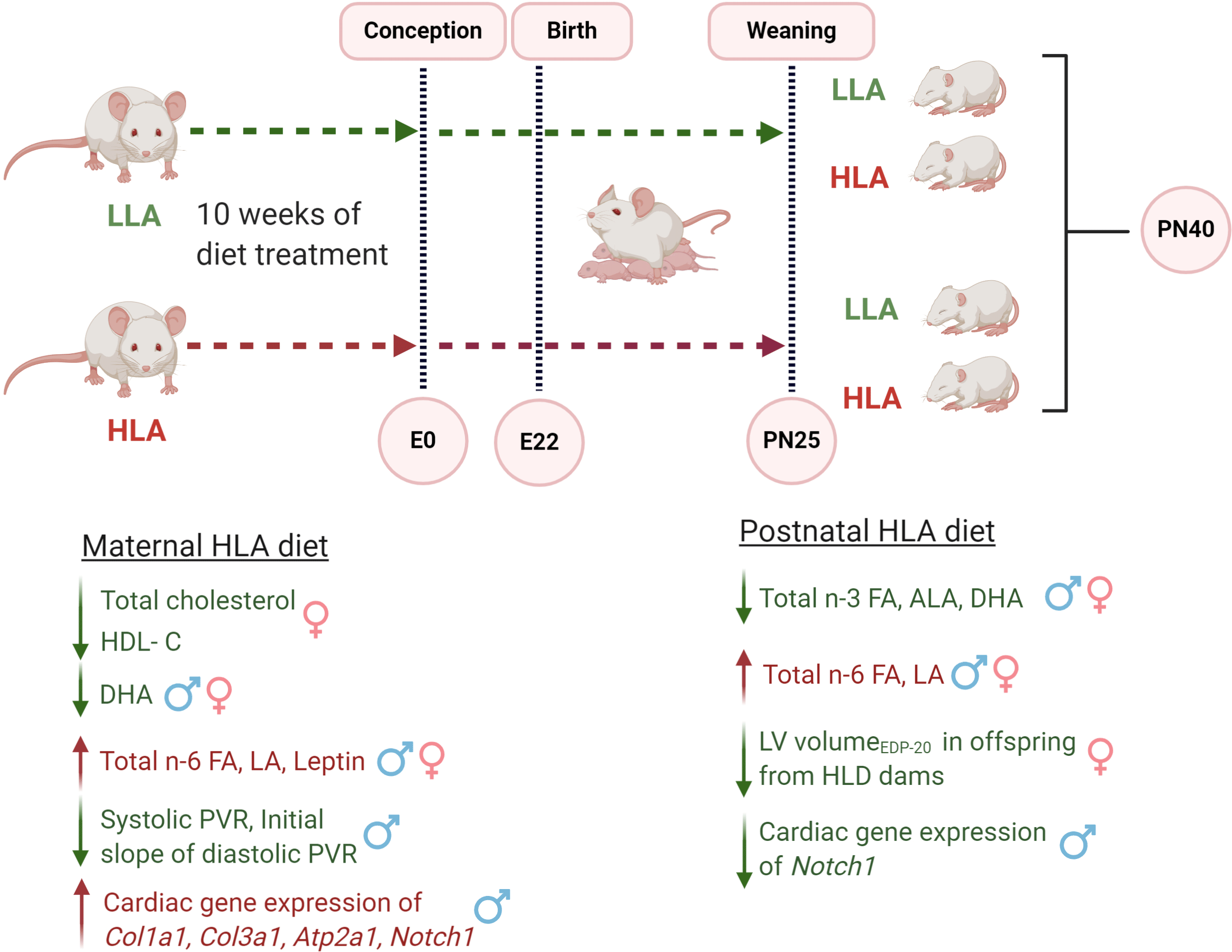
CVD is the most common non-communicable disease globally, with an estimated 17·8 million death in 2017(Reference Roth, Abate and Abate1). The WHO has focused the impact of lifestyle, such as unhealthy diet, physical inactivity and psychological stress, in the development of CVD and stated that three quarters of all CVD-related mortality may be avoided by the lifestyle modifications(Reference Nishida, Uauy and Kumanyika2). The aetiology of this global health problem is complex and may commence in early life or childhood. Epidemiological studies demonstrate that atherosclerosis can have its origin in childhood, and that this major risk factor for CVD can persist into adulthood(Reference Berenson, Srinivasan and Bao3,Reference Gidding, McMahan and McGill4) . Further, childhood CVD risk factor clustering is reported to be predictive of CVD in adulthood(Reference Jaquith, Harris and Penprase5). Both epidemiological data and animal model studies provide evidence of the fetal origin of CVD risk(Reference Gademan, van Eijsden and Roseboom6–Reference Blackmore, Niu and Fernandez-Twinn8). For example, intra-uterine growth restriction that occurred in response to the Dutch famine increased the risk of death from CVD in adult life in affected offspring(Reference Barker, Osmond and Simmonds9). The effects of maternal environment on in utero development are termed as fetal programming or developmental origin of health and disease(Reference Hoffman, Reynolds and Hardy10,Reference Barker and Osmond11) . Adverse maternal conditions such as overnutrition(Reference Wang, Ma and Tong12), obesity(Reference Blackmore, Niu and Fernandez-Twinn8), hypoxia(Reference Xu, Williams and O’Brien13) and protein restriction(Reference Elmes, Haase and Gardner14) during pregnancy are also associated with the development of cardiovascular dysfunction in later life of offspring.
Linoleic acid (LA; 18:2n-6; cis, cis-9,12-octadecadienoic acid) is the most abundant n-6 PUFA in modern human diets. It can be elongated and desaturated to produce arachidonic acid (AA, 20:4n-6)(Reference Whelan and Fritsche15), a longer chain n-6 PUFA. AA is a precursor of eicosanoid mediators such as PG, thromboxanes and leukotrienes, which influence inflammatory processes(Reference Innes and Calder16) and are essential for the normal metabolic function of cells and tissues. When these eicosanoid mediators are in excess, they promote inflammation and chronic disease(Reference Whelan and Fritsche15). In recent years, worldwide dietary guidelines have encouraged replacing animal-based SFA with plant-based PUFA, the most abundant being LA(Reference Naughton, Mathai and Hryciw17). As a result, the current intake of LA is significantly higher than required to prevent deficiency(Reference Naughton, Mathai and Hryciw17). The excess in LA may decrease the ability of the n-3 PUFA α-linolenic acid (ALA), to produce longer chain anti-inflammatory n-3 PUFA such as EPA (20:5n-3) and DHA, as both are metabolised by the same enzymes, with the enzymes that metabolise LA and ALA having a higher enzymatic affinity for LA(Reference Choque, Catheline and Rioux18). A recent meta-analysis and analysis of recovered data from a past clinical trial found that substitution of dietary LA for SFA increases CHD, CVD and all-cause mortalities(Reference Ramsden, Zamora and Leelarthaepin19). However, there is evidence from randomised control trials that a high LA and low SFA diet reduces cardiac events and mortality in post-infarct patients(Reference Leren20), suggesting the conflicting role of LA in CVD risk.
At this time, there is significant research which describes the effect of a high LA diet on cardiac health. Emerging research has indicated that LA is critical for normal development(Reference Shrestha, Cuffe and Holland21). Cardiac development commences in utero and perturbations during this time impact cardiovascular function in later life. Despite the increased consumption of LA in Western cultures, including in women of reproductive age, there is paucity of data investigating the role of maternal diet high in LA on the cardiovascular system and incidence of CVD on offspring development. The fetal and early life exposure to a suboptimal maternal diet programmes CVD risk due to altered cardiac development, with additional studies also demonstrating an important role for the postnatal environment. Development of the cardiomyocyte commences prior to birth, with a full complement of binucleated cardiomyocytes present at birth(Reference Paradis, Gay and Zhang22). Following this period of hyperplasia, postnatal cardiomyocytes undergo hypertrophy(Reference Kreipke and Birren23). Therefore, two critical windows of development of the heart occur both before and after birth in humans. In rodents, the post-weaning period is also important for development, with a critical period in adolescent rats, between 5 and 9 weeks of life where perturbations have the capacity to influence final cardiac development and maturation(Reference Asif, Wlodek and Black24). Rodent studies also support a role for the post-weaning diet on disease risk, with a study demonstrating that a post-weaning obesogenic diet can exacerbate the detrimental effects of maternal diet-induced obesity on offspring body weight gain(Reference Howie, Sloboda and Kamal25). Thus, in addition to investigating the effects of a high LA maternal diet in utero on offspring CVD risk, it is important to identify any effects of a post-weaning diet on cardiovascular function. It is evident that the risk of development of the CVD increases with ageing; however, emerging research suggests an increasing incidence of CVD in young adults(Reference Andersson and Vasan26). Early markers of cardiomyopathy, such as left ventricular hypertrophy and left ventricular dysfunction, are frequently observed during childhood and adolescence(Reference Mitsnefes27) and may represent early heart damage associated with the later development of CVD.
In the present study, our aim was to investigate the effects of an elevated maternal LA diet on offspring heart function, and the ability of the postnatal diet to reverse or exacerbate any adverse phenotype. Further, we aimed to characterise any molecular changes that may contribute and the ability of the post-weaning diet to reverse any deleterious cardiovascular changes. Since it is well established that programmed disease outcomes are sexually dimorphic, we aimed to analyse male and female offspring outcomes. Therefore, the hypothesis of the present study was that a maternal diet high in LA would programme cardiovascular sex-specific deficits that will be reversed by a low LA diet following weaning.
Methods
Ethical statement
Ethical approval for all experimental protocols was granted by the Griffith University Animal Ethics Committee (NSC/01/17/AEC) prior to experimentation. All experiments were conducted humanely. Rats were housed in accordance to the Australian Code of Practice for Care and Use of Animals for Scientific Purpose, following the ARRIVE Guidelines for Reporting Animal Research.
Experimental design, animals and experimental diet
Eight-week-old female Wistar Kyoto rats were purchased from the Australian Resource Centre). Rats were housed in individually ventilated cages under 12 h light-dark cycle at a temperature of 20–22°C and provided with standard food pellets during acclimatisation and tap water ad libitum throughout the study. After a week for acclimatisation, female rats were randomised to consume either a control low LA (LLA: 1·44 % of energy from LA, n 8) or high LA (HLA: 6·21 % of energy from LA, n 8) diet for 10 weeks. The minimum requirement for LA in the rodent diet is between 1 and 1·5 %(Reference Choque, Catheline and Delplanque28). The composition of the custom diet has been previously reported(Reference Shrestha, Cuffe and Holland21). These diets were matched for n-3 PUFA and total fat content. The HLA diet used is comparable to the diet consumed in Western society(Reference Jandacek29). After 8 weeks of dietary exposure, vaginal impedance was measured daily for at least two oestrous cycles using a rodent vaginal impedance reader (Muromachi Kikai Co. Ltd.). Rats were considered ready for mating after 10 weeks of dietary exposure and when vaginal impedance was greater than 4·5 × 103 Ω and at this time were placed with a Wistar Kyoto male rat overnight. Male rats were maintained on standard chow, but during mating (∼8 h), the male rats consumed the similar diet as female rats. After rats gave birth, offspring were left unhandled to minimise stress and weaned at postnatal day 25. From weaning, offspring were housed with one or two other litter mates of the same sex and fed either LLA or HLA diets (n 6–8/litter per sex). Thus, offspring were exposed to the maternal diet until weaning at postnatal day 25 before being placed on either LLA or HLA for 15 additional days. The offspring were euthanised at postnatal day 40. Rats were terminally anaesthetised with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) administered once and monitored until abolishment of reflexes. A thoracotomy was performed, and hearts rapidly excised into ice-cold fluid for Langendorff heart perfusion (details below). After perfusion experiments, hearts were weighed and fixed and frozen for gene analysis. Blood samples were placed in EDTA tubes and centrifuged at 5000 × g for 10 min to separate plasma, which was then stored at –80°C until analysis.
Quantification of fatty acids in plasma
Concentrations of fatty acid (FA) in plasma were measured using GC(Reference Liu, Muhlhausler and Gibson30). The samples were analysed by the South Australian Health and Medical Research Institute, following the method described in(Reference Liu and Gibson31). Briefly, 50 μl of plasma sample was spotted onto Whatman SG81 ion exchange paper (1·5 cm × 1·5 cm) and dried in air at room temperature for ∼30 min. The dried spot was then soaked in acetone for 10 min, then placed into a scintillation vial. The spotted plasma was added to 2 ml of 1 % (v/v) H2SO4 in anhydrous methanol in a 6 ml sealed scintillation vial, and heated at 70°C for 3 h. The resultant fatty acid methyl esters were extracted into n-heptane for GC analysis(Reference Liu and Gibson31). The FA content was expressed as total lipid FA as percentage.
Biochemical analysis of plasma glucose and lipids
Plasma glucose and lipid profile were assessed using an automated chemistry analyser (Integra 400 plus, Roche Diagnostics) in EDTA anticoagulated plasma. All biochemistry assays were performed using Roche certified assay kits, which were calibrated using Calibrator for Automated Systems reagent. Quality control material (PreciControl ClinChem Multi 1 and 2; Roche Diagnostics) was run prior to sample analysis to ensure accuracy of results. All analyses were performed in duplicate. Leptin concentration in offspring plasma was estimated by ELISA following manufacture’s guidelines (Mouse/Rat Leptin Quantikine ELISA Kit, R&D Systems). The intra-assay CV was < 3·0 %.
Langendorff heart perfusion
Hearts were isolated from male and female rat and perfused in a manner similar to that recently detailed for adult mouse hearts(Reference Kaakinen, Reichelt and Ma32). Briefly, following removal of hearts via thoracotomy and placement in ice-cold perfusion fluid, the aorta was cannulated for perfusion in a Langendorff mode at an aortic pressure of 80 mmHg. A modified Krebs–Henseleit buffer was used, and contained (in mM): NaCl, 119; NaHCO3, 22; KCl, 4·7, MgCl2, 1·2; KH2PO4, 1·2; EDTA, 0·5; CaCl2, 1·85; D-(+)-glucose, 11; and Na+- pyruvate, 2 (all from Sigma Aldrich). Buffer was equilibrated with 95 % O2/5 % CO2 to maintain pH at 7·4 at 37°C. A fluid-filled plastic balloon inserted in the left ventricle (LV) and connected to a pressure transducer was used to monitor contractile function, while an in-line Doppler flow probe measured coronary flow (Transonic Systems Inc.,). Data were recorded using a 4-channel MacLab system (ADInstruments Pty Ltd.). Following initial preparation, the hearts were perfused within a 10-ml water jacketed chamber (37°C), with perfusate and chamber temperatures measured via needle thermistors to ensure a constant temperature of 37°C was maintained.
Coronary functional assessment
After 20 min stabilisation to assess baseline function, the LV balloon was deflated to limit contractile workload, thus O2 demand. After 10 min, hearts were subjected to 10 or 20 s occlusions (5 min recovery periods between), with coronary flow changes monitored throughout. Peak hyperaemic flows were measured, and percentage flow repayment over 3 min reperfusion (total reperfusion flow − pre-occlusion flow/flow debt × 100 %) determined.
Left ventricle pressure–volume relationships
Following coronary analysis, LV end-diastolic and systolic pressure–volume relationship (PVR) were assessed via stepped increases in LV balloon volume(Reference Kaakinen, Reichelt and Ma32). The ventricular balloon volume was initially adjusted to yield a zero systolic pressure over a 10 min stabilisation period. Balloon volume was then increased in 2·65 μl increments using a 500 μl calibrated syringe (Hamilton Co), with function assessed after 2 min at each volume. Experiments were terminated when end-diastolic pressure exceeded 30 mmHg. Hearts were then removed from the perfusion apparatus, blotted and weighed and the LV dissected and frozen in liquid N2 before storage at –80°C until later analysis. The PVR data were analysed to extract information on both LV volumes generating specific systolic and diastolic pressures, and slopes of PVR (rate of rise in systolic or diastolic pressure per unit volume change). The linear slope of the systolic PVR (between 0 and 25 µl/g LV volumes) provides a measure of LV inotropic state (Δ pressure/Δ volume). The curvilinear diastolic PVR were analysed to extract distinct early and late slopes: the initial slope at low LV volumes primarily reflects compliance (conversely stiffness) of intracellular elements (predominantly myofibrillar apparatus), with the extracellular matrix having negligible influence on passive tension over normal loads or stretch(Reference Linke, Popov and Pollack33). The final slope at abnormally high stretch or load reflects additional contributions from extracellular elements such as the extracellular matrix.
Quantitative PCR
Total RNA was extracted from cardiac tissues using the RNeasy® mini kit (Qiagen) following the manufacturer’s guidelines. Quantification and evaluation of RNA purity were carried out on a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). Reverse transcription of RNA to synthesise complementary DNA was performed using the iScript gDNA clear cDNA synthesis kit (Biorad) following manufacturer’s guidelines. Quantitative PCR was performed using QuantiNova SYBR® green master mix (Qiagen) following manufacturer’s guidelines, in line with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines(Reference Bustin, Benes and Garson34). PCR initial heat activation was run for 2 min at 95°C, then qPCR reactions were run for 40 cycles of 95°C for 5 s (denaturation) and 60°C for 10 s (combined annealing/extension) using StepOneTM real-time PCR systems (Applied Biosystems). Gene expression was quantified using the 2−ΔΔCq method normalised to the geometric mean of β-actin and β-2 microglobulin as reference genes. Each sample was tested in duplicate for the average of Cq value. The reference genes were stable across the treatment groups. All the primers used for the present study were KiCqStartTM predesigned primers from Sigma-Aldrich: leptin receptor (Lepr; NM_012596), Myh6 (XM_006251951.2), Myh7 (XM_017599642.1), Atp2a1 (NM_058213.1), Nppa (NM_012612.2), Nppb (NM_031545.1), Notch1 (NM_001105721.1), Col1a1 (NM_053304.1) and Col3a1 (NM_032085.1).
Statistical analysis
All data were analysed using GraphPad Prism 8.3.1 (GraphPad software). One male and one female offspring from each litter were analysed. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Only one male and one female were used from each litter for each experiment to remove potential litter effects, as recommended in guidelines on experimental design in developmental origin of health and disease studies(Reference Dickinson, Moss and Gatford35). In essence, each rat in the same litter is a replicate, and thus only one male and one female are analysed for each parameter.
Data were analysed to determine if they were normally distributed. Data were analysed separately for males and female with each sex analysed by two-way ANOVA, with maternal and postnatal diets as the factors. Specific comparisons were made using Tukey’s post hoc multiple comparison test. Data are presented as mean values with their standard error of the mean. P-values < 0·05 were considered evidence of significant differences.
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
Results
Effect of maternal and postnatal diets high in linoleic acid on offspring body weight and organ weights
In both sexes, there were different interaction effects of maternal and postnatal diets (Table 1, n 6–8). In female offspring, a maternal LLA diet followed by a postnatal LLA diet, or a maternal HLA diet followed by a postnatal HLA diet, led to increased body weight (effect of interaction P = 0·02). In offspring of both sexes, neither a maternal nor postnatal diet HLA altered weight of brain, left kidney and right kidney (Table 1, n 6–8).
Table 1. Effect of maternal and postnatal diet high in LA on body weight and organ weight of offspring*
(Mean values with their standard errors of the mean)
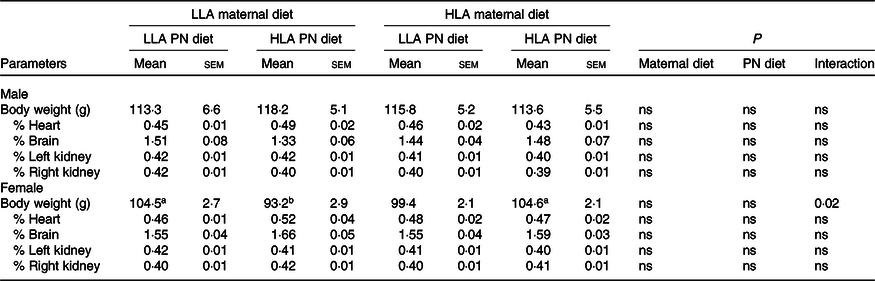
LLA, low linoleic acid; HLA, high linoleic acid; PN, postnatal.
* Two-way ANOVA was performed for statistical analysis with maternal diet and postnatal diet as two factors. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. n 6–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Differences across the groups are denoted by different superscript letters, so that a is different than b.
Effect of maternal and postnatal diets high in linoleic acid on circulating glucose, lipids and leptin
The concentrations of glucose, TAG, total cholesterol (TC), HDL-cholesterol and TC/HDL-cholesterol were unchanged in the plasma of male offspring (Table 2, n 5–8). However, maternal HLA diet decreased TC (effect of maternal diet P = 0·01) and HDL-cholesterol (effect of maternal diet P = 0·03) concentrations in female offspring (Table 2, n 5–8). Circulating leptin in offspring was elevated by a maternal diet high in LA but not by postnatal diet in both sexes. Leptin concentrations in female offspring from LLA dams fed a HLA postnatal diet were reduced, and the maternal diet affected the influence of the postnatal diet in female offspring, indicative of an interaction effect (effect of maternal diet P = 0·001, effect of interaction P = 0·01) (Table 2, n 5–8).
Table 2. Effect of maternal and postnatal diet high in LA on plasma glucose level, lipids and leptin level in offspring*
(Mean values with their standard errors of the mean)
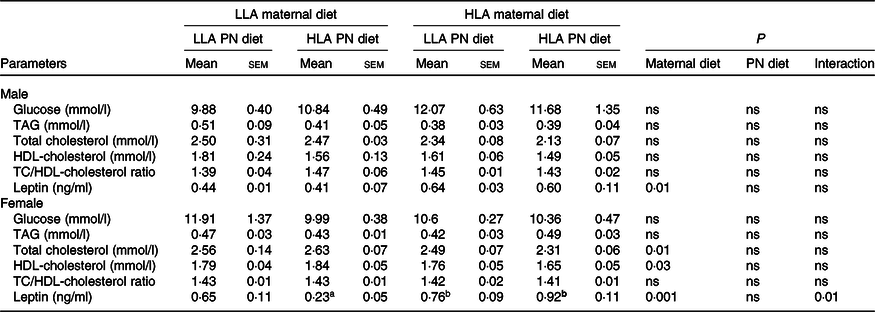
LLA, low linoleic acid; HLA, high linoleic acid; PN, postnatal; TC, total cholesterol.
* Two-way ANOVA was performed for statistical analysis with maternal diet and postnatal diet as two factors. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. n 5–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Differences across the groups are denoted by different superscript letters, so that a is different than b.
Effect of maternal and postnatal diets high in linoleic acid on offspring plasma fatty acid composition
There was no difference in total plasma SFA across dietary groups in both male and female offspring (Tables 3 and 4, n 5–8). Total plasma trans-FA was unchanged among the groups in male offspring (Table 3); however, maternal HLA diet increased trans-FA in female offspring (Table 4, effect of interaction P = 0·04). Both maternal and postnatal HLA diet significantly decreased total plasma monounsaturated fatty acid in both male (Table 3, effect of maternal diet P = 0·003, effect of postnatal diet P = 0·0005, effect of interaction P = 0·0002) and female (Table 4, effect of maternal diet P < 0·0001, effect of postnatal diet P < 0·0001, effect of interaction P < 0·0001) offspring. In male offspring, only postnatal HLA diet decreased total plasma n-3 PUFA (Table 3, effect of postnatal diet P = 0·0007); however, in female offspring, both maternal and postnatal HLA diet decreased total plasma n-3 PUFA (Table 4, effect of maternal diet P = 0·001, effect of postnatal diet P < 0·0001). Interestingly, plasma ALA and EPA were significantly decreased by postnatal HLA diet but not by maternal diet in either sex. In male offspring, plasma docosapentaenoic acid (DPA) was significantly increased by a maternal HLA diet (Table 3, effect of maternal diet P = 0·02); however, in females, the level of plasma DPA was significantly decreased by a postnatal HLA diet (Table 4, effect of postnatal diet P = 0·002). As expected, both maternal and postnatal diets high in LA increased the concentration of total plasma n-6 and LA in both sexes. Interestingly, offspring from HLA dams fed with LLA had a higher concentration of total plasma n-6 FA, suggesting that a LLA diet postnatally does not reverse the effect of high maternal LA on plasma n-6 concentration. Plasma AA, a derivative of LA, was unchanged across the dietary groups; however, the ratio of AA/DHA was significantly increased in the plasma by both maternal and postnatal HLA diets in both sexes. Further, maternal and postnatal diets high in LA increased the ratio of LA/ALA in the plasma of both male and female offspring. Maternal and postnatal diets high in LA decreased the plasma DHA/DPA ratio in male but not female offspring.
Table 3. Effect on maternal and postnatal diet high in LA on plasma fatty acid composition in male offspring*
(Mean values with their standard errors of the mean)
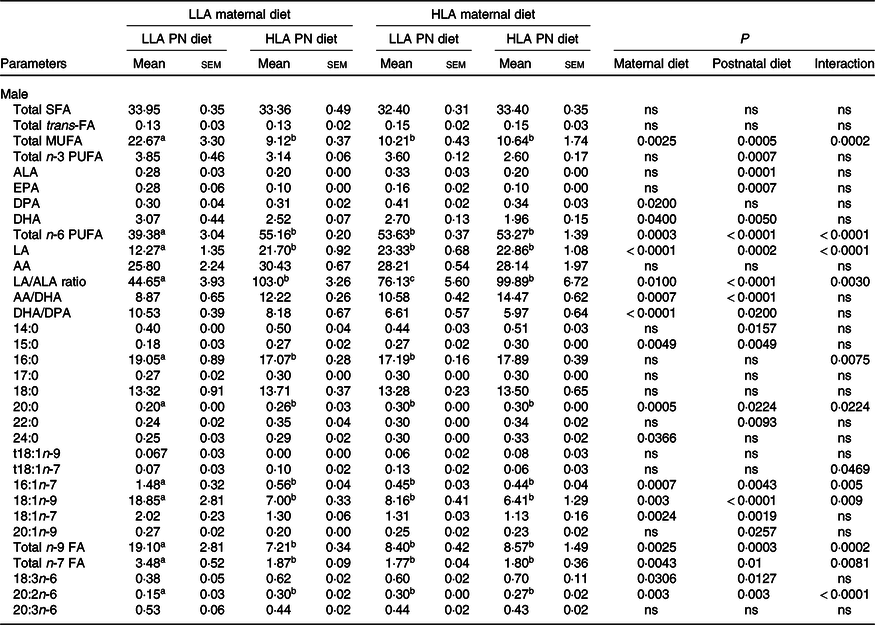
LLA, low linoleic acid; HLA, high linoleic acid; PN, postnatal; FA, fatty acid; MUFA, monounsaturated fatty acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid.
* Two-way ANOVA was performed for statistical analysis with maternal diet and postnatal diet as two factors. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. n 5–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Data are expressed as total lipid fatty acids as percentage. Differences across the groups are denoted by different superscript letters.
Table 4. Effect on maternal and postnatal diet high in LA on plasma fatty acid composition in female offspring*
(Mean values with their standard errors of the mean)
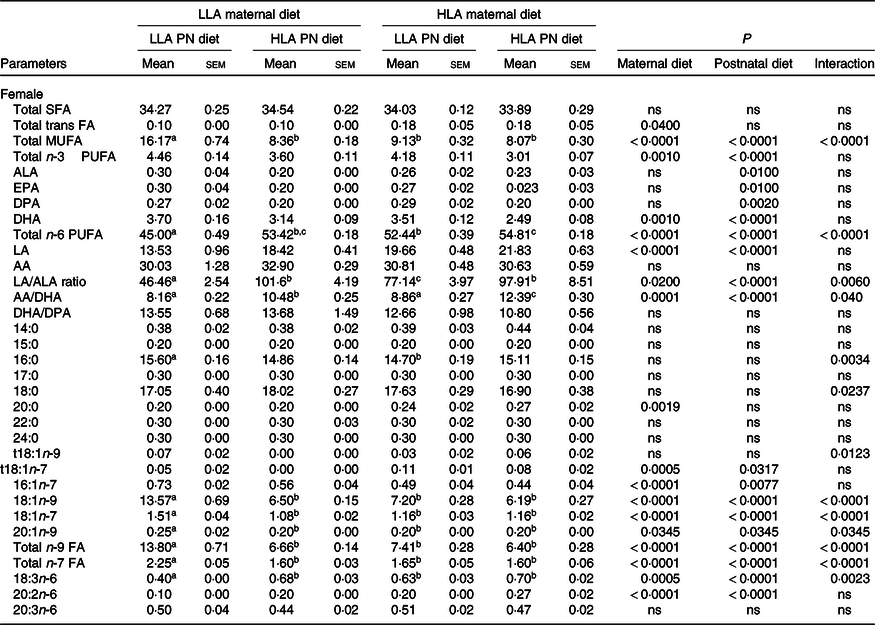
LLA, low linoleic acid; HLA, high linoleic acid; PN, postnatal; FA, fatty acid; MUFA, monounsaturated fatty acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid.
* Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. n 5–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Data are expressed as total lipid fatty acids as percentage. Differences across the groups are denoted by different superscript letters.
Further, in males, 24:0 SFA was significantly increased by the maternal HLA diet (Table 3, effect of maternal diet P = 0·0366). 14:0 and 22:0 SFA were significantly increased by postnatal HLA diets (Table 3, effect of postnatal diet P = 0·00157 and 0·0093, respectively). 15:0 SFA was significantly increased by both the maternal and postnatal HLA diets (Table 3, effect of maternal and postnatal diets P = 0·0049 and 0·0049, respectively). 16:0 SFA had an interaction effect for the HLA diet (Table 3, P = 0·0075). 20:0 SFA was significantly altered by HLA diet (Table 3, effect of maternal diet P = 0·0005, effect of postnatal diet P = 0·0224, effect of interaction P = 0·0224). For monounsaturated fatty acid, 20:1n-9 was significantly reduced by postnatal HLA diet (Table 3, effect of postnatal diet P = 0·0257), 18:1n-7 was affected by both maternal and postnatal HLA (Table 3, effect of maternal diet P = 0·0024, effect of postnatal diet P = 0·0019) and 16:1n-7 and 18:1n-9 were affected by maternal and postnatal HLA diet, with an interaction effect. Trans-fat t18:1n-7 had an interaction effect for the HLA diets (Table 3, effect of interaction P = 0·0469). Other n-6 PUFA were also altered, with 18:3n-6 affected by both maternal and postnatal HLA diet (Table 3, effect of maternal and postnatal diets P = 0·0306 and 0·0127, respectively). 20:2n-6 was affected by maternal and postnatal diets and an interaction effect. Finally, total n-9 and n-7 FA were affected by maternal and postnatal diets and an interaction effect.
In females, 20:0 SFA was significantly increased by maternal HLA (Table 4, effect of maternal diet P = 0·019), while there was an interaction effect for 16:0 SFA and 18:0 SFA (Table 4, effect of interaction P = 0·0034 and 0·0237, respectively). For monounsaturated fatty acid, 16:1n-7, both maternal and postnatal HLA diet had an effect (Table 4, effect of maternal diet P < 0·0001 and effect of postnatal diet P = 0·0077). 18:1n-7, 18:1n-9 and 20:1n-9 were affected by maternal, postnatal as well as an interaction effect. Trans-fat t18:1n-9 had an interaction effect for the HLA diets (Table 4, effect of interaction P = 0·0123). t18:1n-7 had an effect from both maternal and postnatal HLA diets (Table 4, effect of maternal P = 0·0005, effect of postnatal P = 0·0317). Other n-6 PUFA were also altered, 18:3n-6 had a maternal effect, postnatal effect and an interaction (Table 4). 20:2n-6 was also affected by the maternal and postnatal HLA diets (Table 4, effect on maternal and postnatal P < 0·0001, respectively). Finally, total n-9 and n-7 FA were affected by maternal and postnatal diets and an interaction effect.
Effect of maternal and postnatal diets high in linoleic acid on cardiovascular function of adolescent offspring
Baseline function
Baseline contractile function in ex vivo hearts did not differ, regardless of maternal and postnatal diets (Fig. 1, n 5–8). However, interaction between maternal and postnatal diets (combination of the two factors) in female rats significantly affected the baseline coronary flow rate (Fig. 1(d), n 5–8).
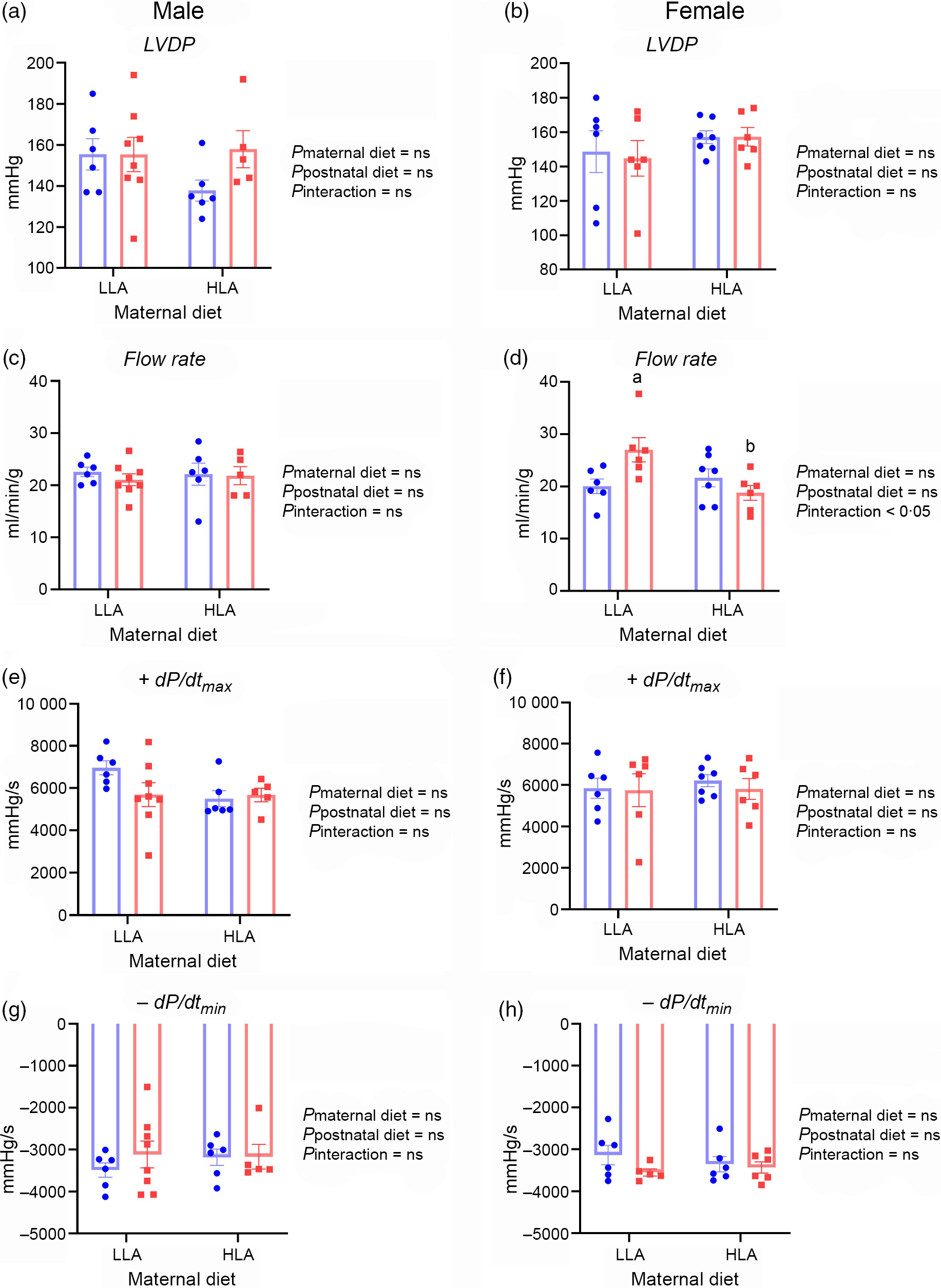
Fig. 1. Effect of maternal and postnatal diets high in linoleic acid on baseline functional parameters for Langendorff perfused hearts. Data were analysed with two-way ANOVA with maternal and postnatal diets as two factors. Data are presented as mean values with their standard error of the mean. n 5–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. LLA, low linoleic acid; HLA, high linoleic acid; LVDP, left ventricular diastolic pressure. (a) ![]() , LLA-postnatal;
, LLA-postnatal; ![]() , HLA-postnatal. (b)
, HLA-postnatal. (b) ![]() , HLA-postnatal. (c)–(h)
, HLA-postnatal. (c)–(h) ![]() , LLA-postnatal;
, LLA-postnatal; ![]() , HLA-postnatal.
, HLA-postnatal.
Systolic and diastolic pressure–volume relationship
The PVR data were interrogated to assess slopes of relationships, and LV volumes generating specific diastolic and systolic pressures. Systolic curves did not differ noticeably with maternal or postnatal diets in either sex. This was confirmed by lack of change in LV volumes yielding 100 or 120 mmHg systolic pressures (Table 5, n 5–8). However, a maternal HLA diet resulted in a fall in the initial slope of the systolic PVR in male offspring (Fig. 2(a), n 5–8, effect of maternal diet P = 0·02). No changes in LV volumes yielding 10 or 20 mmHg diastolic pressures (or diastolic pressure at a LV volume of 250 µl/g) were evident in male offspring (Table 5, n 5–8). However, the initial slope of the diastolic PVR in male hearts was moderately reduced by a maternal HLA diet in male hearts (Fig. 2(c), n 5–8, effect of maternal diet P = 0·005). In contrast to males, a maternal followed by postnatal HLA diet substantially shifted the diastolic PVR to the left in female offspring (Table 5, n 5–8). This resulted in significant falls in LV volumes yielding 10 or 20 mmHg diastolic pressure, and a rise in diastolic pressure at a LV volume of 250 µl/g in females (Table 5, n 5–8, effect of maternal diet P = 0·03, effect of postnatal diet P = 0·03). An interaction was also evident between maternal and postnatal diets in terms of diastolic parameters in female rats (Table 5, n 5–8, effect of interaction P = 0·02).
Table 5. Effect of maternal and postnatal high LA diet on offspring left ventricular volume at systolic pressure and end-diastolic pressure*
(Mean values with their standard errors of the mean)
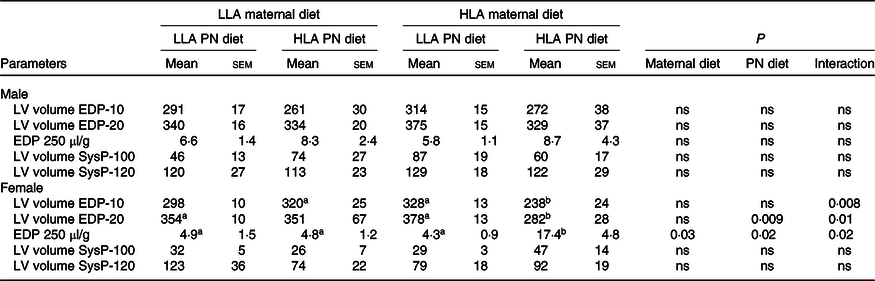
LV, left ventricular; EDP, end-diastolic pressure.
* Two-way ANOVA was performed for statistical analysis with maternal diet and postnatal diet as two factors. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. n 5–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Differences across the groups are denoted by different superscript letters.
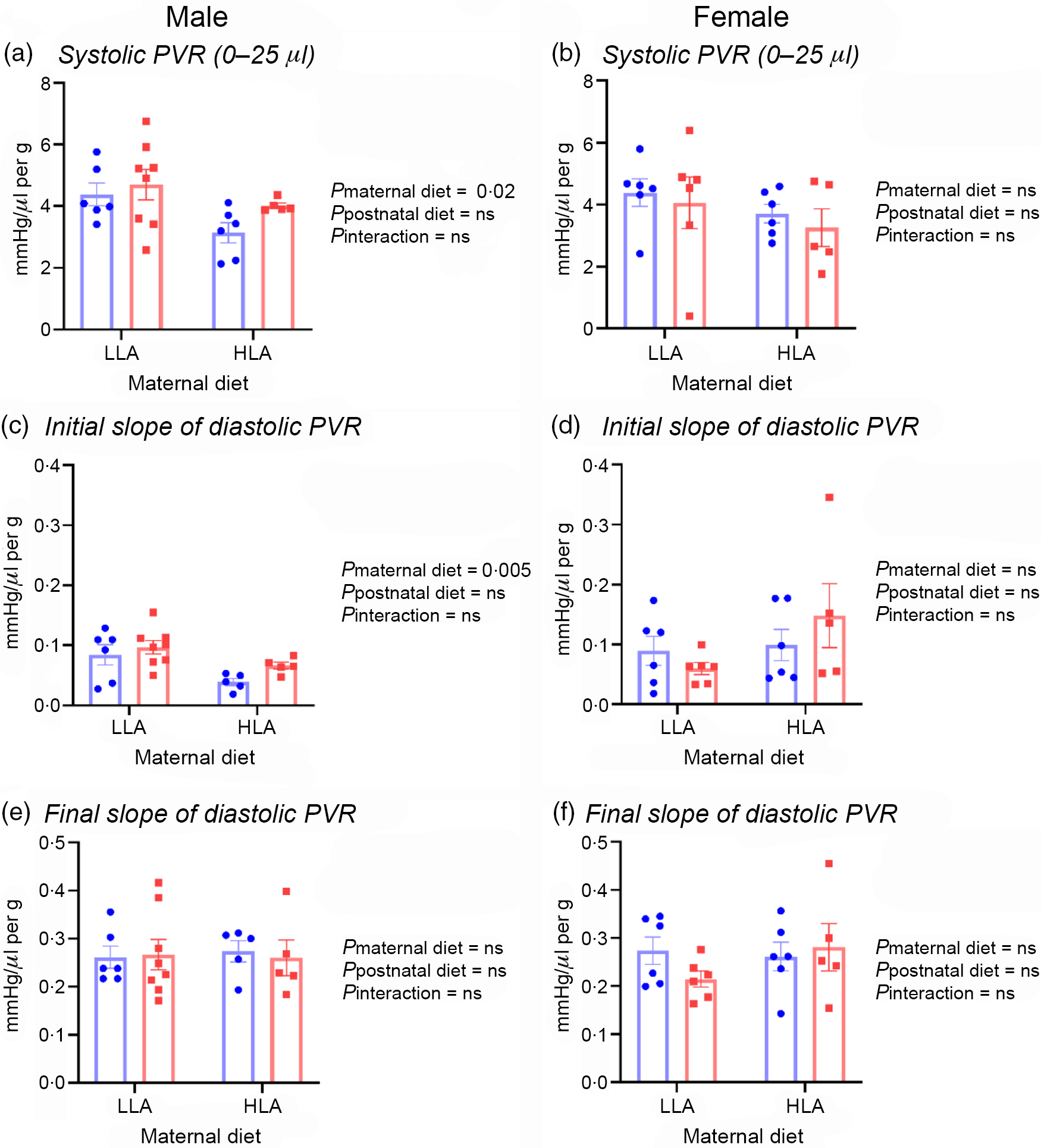
Fig. 2. Effect of maternal and postnatal diets high in linoleic acid on offspring systolic PVR and slopes of diastolic PVR. (a) Systolic PVR in male offspring. (b) Linear slope of the systolic PVR in female offspring. (c) Initial slope of the diastolic PVR in male offspring. (d) Initial slope of the diastolic PVR in female offspring. (e)–(f) Final slopes of the diastolic PVR in male and female offspring. Data were analysed with two-way ANOVA with maternal and postnatal diets as two factors. Data are presented as mean values with their standard error of the mean. n 5–8. The sample size (n) represents the number of litters, and one male and one female were used from each litter. LLA, low linoleic acid; HLA, high linoleic acid; PVR, pressure–volume relationship. (a)–(f) ![]() , LLA-postnatal;
, LLA-postnatal; ![]() , HLA-postnatal.
, HLA-postnatal.
Coronary vascular reactivity
Peak coronary flows after 10 or 20 s occlusions were comparable in male rat hearts, regardless of maternal or postnatal diet (Fig. 3, n 5–7). Debt repayment was substantially less after 20 v. 10 s occlusion in both male (Fig. 3(c) and (g), n 5–7) and female rats (Fig. 3(d) and (h), n 5–7). There appeared to be no diet effects on flow repayment in males (Fig. 3(c) and (g), n 5–7). Maternal HLA diet increased debt repayment after 10 s in female rats with interactive effect of maternal and postnatal diets (Fig. 3(d), n 5–7, effect of maternal diet P = 0·002, effect of interaction P = 0·03). Postnatal HLA diet decreased debt repayment after 20 s in female offspring from dams fed with LLA diet demonstrating the interactive effect of maternal and postnatal diets (Fig. 3(h), n 5–7, effect of postnatal diet P = 0·02, effect of interaction P = 0·03).
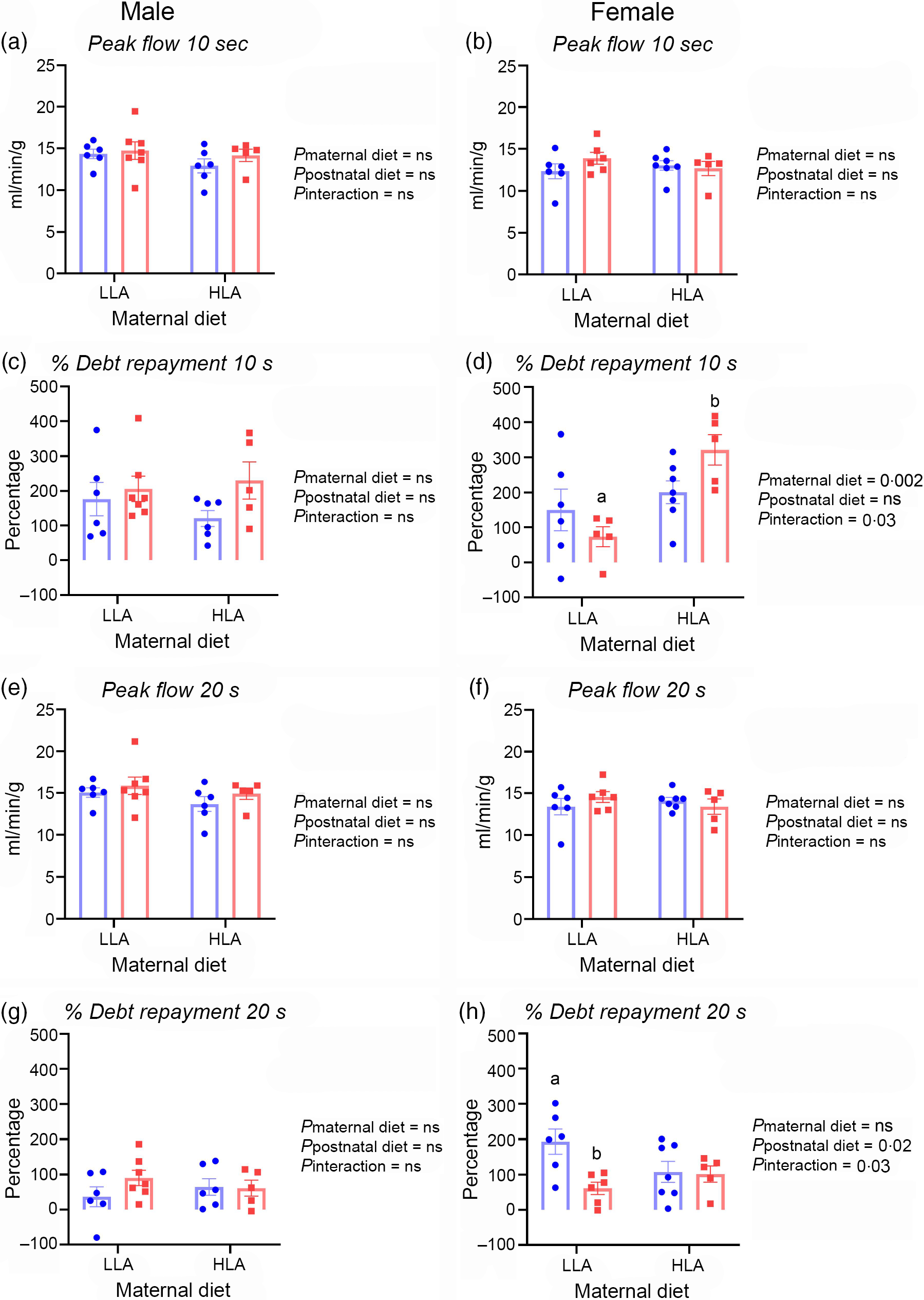
Fig. 3. Effect of maternal and postnatal diets high in linoleic acid on offspring coronary reactivity after 10 and 20 s occlusions. (a)–(b) Peak coronary flow after 10 s occlusions in male and female hearts. (c) Flow repayment after 10 s occlusions in male offspring. (d) Debt repayment after 10 s in female rats. (e)–(f) Peak coronary flows after 20 s occlusions in male and female rat hearts. (g)–(h) Debt repayment after 20 s occlusions in male and female hearts. Data were analysed with two-way ANOVA with maternal and postnatal diet as two factors. Data are presented as mean values with their standard error of the mean. n 5–7. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. LLA, low linoleic acid; HLA, high linoleic acid. (a)–(h) ![]() , LLA-postnatal;
, LLA-postnatal; ![]() , HLA-postnatal
, HLA-postnatal
Effect of maternal and postnatal diets high in linoleic acid on cardiac gene expression in adolescent offspring
We measured the expression of various genes involved in cardiac hypertrophy and cardiac fibrosis to investigate potential molecular responses and remodelling in response to maternal or postnatal diets high in LA. Cardiac expression of myosin heavy chain 6 (Myh6) and myosin heavy chain 7 (Myh7) genes was not different between any dietary group (Table 6, n 4–7). Male offspring born to HLA mothers fed with LLA postnatal diet showed elevated expression of natriuretic peptide type A (Nppa) compared with male offspring born to LLA mothers (Table 6, n 4–7, effect of postnatal diet P = 0·04, effect of interaction P = 0·04). However, the gene expression of Nppa was unchanged across dietary groups in female offspring (Table 6, n 4–7). There were no changes in expression of natriuretic peptide type B transcript (Nppb) in male offspring (Table 6, n 4–7); however, a high LA diet postnatally significantly up-regulated the expression of Nppb in female offspring from dams fed with LLA diet and down-regulated its expression in offspring from dams fed with a HLA diet demonstrating an interaction between maternal and postnatal diets (Table 6, n 4–7, effect of maternal diet P = 0·04, effect of postnatal diet P = 0·04, effect of interaction P = 0·007). Cardiac fibrosis is a hallmark of cardiac hypertrophy; therefore, we further investigated the relative expression of genes related to fibrosis. Maternal diet high in LA significantly up-regulated expression of collagen type I α 1 (Col1a1; effect of maternal diet P = 0·04) and collagen type III α 1 (Col3a1; effect of maternal diet P = 0·03) transcripts in male offspring (Table 6, n 4–7); however, the expression of these fibrosis-related genes remained unchanged in female offspring (Table 6, n 4–7). Further, we assessed gene expression of ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 1 (Atp2a1) which is important in Ca signalling in the heart. The expression of Atp2a1 was significantly increased in male offspring of mothers fed a HLA diet (effect of maternal diet P = 0·03); however, expression was unchanged in female offspring (Table 6, n 4–7). The Notch pathway is also involved in cardiac hypertrophy and regulates fibrotic and regenerative repair in the heart; we therefore measured the expression of the Notch1 gene. Interestingly, a high LA maternal diet up-regulated expression of Notch1, while a high LA postnatal diet down-regulated its expression in male offspring (Table 6, n 4–7, effect of maternal diet P = 0·01, effect of postnatal diet P = 0·03). In female rats, maternal and postnatal diets have an interactive effect in the expression of Notch1 (Table 6, n 4–7, effect of interaction P = 0·04).
Table 6. Effect on maternal and postnatal diet high in LA on the expression of genes involved in leptin signalling, cardiac hypertrophy, fibrosis, Ca signalling and Notch pathway in offspring*
(Mean values with their standard errors of the mean)
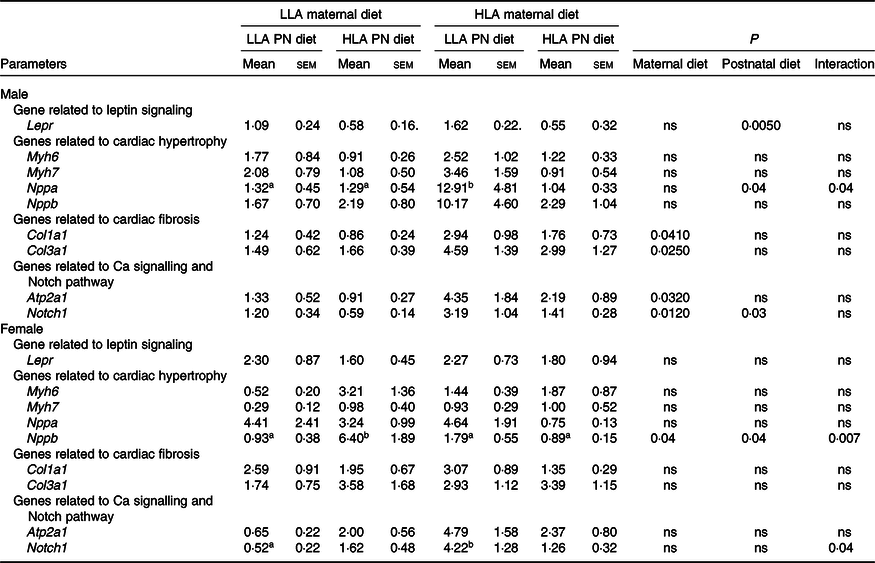
LLA, low linoleic acid; HLA, high linoleic acid; MYH, myosin heavy chain 6; NPPA, natriuretic peptide type A; NPPB, natriuretic peptide type B; COL1A1, collagen type I α 1; COL3A1, collagen type III α 1; ATP2A1, ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 1.
* Data were analysed with two-way ANOVA with maternal and postnatal diet as two factors. Mean values with different superscript letters are significantly different with Tukey’s post hoc multiple comparison test. n 4–7. The sample size (n) represents the number of litters, and one male and one female were used from each litter. Differences across the groups are denoted by different superscript letters.
Discussion
Global dietary recommendations regarding LA, and its cardiovascular effects, remain debated(Reference Marklund, Wu and Imamura36), while consumption is increasing worldwide(Reference Naughton, Mathai and Hryciw17). Further, in utero development is important in determining CVD risk factors(Reference Roseboom, van der Meulen and Osmond7), and there is paucity of data regarding effects of a maternal diet high in LA on offspring cardiac development or of the impacts of a subsequent change in postnatal diet. Recent studies have shown that maternal and postnatal diets supplemented with conjugated LA affect cardiac lipid profile and serum lipoxygenase metabolite of PUFA in rat offspring with mammary tumours(Reference Białek, Jelińska and Tokarz37,Reference Białek, Białek and Czauderna38) . Our study demonstrated that a maternal diet high in LA programmed cardiovascular sex-specific deficits that were not reversed by a low LA diet in the male. For females, the post-weaning low LA diet able to restore the functional changes, in the absence of changes to the gross heart weight.
To our knowledge, this is the first study to elucidate the roles of both maternal and postnatal LA intake on offspring cardiovascular health.
Plasma lipid profile and leptin changes in female offspring
A key finding of the present study is that a maternal diet high in LA significantly decreased both TC and HDL-cholesterol in the plasma of female but not male offspring, regardless of LA amounts in the postnatal diet. In our previous study, we reported that a maternal diet high in LA before pregnancy and during gestation decreased maternal plasma TC, HDL-cholesterol and LDL-cholesterol(Reference Shrestha, Cuffe and Holland21). A systemic review of randomised control trials demonstrated that increasing n-6 FA reduces serum TC, with little or no difference in HDL or LDL-cholesterol(Reference Hooper, Al-Khudairy and Abdelhamid39). One of the proposed mechanisms behind the protective effect of LA in CVD is its TC and LDL-cholesterol lowering properties(Reference Harris40); however, in the present study, a maternal HLA diet also decreased HDL-cholesterol in female offspring, which may limit cardiovascular benefit.
Maternal HLA diet increased leptin concentrations in the plasma of both male and female offspring in the present study and had an interaction effect in females. These changes occurred in the presence of body weight changes in the female, but an absence in the male. We have previously demonstrated that maternal leptin is reduced in response to an elevated LA diet in the absence of changes to body weight(Reference Shrestha, Cuffe and Holland21). Circulating leptin concentrations have a contradictory role in cardiovascular health, showing both protective and deleterious effect(Reference Genoux and Bastard41). Further, as changes in Lepr expression were associated with functional changes in males only, this suggests that altered cardiac Lepr expression does not mediate the changes in cardiac function in this model.
Diet affects offspring plasma fatty acid composition in a sex-specific manner
Maternal and postnatal diets high in LA have significant impacts on offspring plasma FA composition. Despite matching total n-3 PUFA across the diets(Reference Shrestha, Cuffe and Holland21) , a significant effect of diet on total n-3 PUFA, ALA and other longer chain n-3 PUFA in offspring plasma was evident. ALA and LA share enzymes for metabolism, and high LA will compete with ALA for its conversion, potentially reducing DHA and EPA production from ALA(Reference Choque, Catheline and Rioux18). This may have important effects on physiology, as long-chain n-3 PUFA such as EPA and DHA have anti-inflammatory characteristics and are beneficial for cardiovascular health(Reference Swanson, Block and Mousa42).
We observed effects of maternal and offspring diet on offspring plasma FA composition, with some changes sex specific. Male offspring weaned on a HLA diet had lower total n-3 FA irrespective of maternal diet; however, total n-3 FA in female offspring was decreased with both maternal and postnatal diets high in LA. Whereas in both male and female offspring, a postnatal diet high in LA decreased ALA and EPA, while DHA was reduced in both sexes with maternal and postnatal diets high in LA. High concentrations of ALA, EPA and DHA in serum lipids are associated with lower risk of death from CHD in men and women(Reference Erkkilä, Lehto and Pyörälä43). The decreased total n-3 FA along with ALA, EPA and DHA in the present study suggest an increased CVD risk profile in offspring exposed to HLA either pre- or postnatally. Both maternal and postnatal diets high in LA considerably up-regulated total n-6 PUFA and LA in both male and female offspring, as expected. It is important to note that offspring from HLA dams have increased total n-6 PUFA and LA even if weaned onto a LLA diet, suggesting a dominant impact of maternal over postnatal LA intake. A consideration in interpreting these data is that the data were derived from postnatal exposure which was short, and a longer exposure may induce more severe effects that could interact with ageing to exacerbate disease. Notwithstanding this, ∼2 week exposure to a HLA diet did alter some aspects of cardiometabolic function.
Concentrations of AA remained unchanged across the dietary groups studied. However, the AA/DHA ratio increased in male and female offspring in response to both maternal and postnatal HLA diets. The relationship between the AA/DHA ratio and coronary events is controversial(Reference Nishizaki, Shimada and Tani44,Reference Ninomiya, Nagata and Hata45) . Some studies show a positive correlation between AA:DHA and coronary events, while others find no such association(Reference Ninomiya, Nagata and Hata45,Reference Shoji, Kakiya and Hayashi46) . Recent analysis suggests that a higher serum AA/EPA is associated with a greater risk of CVD in the general Japanese population; however, no such association is observed for the AA:DHA ratio(Reference Ninomiya, Nagata and Hata45). Interestingly, the DHA/DPA ratio is specifically decreased by maternal and postnatal diets high in LA in male offspring only, suggesting that both postnatal and maternal LA intake can reduce conversion of DPA to DHA (in a sexually dimorphic manner).
In addition, there the HLA diet altered n-7 and n-9 FA. There have been a few studies that have suggested that n-7 FA are protective for CVD, and that they reduce the effects of CVD risk factors(Reference Yang, Pryor and Noguchi47). It is believed that this occurs via the restoration of normal metabolic function. Further, n-9 PUFA negatively impacts cardiovascular function, due to its ability to promote inflammation and endothelial dysfunction(Reference Delgado, Krämer and Lorkowski48). Both n-7 and n-9 studies have been limited in their scope, with most of the research focused on n-3 and n-6 imbalances and their effects on cardiovascular health. Further studies thus need to be undertaken to explore the biological and prognostic properties of both n-7 and n-9 and how they relate to CVD.
Sex-specific cardiovascular changes
We assessed intrinsic cardiac and coronary functionality in isolated perfused hearts. There was no difference in baseline contractile function among groups. However, a combination of HLA diets in dams and offspring resulted in a shift in the diastolic PVR to lower LV volumes in female offspring, increasing diastolic pressures at LV volumes above ∼200 µl/g. This pattern suggests the change is extracellular in nature potentially due to extracellular rather than myofibrillar elements. Interestingly, in male offspring a maternal HLA diet decreased the initial slopes of systolic and diastolic PVR, indicating moderately reduced inotropic state coupled with a shift in diastolic compliance largely independent of postnatal diet. This dominant effect of maternal diet on cardiac function is supported by a recent study in mice, which showed that maternal diet-induced obesity programmes cardiac dysfunction in male offspring independently of post-weaning diet(Reference Loche, Blackmore and Carpenter49). Coronary reactivity changes also support a dimorphic impact of a HLA maternal diet, in female offspring.
Cardiac gene expression changes and potential relevance to function
Maternal and postnatal diets have interactive effect in the heart weight in male offspring. Nppa and Nppb are biomarkers of cardiac hypertrophy, though they may not be mechanistically involved in cardiac remodelling(Reference Calamaras, Baumgartner and Aronovitz50). A maternal HLA diet increased Nppa expression in male offspring fed a LLA diet. Conversely, expression of Nppb was up-regulated by a HLA postnatal diet in female offspring of dams fed a LLA diet v. down-regulated in female offspring of dams fed a HLA diet. Thus, the sexual dimorphic responses to high LA may modulate heart function in males and females via seperate cellular pathways.
Cardiac fibrosis distorts the architecture of the myocardium and leads to cardiac dysfunction(Reference Li, Zhao and Kong51). Col1a1 and Col3a1 expressions are increased in male offspring of dams fed a high LA diet. Collagen is the primary extracellular protein in the heart(Reference Xu, Williams and O’Brien52), and these increases in collagen I and III transcripts suggest a maternal HLA diet may selectively promote fibrosis in male offspring. A maternal diet high in LA up-regulated cardiac expression of Atp2a1/Serca1 in male offspring independently of postnatal diet, which may be associated with later cardiac dysfunction as the transition from hypertrophy to heart failure is often associated with dysregulation of sarcoplasmic reticulum Ca ATPase(Reference Wilkins and Molkentin53). Increased Atp2a1/Serca1 increases rates of contraction and relaxation; thus, it is not predicted to contribute to the shift in the systolic PVR slope in males. However, this may reflect an adaptation, as supported by studies of heart failure(Reference Summerfield, Peters and Hercock54).
Additionally, a maternal diet high in LA increased expression of Notch1 in male offspring. Maternal and postnatal diets have interactive effect on Notch1 expression in female offspring. Notch1 prevents the uncontrolled hypertrophic growth of cardiomyocytes during cardiac adaptation by inhibiting the transcription of GATA4 (Reference Ninh, El Hajj and Mouton55). However, this potentially adaptive response was negated by a postnatal diet high in LA. Notch signalling is up-regulated following infarction, potentially contributing to remodelling responses, and is also implicated in cardioprotective responses(Reference Gude and Sussman56). The present study demonstrated an up-regulation of Notch1 in male offspring, and an interaction affect in females where the maternal diet altered the effects of the postnatal diet. This finding suggests that the maternal HLA diet may contribute to remodelling and stress resistance supports prior studies that suggest male offspring may be more sensitive to nutrient supply during pregnancy(Reference Kuzawa and Adair57,Reference O’Tierney-Ginn, Presley and Minium58) . However, it is possible that the transcriptional modifications observed here are yet to substantially influence myocardial architecture and function, which may require development beyond the 40-d timepoint assessed here. Thus, further investigations should be focused on the long-term health outcomes to offspring born to mothers consuming an elevated LA diet.
A potential limitation in the present study is a lack of a control group, which would be consuming standard rat chow. Standard rat chow has 1·3 % LA and 0·3 % ALA (https://www.specialtyfeeds.com/new/wp-content/uploads/2016/06/meat_free_rm.pdf.). The LLA diet used in the present study has 1·44 % LA and 0·36 % ALA. Fat concentration in both of our speciality diets is matched (9 % in LLA and HLA v. 4·8 % in standard chow). However, some researchers consider 11 % as the fat content in standard chow, for example(Reference Buettner, Parhofer and Woenckhaus59). Thus, there may be an effect due to the elevated fat (assuming 4·8 %). However, as the aim of the present study was to investigate the effects of a maternal diet with low (control) LA v. high LA concentrations, and the components of the diet were matched apart from the LA content, the control group consuming standard chow is unlikely to be different from the LLA group.
Conclusion
We have shown that both a maternal and a postnatal diet high in LA influence offspring plasma FA, lipid profiles and cardiac function in a sex-specific manner. These direct functional changes are associated with sex-specific changes in fibrogenic gene expression in male offspring in response to a HLA maternal diet. Further studies in adult offspring are warranted to explore how maternal and early postnatal intake of LA affect cardiovascular phenotype in adulthood.
Acknowledgements
The authors would like to thank members of the laboratory and our collaborators for helpful discussions.
This research was supported by the Allen Foundation, Inc. (D. H. H., A. J. M.), and through the Australian Government’s Collaborative Research Networks (CRN) program (A. J. M.). Scholarship funding is provided by Griffith University International Postgraduate Research Scholarship (GUIPRS-NS), Griffith University Postgraduate Research Scholarship (GUPRS-NS) and Griffith Health Top Up Scholarship (NS). This research was in part funded by the Allen Foundation (USA).
N. S., J. C., A. M., J. H. and D. H. designed the study; N. S., S. S., T. H., O. H. and J. H. collected the data; all authors analysed the data; N. S., S. S. and T. H. wrote the first draft of the manuscript and all authors revised the manuscript. All the authors read and approved the final manuscript.
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.