INTRODUCTION
Hepatitis C virus (HCV) infection is common with serious sequelae. Globally an estimated 170 million persons are chronically infected with HCV and 3–4 million persons are newly infected each year [1]. In developed countries HCV is largely transmitted through injecting drug use. In England and Wales an estimated 200 000 people have been infected, 80% of whom are current or former injecting drug users (IDUs) [Reference Sweeting2]. In some UK cities over one in two IDUs have been infected with HCV [3]. Chronic HCV infection is the leading indication for liver transplantation in developed countries and presents an important health and economic burden [Reference Patel, Muir and McHutchison4]. While there is good evidence that opiate substitution treatment (OST) reduces incidence of HIV [Reference Metzger and Navaline5, Reference Gibson, Flynn and McCarthy6], there is only limited evidence for the impact of OST on the incidence of HCV [Reference Wright and Tompkins7]. Research from Italy reported a non-significant protective effect of methadone treatment [Reference Rezza8], recent research from Amsterdam reported an association of full participation in harm reduction programmes with a lower HCV incidence but no effect of methadone alone [Reference Van Den Berg9]. Previous Australian studies in high HCV-prevalence community (~66% HCV infected) or prison (~80% HCV infected) settings reported no significant effect of methadone maintenance on the incidence of HCV [Reference Crofts10–Reference Dolan12]. Homelessness in IDUs has been shown to be an important risk factor for HCV and is associated with greater injecting risk behaviour [Reference Hickman13].
In the present study we sought to estimate HCV incidence in IDUs in South Wales, which may be considered as part of a low-prevalence area (<25% HCV infection from routine surveillance programmes [3]) and to test the impact of OST and homelessness and other potential risks on HCV incidence.
METHODS
We carried out a prospective cohort study of IDUs in South Wales. Dried blood spot samples and behavioural surveillance data were collected at study baseline and again at 1 year follow-up. Dried blood spot samples were tested for evidence of anti-HCV antibodies. The study received ethical approval (MREC 04/MRE09/21).
Participants
The target criteria for inclusion in the study were being a current or a recent drug injector. Over 99% of seronegative individuals followed up reported injecting in the month before baseline interview, the remaining two individuals had injected in the previous year and reported injecting in the year of follow-up.
Recruitment
IDUs were recruited from a range of field stations across South Wales. These included treatment services, needle and syringe exchange services and homeless hostels. Individuals were also approached on the street. Data collection occurred in towns and cities along the main transport links in South Wales (Newport and Calidicot, Cardiff and Barry, Bridgend, Neath & Porth Talbot, Swansea), the South Wales valleys (Merthyr Tydfil, Pontypridd, Rhydfelin, Treorchy, Aberdare) and one outlying market town (Abergavenny). Drug injectors were invited to enter the study by professional staff, by researchers and by word of mouth between study participants. Recruitment was thus opportunistic but made use of existing social and drug-using networks. Dried blood spot samples (DBS) were taken from 700 unique study participants at baseline. Each subject underwent a structured interview that asked questions concerning possible risk factors for the acquisition of HCV infection. All study participants were paid £10 for participating and gave written informed consent. Blood samples were collected and structured interview questionnaires were administered and completed by interviewers working as part of the research team. The study was conducted between 2004 and 2006.
Sample collection and testing
DBS were transported to the Virus Reference Department (Health Protection Agency), and tested for the presence of anti-HCV antibodies using published methods [Reference Judd14]. Sample testing was delinked from subject-identifying data and study participants were not given their test results. A delinked research strategy was used as it was decided, at the project planning stage, that testing within the context of a research study with incentives to take part, and without the opportunity to ensure pre- and post-test discussion, made diagnostic testing inappropriate. Individuals wishing to receive a diagnostic test were signposted to local testing sites. The study team sought to follow-up the sample (re-interviewing and re-testing) approximately 1 year later, using personal details given at study baseline, both seropositive and seronegative individuals were sought and when found recruited to the follow-up. Follow-up of only seronegative individuals would have led to deductive disclosure of infection in those not sought for follow-up. At follow-up all interviewers in the research team were unaware of the baseline serostatus of the individual being interviewed.
Statistical analysis
Analysis was performed on data from individuals who were seronegative at baseline and then successfully followed up. An incident HCV case was defined as an individual who was HCV seronegative at baseline and seropositive at follow-up. This definition will, due to the window period between initial infection and production of detectable levels of antibody, have falsely classified individuals who were infected at baseline yet, at the time of sampling, had failed to mount a serological response detectable by DBS. Similarly, individuals infected in the follow-up period and seronegative at follow-up will also have been misclassified. Implicit in the measure of incidence used is the assumption that these sources of error will have cancelled each other out.
HCV incidence was expressed using person-years of follow-up calculated to the nearest month of follow-up. HCV incidence was estimated by community size and by a range of self-reported behavioural and participant characteristics in univariable analysis using Poisson regression analysis. Homelessness in the year and OST at follow-up were obtained from self-reports. Paraphernalia sharing and needle sharing, respectively, were combined into a dichotomous variable termed equipment sharing generated from any report of these behaviours with either partners, core group of individuals injected with, or new people injected with, since the baseline interview. Imprisonment, stimulant injection (any cocaine, crack-cocaine or amphetamine injection), and injection-site infections (ranging from redness to septicaemia) in the follow-up year and ever cleaning needles and syringes before reuse and gender were entered as dichotomous variables (male/female or yes/no).
The communities from which individuals were recruited were grouped by population size. One region comprised the two large cities (>200 000 population) of Swansea and Cardiff and the other region comprised all other smaller population areas (<200 000 population) and included Neath, Bridgend, sites in the Rhondda Cynon Taff valleys, Pontypridd, Rhydfelin, Merthyr Tydfil, Barry, Newport, Caldicot and Abergavenny. Additional analysis was performed to explore the relationship the between the distal variables of community size, homelessness and OST and the more proximal variables addressing injecting practices.
Variables with a significant univariable effect on HCV incidence (estimated by P<0·05) and variables with non-significant effects thought to be possible confounders (age group, duration of injection, stimulant injection in year and imprisonment in year) or with a highly plausible causal association (needle and syringe sharing) were entered into a multivariable Poisson regression model with incident HCV infection as the dependent variable. Using backwards stepwise estimation, variables were dropped from the model where the P value was <0·05 to inform the final multivariable model. The variable reporting needle and syringe sharing was included in the final model despite a P value of >0·05 due to the likely causal association between needle sharing and disease transmission.
A number of models were examined that included interaction terms to examine potential interactions between community size and homelessness and between community size and OST; we hypothesized that geographical differences in the nature of service provision for treatment and support for homeless individuals may have varied across the community size groupings. Likelihood ratio tests (LRT) were used to test for differences between models with or without interaction terms; an LRT χ2 probability of >0·05 was taken to indicate no significant difference between models. Interaction terms were not included in the final model if they did not improve goodness of fit.
In order to reduce follow-up bias and use all HCV seronegative IDUs in the study we used multiple imputation of missing data using switching regression [Reference Royston15, Reference Rubin16]. All variables in the analysis plus information on geographical area, past imprisonment, homelessness and treatment were used in the imputation model. Analyses were carried out on 20 imputed datasets and the results combined appropriately using Rubin's Rules [Reference van Burren17].
RESULTS
Baseline sample
A total of 744 interviews were conducted at baseline. Forty-four interviews were discounted as invalid due to concerns of data reliability; the collection of identifying data allowed the identification of 41 interviews that were repeats (due to the same individual returning to take part in the study on more than one occasion), and a three further interviews where responses suggested they did not meet inclusion criteria. These interviews were discarded. Of the remaining 700 eligible individuals at baseline, 516/700 (73·7%) were HCV seronegative and 184/700 (26·3%) were seropositive (Fig. 1). Just under half 327/700 (47%, 10 non-responses) reported being in OST; of these 88% reported being prescribed methadone and 12% reported being prescribed buprenorphine.
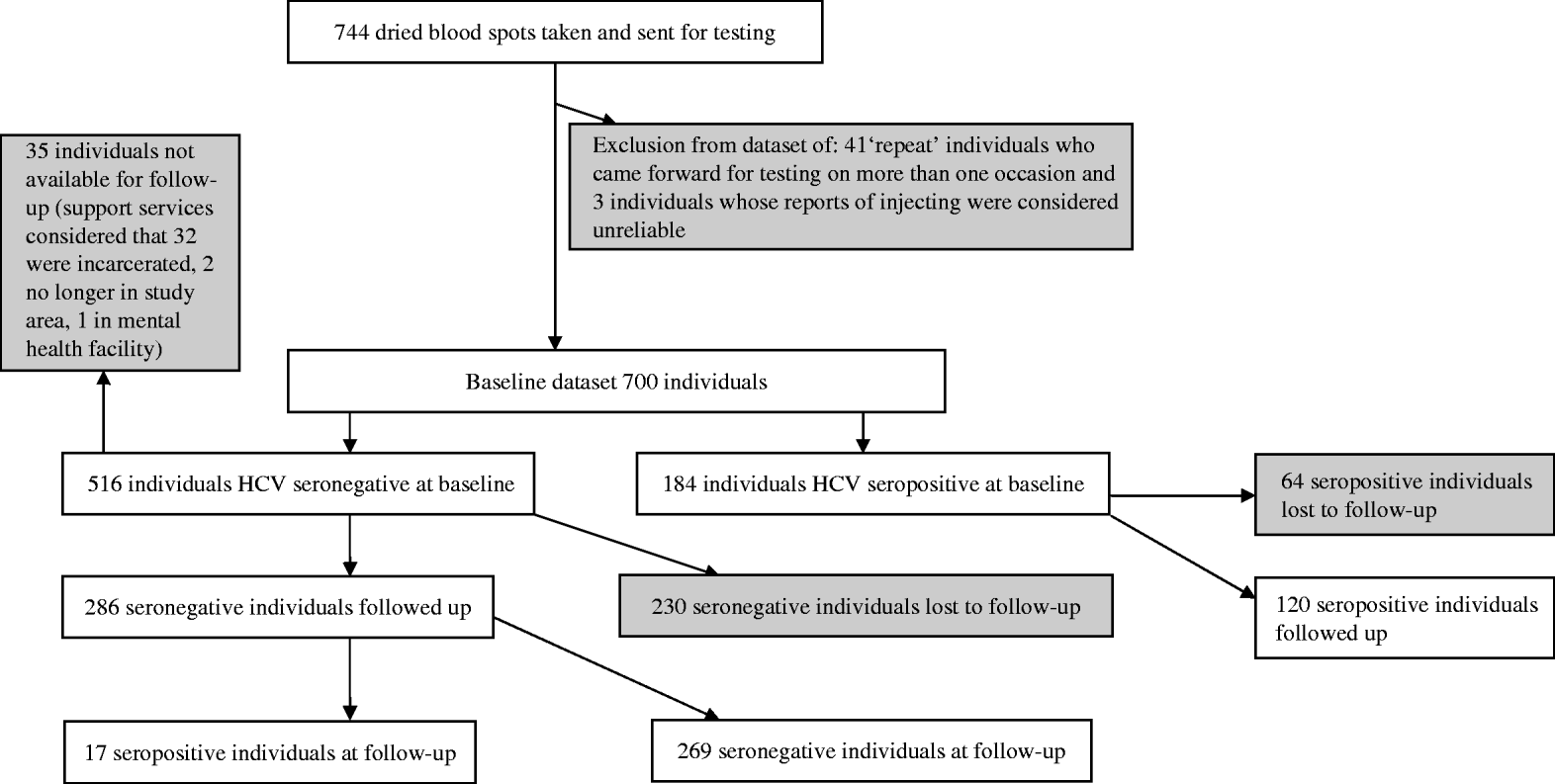
Fig. 1. Participant flow diagram.
Follow-up sample
Approximately 12 months later 286 anti-HCV seronegative individuals, and 120 seropositive individuals, were followed up and re-interviewed. The response rate in seronegatives was 55·4% (286/516). The overall response rate was 57·9% (406/700). The response rate in seronegatives excluding 35 individuals unavailable for follow-up was 59·5% (286/481) (Fig. 1). The 286 antibody-negative participants generated 287·33 person-years of follow-up.
There was evidence of differential follow-up within the sample. The prevalence of HCV at baseline was higher in those followed up [29·6%, 95% confidence interval (CI) 25·1–34·0] than in those who were lost to follow-up (21·8%, 95% CI 17·0–26·5%) (Pearson χ2=5·34, P=0·021). The seronegative individuals who were followed up were more likely to be in OST at the time of baseline interview, were slightly older and injected more frequently in the previous 4 weeks than those who were not. They had similar reported experiences of homelessness in the previous 12 months and of prison. The age of first injecting, years injecting and the sex ratio were similar (Table 1). The 25 individuals that reported not injecting in the year of follow-up were included in the analysis.
Table 1. Characteristics of seronegative study participants who were successfully followed up, and who were lost to follow-up
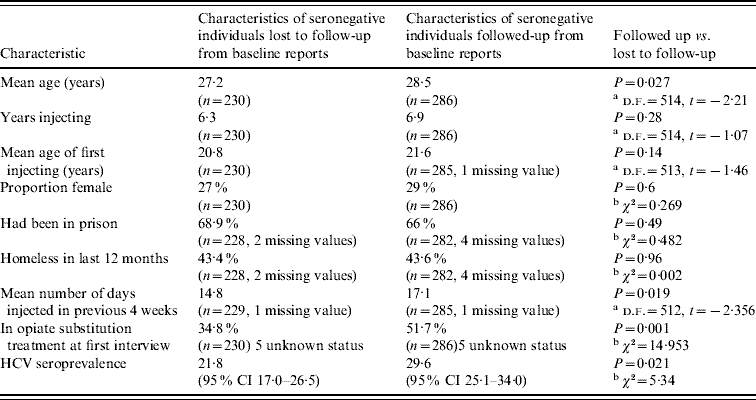
CI, Confidence interval.
Information drawn from baseline data collection.
a t test, b Pearson χ2 test.
Incidence of HCV
During the follow-up period 17 individuals seroconverted, yielding an overall HCV incidence rate of 5·9/100 person-years (95% CI 3·4–9·5). After imputation for missing data the estimated incidence of HCV was 8·5/100 person-years (95% CI 5·4–12·7).
Univariable analysis
Univariable associations between variables and HCV incidence are shown in Table 2. There was a significant difference in the incidence of HCV between the community sizes; in the large city region (Swansea and Cardiff, population of each >200 000) the HCV incidence rate was 15·2/100 person-years (95% CI 8·1–26·0); in the sample drawn from the smaller population centres the incidence rate was 2/100 person-years (95% CI 0·5–5·1). We examined the incidence by smaller geographical groupings (e.g. town vs. town vs. city); whilst lacking in statistical power, the data were consistent with a varied incidence of HCV across South Wales (data not shown).
Table 2. HCV incidence described by behavioural and demographic variables in univariable analysis
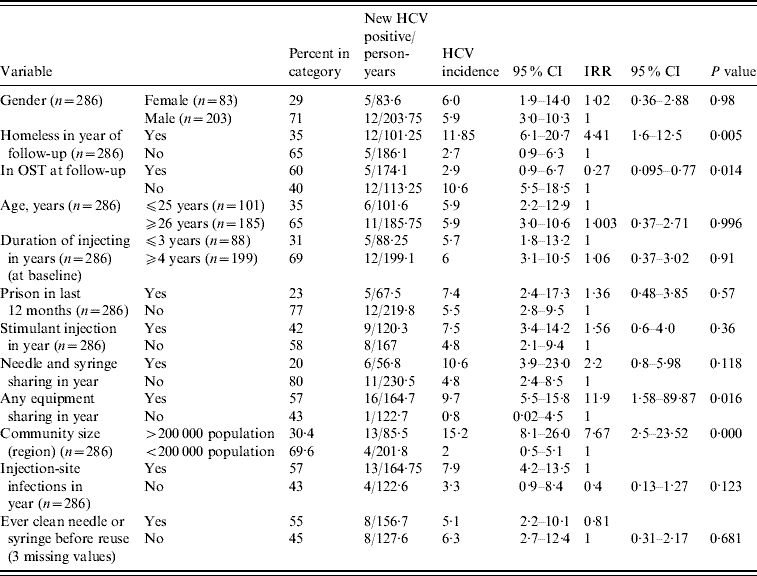
CI, Confidence interval; IRR, incident rate ratio; OST, opiate substitution treatment.
With the exception of gender, age and duration of injection all data are drawn from follow-up data collection.
Any homelessness in the previous year was associated with elevated HCV incidence [11·9/100 person-years (95% CI 6·1–20·7) in homeless compared to 2·7/100 person-years (95% CI 0·9–6·3) in those housed]. OST at follow-up was associated with a decrease in HCV incidence [2·9/100 person-years (95% CI 0·9–6·7) in those in OST at follow-up compared to 10·6 (95% CI 5·5–18·5) in those not in OST at follow-up].
HCV incidence was elevated in individuals reporting sharing injecting equipment in the year of follow-up [9·7/100 person-years (95% CI 5·5–15·8) in those reporting sharing, 0·8/100 person-years (95% CI 0·02–4·5) in those reporting no sharing]. Incidence of HCV infection was not significantly elevated in those reporting needle and syringe sharing. There was no detectable effect on HCV incidence of duration of injection, injection-site infections, stimulant injection or prison in the last 12 months or of ever cleaning injecting equipment before reuse, or age, or gender (Table 2). Ethnicity was not analysed as a covariate. A variable reporting the selling of sex, although significant in univariable analysis, was dropped from the analysis as it was reported by only 2·5% of the sample.
Adjusted analysis
The final variables entered into the multivariable model were: region, whether homeless at any time in the follow-up period, whether in OST at follow-up, sharing of injecting equipment in the follow-up period and sharing of needles and syringes in the follow-up period; this last variable was included despite not fulfilling the significance criteria. In the adjusted model (Table 3) elevated HCV incidence was predicted by larger community size [incident rate ratio (IRR) 6·6, 95% CI 2·11–20·51, P=0·001], homelessness in year (IRR 2·9, 95% CI 1·02–8·28, P=0·047) and equipment sharing in year (IRR 12·7, 95% CI 1·62–99·6, P=0·015). Being in OST at follow-up was associated with lower HCV incidence (IRR 0·34, 95% CI 0·12–0·99, P=0·047).
Table 3. Adjusted IRRs for HCV incidence from multivariable Poisson regression model (n=286)
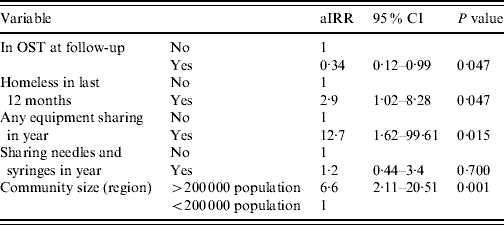
aIRR, Adjusted incident rate ratio; CI, confidence interval; OST, opiate substitution treatment.
Test for interaction between variables
Interaction terms for interaction between region and OST and also for interaction between region and homelessness were entered individually into the multivariable model used in the adjusted analysis. Addition of the interaction terms did not improve the fit of the multivariable model (region x homeless: LR χ2=0·72, P=0·4; region×OST: LR χ2=0·63, P=0·4); these interaction terms were thus not included in the final model.
HCV incidence stratified by homelessness and OST
In homeless individuals in OST at follow-up interview the HCV incidence rate was 7·2/100 person-years (95% CI 2·0–18·5), whilst in homeless individuals out of treatment at follow-up it was 17·4/100 person-years (95% CI 7·5–34·3). The incidence in housed individuals in OST at follow-up was 0·8/100 person-years (95% CI 0·02–4·7) whilst the incidence in housed individuals at follow-up who were not in OST was 5·9/100 person-years (95% CI 1·6–15·2).
Injecting risk associated with homelessness
In seronegative individuals followed up, the average number of days injecting in the last month was higher in those who reported being homeless in the last year (13·6, 95% CI 11·1–16·2) than in those housed (9·8, 95% CI 8·2–11·5) (t test: P=0·011, t=−2·55, d.f.=283, one missing value). Reports of sharing of needles and syringes in the previous year were higher in individuals who reported being homeless in the last year (29%, 95% CI 20–38) than in those housed (14·5%, 95% CI 9·4–19·6, Pearson χ2=8·66, P=0·003). Reports of sharing injecting equipment in the previous year were higher in individuals who reported being homeless in the last year (67%, 95% CI 57·6–76·4) than in those housed (51·6%, 95% CI 44·4–58·9%, Pearson χ2=6·28, P=0·012).
Homelessness was positively associated with self-reported public injecting (Pearson χ2=30·6, P<0·001), having been in prison in the year (Pearson χ2=11·5, P=0·001), and with stimulant injection (Pearson χ2=3·13, P=0·08). There was no difference in self-reported injection-site infections or needle and syringe exchange use in homeless and housed individuals (Pearson χ2=2·3, P=0·132, Pearson χ2=0·002, P=0·96, respectively).
Injecting risk associated with OST
In baseline seronegative individuals who were followed up, the average number of days injected in the month prior to interview was lower for individuals in OST at the time of the interview (8·9, 95% CI 7·2–10·535) than for those out of OST (14·6%, 95% CI 12·3–17·01) (t test: P<0·000, t=4·05, d.f.=283, one missing value).
OST was associated with a reduced probability of having been in prison (Pearson χ2=19, P<0·001). There was no detectable difference on reported stimulant injection (Pearson χ2=0·04, P=0·84), needle and syringe exchange use (Pearson χ2=0·4, P=0·523), needle and syringe sharing (Pearson χ2=1·73, P=0·188), or equipment sharing in the last year (Pearson χ2=2·1, P=0·145) between those in or out of OST. However, there was an increase in self-reporting of injection-site infections (Pearson χ2=5·9, P=0·015) in individuals in OST at follow-up.
Injecting risk associated with community size
In the study sites of communities of >200 000 people (Swansea and Cardiff) the average number of days injected in the month prior to interview was similar to that reported from the region defined by smaller population size (<200 000 people) [mean days per month 10·9 (95% CI 9·2–12·5) and 11·8 (95% CI 9·2–14·4), respectively; t test: t=−0·06, d.f.=283, P>0·5]. There was no detectable difference in self-reported needle and syringe sharing or equipment sharing between the two regions (Table 4).
Table 4. Key variables defined by community size >200 000 or <200 000
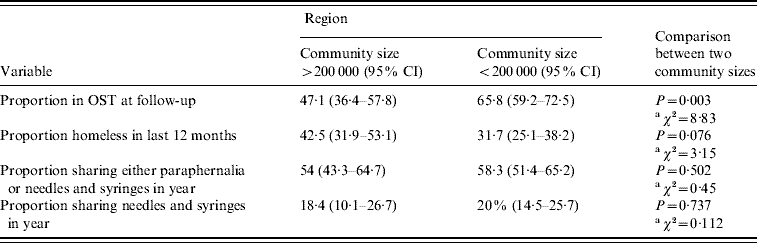
CI, Confidence interval; OST, opiate substitution treatment.
a Pearson χ2 test.
Association between community size and homelessness and OST
Both the proportion of individuals in OST at follow-up and the proportion homeless in the year varied between the two community sizes. The proportion of individuals reporting OST at follow-up was lower, and the proportion reporting homelessness higher, within the region with population centres of >200 000 people than within the region defined by smaller population size (<200 000 people) (Table 4).
DISCUSSION
Principal findings
In South Wales we detected an overall HCV incidence in drug injectors of ~6/100 person-years, with evidence of major variation of incidence between the large cities and the smaller population centres. HCV incidence was about fourfold higher in drug injectors who reported being homeless in the previous 12 months than in those who reported being housed. The association between being in OST at follow-up and reduced HCV incidence remained after adjustment for homelessness, sharing behaviour and region. One clear impact of treatment is that participants report substantially fewer injecting events. The incidence of HCV was greatly elevated in those reporting sharing injecting equipment in the last year. It is highly likely that direct sharing of needles and syringes is a key route of HCV transmission. However, uncertainty exists around the reliability of self-reports of direct sharing [Reference Hickman13].
Additional findings
We estimated an incidence of HCV in drug injectors in South Wales of between 3·4 and 9·5 cases/100 person-years. The estimates produced from this study are substantially lower than estimates of 31·9 to 54·7 cases/100 person-years reported from London in 2001 [Reference Judd18] and 15·7 to 51·2/100 person-years reported from Glasgow in the 1990s [Reference Roy19], and are closer to an estimate from rural Australia of between 4·4 and 21·8/100 person-years [Reference Maher20]. Studies from the USA have reported incidences/100 person-years of 9·8 to 13·5 from the Seattle area [Reference Hagan, Thiede and Des Jarlais21], 16 from Baltimore [Reference Garfein22], 6·7–14·4 from Greater Chicago [Reference Thorpe23] and 13·4–30·6 from New York City [Reference Des Jarlais24] (although within a subsample of the New York cohort from East Harlem incidence was lower at 3·4–20·2). Australian studies have reported incidences/100 person-years of 6·8–16·8 from Melbourne [Reference Crofts and Aitken25], 34·4–56·6 from an urban setting in New South Wales [Reference Maher20], and 35·6–58·8 from Sydney [Reference Maher26].
In addition the study indicated that there is considerable variation in the incidence of HCV across different regions of South Wales. We chose to divide the region into two, the large cities of Swansea and Cardiff forming one group and the smaller populations the second group. The difference in incidence between these regions is striking. Analysing the data by study site, i.e. in smaller regions than the dichotomous geographical variable, greatly reduced the power to determine regional differences in incidence; nonetheless the observed patterns were consistent with geographical differences in HCV incidence occurring within South Wales. Associations between the two regions and the key variables of OST and homelessness (Table 4) were consistent with the observed incidence.
The increase in the frequency of injecting and in reported equipment sharing associated with homelessness is consistent with an elevated risk of HCV acquisition. Similarly, a reduction in the frequency of injecting (estimated by self-reported number of days injected per month) in individuals reporting OST at follow-up is consistent with a protective effect of treatment on HCV incidence. OST had no detectable effect on equipment sharing reports. Region had no detectable effect on any of these injecting variables. These findings suggest that there are other important unmeasured factors (e.g. mixing patterns and social networks) that may influence disease acquisition across different regions and within different population subgroups. The increase in the reports of injection-site infections in individuals in OST at follow-up requires further research; perhaps injection-site infections are a factor in triggering seeking OST.
It is worth noting that the majority of studies on HCV incidence in the UK have been carried out in very large cities with populations measured in millions rather than thousands; the data presented here may be more typical of smaller communities within the UK. The two cities classed as having populations of >200 000 accounted for, based on 2001 census figures, ~18% of the population of Wales; it is thus important that future research, and surveillance of infection is performed within smaller population centres as well as large cities.
After imputation for missing data the estimated incidence of HCV was 8·5/100 person-years. This value is higher than the HCV estimate from full case analysis (although within the 95% CI of the full case estimate) and corroborates the hypothesis and analysis that those lost to follow-up may be more at risk of HCV than those successfully followed up.
Strengths of the study
The study was a large prospective cohort targeted at a high-risk population within the UK. The study was the largest of its type in the UK to date. The study followed up a vulnerable population with a high prevalence of HCV.
Weaknesses of study
The relatively weak associations described in the statistical tests must be treated with caution. However, these findings raise important public health questions. The study was limited by a low follow-up rate; this low follow-up reflects the challenges of relocating vulnerable individuals recruited from a range of sites across South Wales. There was a bias at follow-up towards older individuals, those in OST, more frequent injectors and to those who had been exposed to HCV prior to the onset of the study, whilst years injecting, age of first injecting, gender, homelessness and having ever been in prison were similar in individuals who were successfully followed up and those who were not. We acknowledge that there may have thus been a bias towards older individuals who were in OST; these individuals may be atypical.
The measure of incidence used will have falsely classified individuals who at baseline were recently infected yet at the time of sampling had failed to mount a serological response detectable by DBS; similarly, individuals recently infected and still seronegative at study follow-up will have been misclassified. There is probably a lack of precision in measures of risk behaviours; it is likely that the risk factor data may not exactly correspond to the period for which incidence is measured; risk behaviours reported in the year of follow-up may be irrelevant for individuals already infected yet seronegative at baseline, similar risk in the latter part of the period may not impact on incidence. Recall bias may result in more recent risk being reported with greater precision than risk from early in the follow-up period.
We acknowledge that the selection of sample recruits at baseline may have been biased towards drug injectors in contact with treatment agencies and towards publicly visible drug injectors. These biases may influence the validity of the incidence estimate. Differential follow-up remains a challenge for prospective cohort studies in difficult to reach and vulnerable populations. Currently we lack a clear description of the size and characteristics of the at-risk (i.e. injecting) population from which our sample was drawn. Nonetheless the individuals recruited to the study were drawn from a population of some of the most visible and potentially vulnerable drug injectors in South Wales.
Although we excluded a number of possible confounders it is possible that we failed to identify differences between those in and out of treatment. Of particular concern is that being in OST might arguably reflect more care seeking and lower risk behaviour in the study cohort, rather than an effect of treatment per se.
The study did not record the methadone dose received by study subjects in OST, the dose of which may have influenced the impact of treatment. Higher doses and longer duration of treatment have been associated with lower rates of HIV infection [Reference Hartel and Schoenbaum27]. Higher methadone dose has been associated with reductions in illicit heroin use and retention in treatment [Reference Gossop28]. The definition of homelessness in this study was broad, further work and larger samples are needed to determine the specific influence different types of homelessness such as rough sleeping, hostels, and bed-and-breakfast hotels may have on HCV incidence.
Comparison with other studies
We corroborate the importance of homelessness in increasing the risk of HCV infection. Our findings are consistent with a recent study of HCV prevalence in multiple sites in England that reported an association between homelessness and HCV infection [Reference Hickman13]. Homelessness is associated with an increase in risk factors that elevate the risk of viral transmission. Homeless individuals are more likely to inject in public places and this is associated with frequent and hasty injecting, and needle sharing [Reference Klee and Morris29–Reference Wright and Tompkins31]. We provide evidence that is consistent with the hypothesis that OST can be protective against HCV infection. These data are consistent with recent research from Amsterdam that reported an association of full participation in harm reduction programmes with a lower HCV incidence but no effect of methadone alone [Reference Van Den Berg9] and are consistent with earlier research from Italy that reported a non-significant protective effect of methadone treatment [Reference Rezza8].
Meaning and implications of the study
The implementation of measures to prevent HCV transmission must, in the UK context, be implemented in IDUs if ongoing transmission of HCV infection and the associated mortality and morbidity are to be successfully reduced. Reducing injecting risk and preventing HCV transmission in homeless injectors must be prioritized. The existence of injecting populations in South Wales experiencing a low incidence of HCV infection, as suggested by the regional differences observed in this study, should be a spur to ensuring prevention efforts are optimized in these settings to maintain this epidemiological picture; doing nothing may allow this potential opportunity to prevent infection to be lost. Our data are consistent with the hypothesis that in regions within the UK with comparatively low HCV prevalence comprehensive drug treatment has the potential to alter the course of the HCV epidemic.
Future research
Further research with greater control of potential cofounders and with greater study power is needed to explore the suggested impact of OST upon HCV incidence. Such studies must reflect the range of OST programmes that exist in the UK and that vary in nature of dispensing (on-site, take home, supervised, etc.) and in prescribed dose. Greater clarity is needed on the role of the various components of equipment sharing and of needle and sharing in driving transmission of infection within the UK. We also urge that smaller communities are not neglected in studies of HCV incidence.
ACKNOWLEDGEMENTS
The authors thank: Adref Pontypridd, Bridgend Community Drug and Alcohol team, Cardiff Addictions Unit, Cardiff Night Bus, FADS Pen y Waun, Gwent SSMS Newport, Huggard Centre Cardiff, Inroads Cardiff & Barry, Kaleidoscope Newport & Tredegar, DrugAid & MIDAS Merthyr Tydfil, Newlands Barry, Rhondda Cynon Taff Community Drug Team, SANDS Swansea, Swansea Community Drug Team, Salvation Army Cardiff, TEDS Aberdare, The Medicine Centre Neath, Tresillian House Cardiff, WGCADA Bridgend Neath and Port Talbot, Wallich Clifford Community, the field work team of Hidden Populations Research Ltd, Zoe Couzens, Ceri Harris, Sam Ray, Rosemary Allegier, Gary Porter Jones, Dee Walker, Amy Larkins, Dr Ali Omrani, Elaine McKinney, Dr Humphrey Birley and Isabel Puscas for their assistance. This study was funded in full by the Welsh Assembly Government (The Welsh Assembly Government is responsible for developing the HCV Action plan for Wales and is responsible for commissioning health services in Wales. The sponsor has no financial links with either OST or HCV pharmacological treatment providers).
DECLARATION OF INTEREST
None.







