen 6 ). A limitation of this study is that it is based on an ethnically homogenic population of middle-aged and older men, which may limit the generalisability of our results. In addition, the average hair Hg concentrations are somewhat higher in the KIHD cohort compared with other study populations that have reported Hg exposure( Reference Wennberg, Bergdahl and Hallmans 45 , Reference Mozaffarian, Shi and Morris 46 ). Therefore, our results may not be generalisable to study populations with lower average Hg exposure.
In conclusion, our results suggest that higher circulating concentrations of long-chain n-3 PUFA, mainly a marker of fish consumption in this study population, are associated with higher ECP and VO2max in middle-aged and older men from eastern Finland. As low cardiorespiratory fitness (VO2max) and low ECP are risk factors for CVD( Reference Kurl, Laukkanen and Niskanen 3 – Reference Laukkanen, Kurl and Salonen 6 , Reference Laukkanen, Mäkikallio and Rauramaa 8 ), these results could partially explain how long-chain n-3 PUFA may reduce the risk of cardiac mortality.
Acknowledgements
The authors thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health, University of Eastern Finland, for data collection.
The study was supported by the University of Eastern Finland. This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
The authors’ contributions were as follows: B. T., S. K., T.-P. T. and J. K. V. contributed to the conception and design of the research; S. K., and T.-P. T. acquired the data; B. T. and J. K. V. analysed the data and interpreted the results; B. T. drafted the manuscript; and all the authors critically revised the paper and approved the final version of the manuscript.
There are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516002142







