Introduction
Large-bodied animals are particularly vulnerable to increased hunting facilitated by expansion of access networks. The North American bison Bison bison bison was almost extirpated within a few decades after railways opened up the western USA (Isenberg, Reference Isenberg2000), and large forest animals in Amazonia and central Africa have been depleted as access for hunters has expanded along roads and rivers (Peres & Lake, Reference Peres and Lake2003; Laurance et al., Reference Laurance, Croes, Tchignoumba, Lahm, Alonso and Lee2006; Yackulic et al., Reference Yackulic, Strindberg, Maisels and Blake2011). Effects of increased hunting can be even more drastic when expansion of road networks is associated with disturbances such as large-scale hydrocarbon extraction, as documented for caribou Rangifer tarandus in Alaska (McLoughlin et al., Reference McLoughlin, Dzus, Wynes and Boutin2003) and large mammals in the Ecuadorian Amazon (Suárez et al., Reference Suárez, Morales, Cueva, Utreras Bucheli, Zapata-Ríos and Toral2009). Expansion of hydrocarbon extraction, which has escalated worldwide, brings about social and economic changes that can further affect biodiversity (Jobin, Reference Jobin2003; Berger & Beckmann, Reference Berger and Beckmann2010). One such change is the rapid increase of human populations in urban centres near oil fields, which puts additional pressure on nearby wildlife resources (Suárez et al., Reference Suárez, Morales, Cueva, Utreras Bucheli, Zapata-Ríos and Toral2009).
The guanaco Lama guanicoe is a South American camelid that has suffered a drastic population decline in the last 100 years. The dominant native ungulates of the 750,000 km2 Patagonian steppe of southern Argentina and Chile, guanacos once numbered 7–50 million (Raedeke, Reference Raedeke1979; Torres, Reference Torres1985). Only about half a million remain, with the few large, fragmented populations restricted to remote areas with low plant productivity (Franklin, Reference Franklin, Eisenberg and Kleiman1983; Torres, Reference Torres1985; Pedrana et al., Reference Pedrana, Bustamante, Travaini and Rodríguez2010). The main causes of this population collapse are competition from livestock (Baldi et al., Reference Baldi, Albon and Elston2001, Reference Baldi, Pelliza Sbriller, Elston and Albon2004) and hunting. The impact of hunting has been inferred from numbers of guanaco pelts legally exported until the early 1990s (Mares & Ojeda, Reference Mares and Ojeda1984; Baldi et al., Reference Baldi, Campagna and Saba1997), recovery after protection (Puig et al., Reference Puig, Ferraris, Superina and Videla2003), and an inverse relationship between guanaco presence and distance to urban centres and oil camps (Pedrana et al., Reference Pedrana, Bustamante, Travaini and Rodríguez2010). However, in spite of the regional decline of the guanaco and the expansion of hydrocarbon extraction in Patagonia in recent decades, no assessments of guanaco population changes in areas with increased hunting pressure and oil activity are available.
Here we report temporal and spatial patterns in guanaco density, recruitment and social structure in an area subject to oil exploration and extraction, an assessment of the relative impacts of access for poachers, livestock, and primary productivity, and conservation measures that have been taken to allow guanaco populations to recover. As development continues and roads are opened for hydrocarbon exploration and other purposes, our results may have implications for wildlife conservation throughout Patagonia and elsewhere.
Study area
The 15,000-km2 study area in Neuquén province, Argentina (38°S and 69°W; Fig. 1), comprises basaltic plains and volcanic cones with grassy steppe vegetation and lower valleys with shrub-steppe (León et al., Reference León, Bran, Collantes, Paruelo and Soriano1998). Approximately 250 families have grazed livestock throughout the area during the last 100 years, although the higher slopes of the Auca Mahuida Volcano (the highest peak in the area, at 2,253 m) are used only in summer. Goats were the most common livestock during 2005–2011, with a mean herd size of 730 goats per family, resulting in a mean density of 12 goats per km2 (Gonzalez et al., Reference Gonzalez, Novaro, Funes, Pailacura, Bolgeri and Walker2012). Other large-bodied herbivores in the area are Darwin's rhea Rhea pennata and the mara Dolichotis patagonum, and predators include the puma Puma concolor and culpeo fox Lycalopex culpaeus. Pumas were extirpated in the mid 1900s but have recolonized in the last 2 decades (Gonzalez et al., Reference Gonzalez, Novaro, Funes, Pailacura, Bolgeri and Walker2012).

Fig. 1 Hydrocarbon exploration roads built in recent decades, transects surveyed for the guanaco Lama guanicoe during 1982–1983, and polygons around areas surveyed during 2002–2007 (survey polygons: 1, Cerro Bayo; 2, Chivatos; 3, Sierra Negra; 4, Auca Mahuida Volcano; 5, Chihuidos; 6, Mesa; 7, Chorriaca) in northern Patagonia. Insets show location of main map in South America and increase in number of wells after 1946.
Oil exploration and extraction around Auca Mahuida began in the late 1940s but accelerated in the early 1990s (Fiori & Zalba, Reference Fiori and Zalba2003). Since 1993 the region has produced 46% of the oil and 58% of the gas in Argentina (Instituto Argentino del Petróleo y el Gas, 2012). In the last 3 decades opening of seismic lines by oil companies increased the road density from 0.14 km of open-access dirt roads per km2 to 1.84 km of roads and seismic lines per km2 (Fiori & Zalba, Reference Fiori and Zalba2003; Fig. 1). No new public roads were opened in this period and therefore the increase in road density, primarily seismic lines, was entirely a result of oil activity. Seismic lines are 5 m wide, opened to provide access for oil exploration equipment. Distribution of seismic lines is highly heterogeneous in the landscape (Fig. 1), because areas where rich oil deposits were discovered were explored more intensively. Because of the dry climate and slow vegetation growth 66–70% of these seismic lines remain passable with a 4 × 4 vehicle after 20 years (Fiori & Zalba, Reference Fiori and Zalba2000; Michel, Reference Michel2003). Because most low-lying areas are covered by shrubs with thorns that can puncture a tyre, seismic lines where these plants have been removed provide access for vehicles. The number of access points into an area can affect the ability of poachers to enter the area from public roads and thus the impacts of poachers on wildlife (Yackulic et al., Reference Yackulic, Strindberg, Maisels and Blake2011).
Most poaching is for recreation by urban dwellers using 4 × 4 vehicles, although rural people occasionally kill guanacos for meat. The only town in the area, Rincón de los Sauces (Fig. 1), grew from 1,921 inhabitants in 1980 to c. 15,000 in 2005 (Instituto Nacional de Estadística y Censos, 1991, 2001 & 2010) mainly because of increased hydrocarbon exploration and extraction activities. The number of vehicles registered in the town grew from 275 in the 1980s to 16,750 in 2007, of which 26% were trucks and 4 × 4 vehicles (Dirección Provincial de Estadística, unpubl. data).
Methods
We conducted surveys of the guanaco to assess both temporal and spatial differences in population parameters in the study area. Surveys conducted in 1982–1983 by the Neuquén wildlife management agency (by RG and two additional observers; Fig. 1) and in 2002–2007 by the Wildlife Conservation Society (by NIR and two additional observers; Fig. 2) provided an assessment of long-term temporal changes in population parameters at three sites (Cerro Bayo, Chivatos and Sierra Negra). To assess spatial differences in population parameters at sites with different intensities of hydrocarbon development activity, we located in 2002 four additional sites with year-round guanaco presence (Chihuidos, Mesa, Chorriaca and Auca Mahuida Volcano; Fig. 2) through interviews with 25 wildlife rangers and local herders. We sampled these four additional populations during 2002–2007, giving a total of seven sites. This spatial comparison did not include data for 1982–1983. At the three long-term sites and Auca Mahuida Volcano, where logistical conditions allowed us to carry out repeated annual surveys during 2002–2007 (see survey description below), we also assessed inter-annual changes in population parameters.

Fig. 2 Transects surveyed for the guanaco in 2002–2007 (survey polygons: 1, Cerro Bayo; 2, Chivatos; 3, Sierra Negra; 4, Auca Mahuida Volcano; 5, Chihuidos; 6, Mesa; 7, Chorriaca), location of the Auca Mahuida protected area, and area where oil trails were closed in 2006 and 2010. The insert indicates the number of vehicles detected by traffic counters during 2007–2008 along closed trails and open control trails.
Based on the home range of guanacos in other areas of Patagonia (4 km2 in Chubut, Argentina: Marino & Baldi, Reference Marino and Baldi2008; 0.25 km2 in Torres de Paine, Chile: Young & Franklin, Reference Young and Franklin2004), the seven sites (Fig. 2) were large enough (160–970 km2) to encompass the home ranges of > 40 resident guanaco groups. Densities of guanacos across seasons and years during 2002–2007 did not change significantly within these seven sites (N. Radovani, unpubl. data, and results below), indicating these were stable resident populations.
To examine the influence of and interactions among the various factors that could affect guanaco populations we assessed mean elevation, plant productivity, livestock density, and number of access points per unit of area at the seven sites. Elevation can affect forage availability through its influence on the proportion of grasses and shrubs (León et al., Reference León, Bran, Collantes, Paruelo and Soriano1998) and may also affect vehicle access along seismic lines because roads on higher, steeper slopes are often rendered impassable by water after torrential rain or snow melt. We obtained elevation by overlaying transects on a digitized topographic map, using a geographical information system (GIS). We used the Enhanced Vegetation Index (EVI) as an index of primary productivity, calculating the mean EVI for each site during 2002–2007 from the IDRISI 16 (Clark Labs, 2009) archive of monthly EVI derived from 0.5 × 0.5° MODIS satellite images processed by the NASA Goddard Space Flight Center.
We defined access points for poachers as those where seismic lines intersected public roads, and hypothesized that the density of access points could be a good indicator of the impact of poaching on guanacos. We recorded access points from a GIS layer of seismic lines digitized from satellite images, and confirmed the number and location of access points for each polygon on the ground. We could not estimate absolute livestock density because of the low number of observations, and therefore we used the number of animals encountered per km during the guanaco transects as an estimate of relative abundance. We did not have data for 1982–1983 to assess long-term temporal changes in plant productivity and road and livestock abundance. Therefore, we assessed temporal changes at the scale of the entire study area by analysing trends in mean annual precipitation from weather stations and livestock abundance from agricultural census data. Precipitation is often a good predictor of plant productivity in dry ecosystems (Jobbágy et al., Reference Jobbágy, Sala and Paruelo2002).
In April 1982 and seasonally in 2002–2007 daytime surveys for guanacos were conducted along transects on secondary roads and seismic lines by two observers from the back of a pick-up truck driven at 10–15 km h−1 (Figs 1 & 2). In April 1983 surveys were conducted from a fixed-wing Piper PAI2 aircraft, flying at 160 km h−1 at 300 m (Fig. 1). The three sites studied for the temporal comparison were thus surveyed with ground and aerial transects during 1982–1983 (Fig. 1) and with 3–8 ground transects of 9–20 km at each site during 2002–2007 (Fig. 2). At the four additional sites studied for the spatial assessment during 2002–2007 we surveyed 3–8 ground transects of 5–32 km per site (Fig. 2).
Because transect data in 1982, 1983 and 2002–2007 were collected differently, we only used survey data that allowed estimation of population parameters that were comparable across time. In 1982 the size and composition of guanaco groups were recorded within a 300 m strip on each side of the road, and in 1983 within 2.5 km by 400 m segments along the aerial path. Guanaco social structure includes family groups, bachelor groups and solitary animals (Franklin, Reference Franklin, Eisenberg and Kleiman1983). As distances between groups and transect lines were not recorded in 1982, we could not estimate error for density and proportion of yearlings and the 1983 aerial surveys probably missed solitary guanacos (none were recorded), leading to overestimation of group size. Therefore, for the temporal comparison of population parameters in the first period we used the 1982 data to estimate group size and the 1983 data to estimate density and proportion of yearlings. During 2002–2007 we gathered data at all seven sites, using the line-transect method, recording distance between guanaco groups and transect lines (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Thus, for the second period of the temporal comparison and for the spatial comparison, data from all 2002–2007 transects were used to estimate group size, density, and proportion of yearlings.
For the spatial comparison we estimated densities from line-transect data from the seven sites surveyed during 2002–2007, using Distance v. 5.0 (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Sighting data were modelled using the half-normal function with polynomial adjustment for the Auca Mahuida Volcano (f(0) = 2.78E-03, %CV = 3.55) and the hazard function with cosine adjustment for the other six sites combined (f(0) = 3.01E-03, %CV = 5.21), because the distribution of the data were different, perhaps as a result of differences in guanaco behaviour caused by lower poaching pressure at the Auca Mahuida Volcano (N. Radovani, unpubl. data and results below). The models that best fitted the data were selected according to the Akaike information criterion (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). We could not estimate detection functions for the 1983 aerial counts because distances between groups and transect lines were not recorded. Thus, for the temporal comparison we obtained comparable density estimates for the three sites sampled in 1983 and 2002–2007, using the strip-transect method (Thomas et al., Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2002), re-analysing the 2002–2007 transect data within 400 m wide strips. Therefore, for the three long-term sites during 2002–2007, two density estimates were obtained, by different methods, one for the temporal comparison (strip transect) and one for the spatial comparison (line transect). In addition, because the 1983 data were obtained from the air and the 2002–2007 data from the ground, we converted the 1983 data using a calibration based on comparisons between aerial and ground transects at eight sites in Patagonian steppe and scrub (A. González, unpubl. data). Because this calibration was obtained at sites to the north and south of our study area with similar topography and vegetation we are confident that it is applicable to the detectability conditions of aerial and ground surveys in our study.
We studied long-term temporal trends in density by visual examination of overlap among confidence intervals between 1983 and 2002–2007. At sites where we had sufficient annual surveys (the three long-term sites and the Auca Mahuida Volcano) we also studied short-term trends during 2002–2007 by estimating finite rates of increase (λ = er ), based on exponential rates of increase (r) that were calculated as the slope of the linear regression of the natural logarithm of guanaco population size on time (Caughley & Sinclair, Reference Caughley and Sinclair1994). We estimated population sizes from density estimates and sizes of polygons surveyed during 2002–2007. We did not include the 1983 density data in the linear regression because we could not estimate population size as a result of limitations in the design of the 1983 survey. We could not assess trends in density at the three remaining short-term sites because the number of guanaco groups sighted was too low to estimate annual densities at those sites and we were forced to pool data among years to obtain a precise estimate for 2002–2007.
For the spatial comparison among the seven sites we assessed the relationship between guanaco density, EVI, number of access points, and livestock density, with a generalized linear model (GLM) with Poisson error and log link functions (Hoffmann, Reference Hoffmann2004). We tested for correlations among the dependent variables, using Pearson's correlation coefficient. As we found a significant negative correlation between the number of access points and elevation (R = −0.87, P = 0.010) we did not include elevation in the model. None of the remaining variables were significantly correlated.
We also performed linear regression analysis within sites for the period 2002–2007 to determine if there were significant, short-term trends in group size and proportion of yearlings. Because no short-term trends within sites were significant (see Results) we grouped data for 2002–2007 and analysed long-term changes in group size between 1982 and 2002–2007 using a GLM with Poisson error and log link functions, and changes in proportions of yearlings between 1983 and 2002–2007 with a factorial ANOVA (Zar, Reference Zar1996) in STATISTICA v. 7.1 (StatSoft, Tulsa, USA). These long-term trends were analysed simultaneously for the three long-term sites. To avoid seasonal effects on group size (Franklin, Reference Franklin, Eisenberg and Kleiman1983) and proportion of yearlings, we used data only from April (post-reproductive season) surveys for 2002–2007. Because the 1983 aerial counts probably missed more yearlings than the 2002–2007 ground counts, this comparison provides a conservative test of a decline in the proportion of yearlings.
Results
Guanaco densities declined by 93–96% between 1983 and 2002–2007 at the three long-term sites (Fig. 3a). Densities at these sites were stable during 2002–2007 (P ⩾ 0.105; Table 1) and therefore we averaged densities for this period. Mean guanaco density was 16.9 ± SE4.89 km−2 in 1983 and 1.0 ± SE 0.48 km−2 during 2002–2007. According to our interviews, guanacos were present throughout the study area until the 1980s but during 2002–2007 were restricted to the seven sites mapped in Fig. 1. This was confirmed by our searches during 2002–2007 as we never observed guanacos outside these sites while travelling hundreds of kilometres along all public roads and many seismic lines throughout the study area.

Fig. 3 (a) Mean guanaco density ± SE, (b) mean group size ± SE, and (c) proportion of yearlings at Cerro Bayo, Chivatos and Sierra Negra (Fig. 1) during 1982–1983 (light shading) and 2002–2007 (dark shading). Numbers above bars are sample sizes (guanaco groups for (a) and (b), and number of transects for (c)).
Table 1 Regression analysis of guanaco Lama guanicoe population trends at four sites in northern Patagonia during 2002–2007. λ is the finite rate of guanaco population increase at each site during this period.

* Sample size was 2,556 guanaco groups.
During 2002–2007 annual densities were also stable at the Auca Mahuida Volcano short-term site (P = 0.937; Table 1). We could not assess trends in density during 2002–2007 at the other three short-term sites because of the small number of guanaco groups sighted (Chihuidos, n = 5; Mesa, n = 8; and Chorriaca, n = 30). Mean population sizes estimated at the seven sites during 2002–2007 ranged between 111 ± SE 33 in Chihuidos and 8,683 ± SE2,374 in Auca Mahuida Volcano (Table 2).
Table 2 Guanaco density and abundance, enhanced vegetation index (EVI), density of access points, livestock density, and mean altitude at seven sites in northern Patagonia (Fig. 1) during 2002–2007.
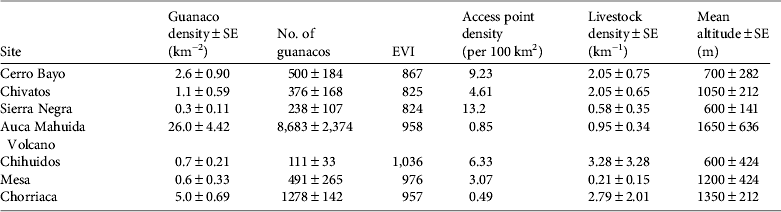
Guanaco densities at the seven sites during 2002–2007 had a negative relationship with the density of access points (Wald = 14.6, P < 0.001) and to a lesser extent with livestock density (Wald = 4.6, P = 0.033; Table 2). During 2002–2007 mean guanaco densities were < 3 km−2 at sites with a high density of access points (> 3 per 100 km2), and > 5 km−2 at sites with a low density of access points (< 1 per 100 km2; Table 2). Guanaco densities were not significantly associated with EVI (Wald = 0.9, P = 0.345).
Mean group size declined significantly from 9.8 guanacos per group in 1982 to 5.0 during 2002–2007 at the three long-term sites (Wald = 165.5, P < 0.001; Fig. 3b). Between 1982 and 2002–2007 the overall percentage of solitary guanacos at these sites increased from 0 to 26%. Linear regression analysis indicated that group size remained stable during 2002–2007 at the Auca Mahuida Volcano (F = 6.8, df = 1, P = 0.060), where mean group size was 7.0 ± SE 0.36 (n = 564) during 2002–2007. Sample sizes at the long-term sites (Fig. 3b) and the three short-term sites were too small to test for trends during 2002–2007. Mean group sizes over all years during 2002–2007 at the other three short-term sites were 2.4 ± SE 0.98 in Chihuidos, 4.1 ± SE 1.03 in Mesa, and 3.5 ± SE 0.35 in Chorriaca.
The mean proportion of yearlings did not change significantly at the three long-term sites (F = 0.21, P = 0.65, df = 1) between 1983 and 2002–2007 (Fig. 3c). Linear regression within sites indicated that the proportion of yearlings remained stable during 2002–2007 at the three long-term sites (Cerro Bayo F = 1.94, P = 0.24, df = 1; Chivatos F = 0.05, P = 0.84, df = 1; Sierra Negra F = 7.4, P =0.11, df = 1) and also at the Auca Mahuida Volcano (F = 0.14, P = 0.72, df = 1; 2002–2007 mean proportion of yearlings was 0.14 ± SE 0.01, n = 6 transects). Sample sizes at Chihuidos, Mesa and Chorriaca were too small to estimate the proportions of yearlings.
Discussion
The precipitous declines in guanaco density and group size at the three long-term sites were probably the result of poaching from seismic lines and, to a lesser extent, the negative effect of livestock. The data from the additional, short-term sites and interviews during 2002–2007 suggest that the decline occurred over a large area and led to fragmentation of the guanaco population. The absence of resident guanaco groups in the matrix surrounding Chorriaca, Mesa and Chihuidos means that these guanaco populations are now relatively isolated from the remaining populations (> 20 km away from Chivatos; Fig. 2) and may receive only occasional dispersing guanacos. The relatively small size of the Chihuidos and Mesa populations (< 500 individuals), combined with their isolation, may expose them to high risk of local extinction.
According to the densities estimated during 2002–2007 the only sites where the precipitous decline did not occur after 1983 were the Auca Mahuida Volcano and, to some extent, the Chorriaca hills, the two highest sites surveyed (Table 2). These two populations may have been least affected by poachers because they had fewer passable access points as a result of the limited oil exploration in these locations, high elevation and steep slopes (N. Radovani, pers. obs.). The Chorriaca hills population, however, probably suffered the combined effects of some poaching and high livestock density. All other sites, including the three long-term sites, had either high access for poachers (> 3 access points per 100 km2) or a combination of high access and high livestock densities (Table 2).
Poaching probably increased in recent decades as a result of increasing access from the extensive network of seismic lines and the number and affluence of residents in nearby towns, many of them hydrocarbon company employees with leisure time and appropriate vehicles for hunting. The frequent use of seismic lines by unauthorized people with 4 × 4 vehicles was confirmed by rural inhabitants, who often lost livestock to thieves, by poachers caught by rangers (S. Goitia & F. Barros, pers. comm.), and by data from traffic sensors (Fig. 2; N. Radovani, unpubl. data).
Other factors, however, may have exacerbated the decline of the guanaco population. The number of goats and sheep declined by 41% in the region, from c. 195,000 in 1978 to c. 116,000 in 2002 (Dirección Provincial de Estadística y Censos, unpubl. data), in part because of range depletion from overgrazing (PROSA, 1988). Although this decline in livestock may have reduced competition with guanacos, overgrazing may also have reduced carrying capacity for guanacos. In addition, total annual precipitation has declined; the annual mean at four stations in the area was 210 ± SD 59 mm during 1980–1983 and 122 ± SD 59 mm during 2002–2007 (Autoridad Interjurisdiccional de Cuencas, unpubl. data), potentially resulting in decreased plant productivity.
Pedrana et al. (Reference Pedrana, Bustamante, Travaini and Rodríguez2010) concluded that higher probability of guanaco presence in areas of low productivity in southern Patagonia was a result of intense competition with livestock and persecution by humans in areas of high productivity. In our study the lack of a significant association between guanaco density and productivity may also be the result of the combined negative effects of poaching and livestock in the most productive ranges. If a long-term decline in productivity is occurring because of reduced precipitation, as predicted by climate models (Vera et al., Reference Vera, Silvestri, Liebmann and González2006), guanaco populations that are pushed into less productive ranges may be affected most severely. The lack of satellite imagery for the early 1980s precluded examination of changes in productivity.
The changes reported in population structure may affect the capacity for guanaco populations to recover. The reduction in mean group size between 1982 and 2002–2007 may limit group vigilance, reducing individual foraging time and increasing vulnerability to predators, particularly pumas (Marino & Baldi, Reference Marino and Baldi2008; Marino, Reference Marino2010). The proportion of yearlings at the long-term sites (1983–2007 mean was 0.25 ± SE 0.04) was high compared to other studies (0.00–0.30; Rey et al., Reference Rey, Novaro, Sahores and Guichón2012; earlier studies cited in Saba et al., Reference Saba, De Lamo, Puig and Puig1995) and also in comparison with the Auca Mahuida Volcano site during 2002–2007, where high density may limit birth rates or yearling survival through density dependence. Small group sizes at the long-term sites, however, may limit recruitment from the abundant yearling class into reproductive adults because of reduced group vigilance, individual foraging and predator avoidance.
Our data suggest that, in areas that have been subject to oil exploration, minimizing the density of access points at intersections between public roads and seismic lines can limit the impact of poaching on wildlife, as has been reported for forested areas where poachers use logging roads for access (Yackulic et al., Reference Yackulic, Strindberg, Maisels and Blake2011). The impact we report on guanacos in Patagonia has probably occurred on other species that are targeted by poachers, such as the rhea and mara (Barri et al., Reference Barri, Martella and Navarro2008; M L. Rivas & S. Walker, unpubl. data). Extensive areas of Patagonia were subject to oil exploration before the late 1990s, when improvements in exploration technology reduced the need for seismic lines. To help guanacos and other species recover we recommend widespread closing of oil trails in those areas of Patagonia explored for oil until the mid 1990s. Under Argentine law open seismic lines must be mitigated by oil companies that currently hold concessions and we therefore recommend that the government use the information we report here to enforce the closing of seismic lines.
Our collaboration with the provincial Department of Protected Areas and the Repsol-YPF oil company may help guanacos recover in the study area. In 1996 an 800 km2 provincial protected area was declared around the Auca Mahuida Volcano and since 2005 four wildlife rangers and two park rangers have patrolled the study area with 4 × 4 vehicles. In 2006, at the request of the Department of Protected Areas, the oil company closed 376 seismic lines with levees and ditches at key access points, blocking access by poachers to an area of 220,000 ha that includes the Cerro Bayo and Chivatos sites (Fig. 1). Local herders collaborated because they expected a reduction in livestock theft by poachers. The oil company, in addition to complying with the mitigation requirement, will benefit from increased security at their wells and camps. We are monitoring the effectiveness of these closures, using magnetic traffic sensors, and the response of the guanaco population by surveying transects seasonally in the closed area and control areas. During the first 3 years the closures led to a considerable reduction in vehicle access but 93 closings needed to be reinforced (Fig. 2); this was done by Repsol-YPF in 2010. Our experience so far indicates that levees and ditches are effective means of preventing access along seismic lines but closures must be reinforced every 2–4 years, depending on the intensity of wind and precipitation, to keep them from becoming passable by 4 × 4 vehicles, and several years of monitoring will be required to observe a guanaco recovery in closed areas.
Our experience also highlights the benefit of collaboration among scientists, government and industry in the design and implementation of conservation actions. In northern Patagonia mitigation of the impact of poaching was successful because scientists provided data on indirect impacts of hydrocarbon exploration and were able to suggest how to mitigate them, the government enforced existing laws regarding mitigation of industry impacts, and the oil company agreed to provide the necessary resources. As others have recognized (e.g. Suarez et al., Reference Suárez, Morales, Cueva, Utreras Bucheli, Zapata-Ríos and Toral2009; Berger & Beckmann, Reference Berger and Beckmann2010), collaborative efforts are essential for minimizing the environmental and social impacts of industry.
Acknowledgements
Funding was provided by the Wildlife Conservation Society, Whitley Fund for Nature, U.S. Fish and Wildlife Service, Disney Worldwide Conservation Fund, and Agencia Nacional de Promoción Científica of Argentina. We are grateful to S. Di Martino, S. Goitía, F. Barros, C. Garcia, L. Heidel, A. González, O. Pailacura, O. Monsalvo, L. Piudo, L. Laffitte, M.L. Guichon, A. Rey, L. Rivas, R. Palacios, F. Milesi, J. Bonaccorso and two anonymous reviewers for their help in various aspects of this work.
Biographical sketches
Natalia Radovani has worked on wildlife management and conservation in Patagonia since 2002. She studies the impact of human activities on guanacos. Martín Funes has worked on wildlife research and management for over 20 years, in government agencies and NGOs. He focuses on ecology of carnivores, raptors and exotic species, and wildlife monitoring techniques. Susan Walker is a wildlife biologist whose research on landscape connectivity is being applied to the development of the Payunia-Auca Mahuida Guanaco Corridor in northern Patagonia. Reinaldo Gader is an agricultural engineer who carried out the first guanaco surveys in the province of Neuquén in the 1980s. Andrés Novaro’s research focuses on impacts of hunting on wildlife and predator–prey interactions. He works with governments and the private sector to address threats to wildlife from extractive industries, livestock grazing and poaching.







