West Nile virus (WNV), a flavivirus of the Flaviviridae family [Reference Burke, Monath, Knipe and Howley1, Reference Beasley2], was first isolated in Uganda in 1937 [Reference Smithburn3]. WNV is endemic in Africa, the Middle East and western Asia and, nowadays, also in North America, where it was first reported in 1999 [Reference Beasley2, Reference Hayes4]. The virus is responsible for encephalitis outbreaks involving birds, horses and humans, and it has caused over 1000 human deaths in the USA (http://www.cdc.gov). Since 1999, WNV has dispersed widely and it has already been isolated in Central and South America [Reference Beasley2, Reference Hayes4, Reference Morales5]. Its natural transmission cycle is maintained in a cycle between mosquitoes, mainly from the Culex genus, and birds. However, other vertebrates such as humans and horses are incidental hosts, as they do not reach viraemia levels high enough to infect feeding mosquitoes [Reference Hayes4].
The first serological evidence of WNV activity in Mexican horses was observed in 2002 in states that border Texas (USA) and the coast of the Gulf of Mexico [Reference Blitvich6–Reference Loroño-Pino8], where migratory birds coming from southeastern USA may have introduced the virus [Reference Deardorff9]. The first isolation of WNV in Mexico was reported in 2003 in an imported common raven (Corvus corax) from the USA [Reference Estrada-Franco8]. At the time of sampling for our study, March–April 2006, enzyme-linked immunosorbent assay (ELISA)-positive serology had been already reported in humans, horses and other mammals, reptiles, and different species of birds from several Mexican states [Reference Estrada-Franco8–Reference Deardorff12], but no data from Chiapas or Puebla states had been published. In Mexico, WNV expansion and infectivity behaviour has been quite different from that in the USA [Reference Deardorff9–Reference Farfán-Ale12]. Nevertheless, surveillance of WNV activity is important in order to assess viral expansion, control zoonotic transmission and to elucidate the pathogenic and epidemiological differences observed between the USA and the rest of the world [Reference Blitvich6–Reference Loroño-Pino7, Reference Blitvich12].
In this study, we analysed 288 equine sera, including 150 from Pichucalco (17° 30′ N, 93° 07′ W) and Juárez (17° 36′ N, 93° 10′ W) municipalities (Chiapas state), and 138 from Hueytamalco (19° 56′ N, 97° 17′ W), Ayotoxco (20° 05′ N, 97° 24′ W) and S. José Acateno (20° 07′ N, 97° 12′ W) municipalities (Puebla state). These sites have very similar climates, i.e. warm and humid. The temperature ranges from 22°C to 27°C and the average rainfall varies from 2500 to 3000 mm3 per year. All locations are between 120 and 300 m above sea level, except Hueytamalco, located at 914 m. Equine sera comprised 272 (94·4%) horses, 12 (4·2%) mules and four (1·4%) donkeys. The sex distribution was equitable, comprising 149 (51·7%) mares and 139 (48·3%) horses. Twenty-two animals were used for breeding, six for recreational activities and the rest for farm labour. The mean age of the animals was 6·8 years (range 1 month to 25 years). None had been vaccinated or presented with signs of West Nile disease (WND).
Analyses were conducted in bio-safety level-3 containment facilities. Equine sera were stored at −20°C and inactivated at 56°C for 30 min prior to testing. Sera were tested for anti-WNV IgG antibodies by ELISA as described previously [Reference Córdoba13, Reference Ebel14]. For antigen production, Vero cells were infected with the WNV NY-99 flamingo 382-99 strain. Cell lysates were heat-inactivated and processed as described previously [Reference Blitvich15]. Uninfected cell lysates, similarly processed, were used as negative controls. The positive cut-off value was assigned using a positive/negative (P/N) ratio ⩾2, calculated by dividing the mean absorbance of the test sera reacted on viral antigen by the absorbance of the negative control serum on viral antigen [Reference Beaty, Calisher, Shope, Schmidt and Emmons16]. Plaque reduction neutralization tests (PRNT) were performed to confirm the ELISA results. The PRNT was conducted on Vero cells with the WNV NY-99 flamingo 382-99 strain using twofold serial sera dilutions, as previously described [Reference Beaty, Calisher, Shope, Schmidt and Emmons16]. Titres were calculated as the reciprocal of the serum dilution, diluted at least 1:40, which reduced plaque formation ⩾90% (PRNT90). Because other flaviviruses circulating in Mexico may cross-react with WNV, a subset of 138 samples was also tested against St Louis encephalitis virus (SLEV) and Ilheus virus (ILHV) by haemagglutination inhibition (HI) tests [Reference Beaty, Calisher, Shope, Schmidt and Emmons16]. A ⩾fourfold antibody titre difference between WNV and the other antigens was used for differential diagnosis.
Our results showed a relatively high seropositivity, 31·6% (91/288) of the samples were IgG-positive for WNV (Table 1). The seroprevalence was higher in Chiapas (53·3%, 80/150; average P/N=3·6, range 2·03–9·8) than in Puebla (8%, 11/138; average P/N=3·6, range 2·5–7·3). No appreciable seroprevalence differences were detected between municipalities within each state. All but one IgG-positive sample from Chiapas were also positive by PRNT (average PRNT90=435, range 40 to ⩾1250) while 15 IgG-negative samples tested positive by PRNT. All ELISA-positive samples from Puebla were also PRNT-positive (average PRNT90=430, range 130 to ⩾1250), and only one case was IgG-negative and PRNT-positive. None of the mules or donkeys from Puebla were positive, while two mules and two donkeys from Chiapas were IgG- and PRNT-positive. Concordance between ELISA and PRNT data was quite good (κ=0·87). Taking the PRNT as the gold standard technique, the sensitivity and specificity of the ELISAs were 84·9% and 99·4%, respectively. Although the seroprevalence was slightly lower in animals aged <2 months (16%), no statistically significant differences were recorded as a consequence of the animals' age or sex.
Table 1. Summary of equine sera tested for evidence of WNV infection by ELISA and PRNTFootnote *
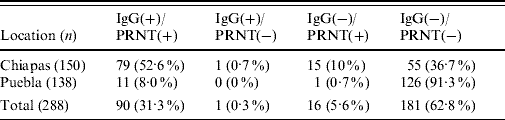
WNV, West Nile virus; ELISA, enzyme-linked immunosorbent assay; PRNT, plaque reduction neutralization test.
Data are expressed as number of samples with percentages given in parentheses.
* IgG-positive samples determined by ELISA; virus neutralization positive samples determined by PRNT.
Analysis by HI tests of a subset of 138 (82 IgG- and/or PRNT-positive and 56 negative) samples against three different flaviviruses (WNV, SLEV, ILHV) currently circulating in Central and South America (Table 2) showed that no reactivity was detected in 39·8% (55/138) of them. On the other hand, 44 (31·9%) of the samples were WNV-specific, either because they only contained antibodies against WNV (23/44, 52·3%) or because they presented fourfold higher HI titres against WNV than against SLEV (16/44, 36·4%) or than against both SLEV and ILHV (5/44, 11·3%). The remaining 39 samples (28·3%) recognized flaviviruses, but their specificity could not be clearly established because they showed similarly positive HI titres against WNV and SLEV (24/39, 61·6%) or against WNV, SLEV and ILHV (13/39, 33·3%). Two cases (5·1%) presented low (1/40) HI titres only against SLEV. In summary, 53% (44/83) of the samples that reacted against flaviviruses by HI were specific for WNV.
Table 2. Number of samples that react against WNV, SLEV and ILHV by haemagglutination inhibition tests
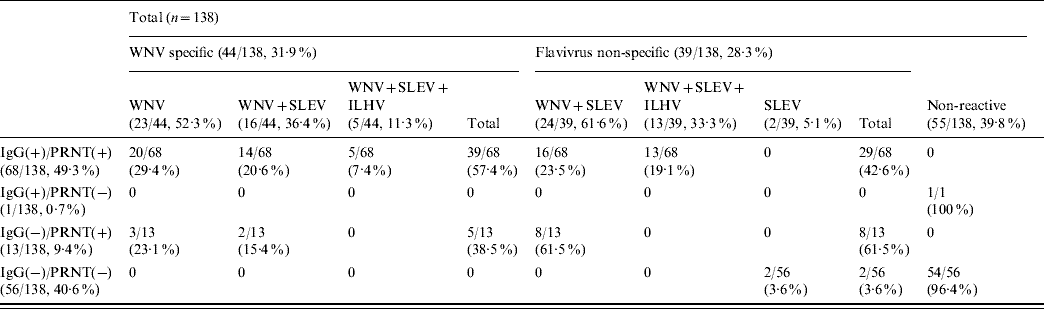
WNV, West Nile virus; SLEV, St Louis encephalitis virus; ILHV, Ilheus virus; PRNT, plaque reduction neutralization test.
Data are expressed as number of positive samples/total samples with percentages given in parentheses.
No reactivity by HI against any of the three flaviviruses tested was observed in the only serum that was IgG-positive and did not neutralize the virus, neither was it detected in 54/56 IgG- and PRNT-negative samples (Table 2). The other two samples that were also IgG- and PRNT-negative reacted only against SLEV. Most (61·5%) of the 13 neutralizing sera that were IgG-negative reacted non-specifically against WNV and SLEV, while the other five sera (38·5%) presented specific reactivity against WNV by HI test. Of the 68 samples that were IgG- and PRNT-positive, 42·6% recognized flaviviruses, but their specificity could not be clearly established, while the remaining 57·4% specifically reacted against WNV by HI.
Occasional disparities between ELISA and PRNT results observed in a few sera could be due to several reasons. For example, the neutralizing capability of the 16 IgG-negative samples might be due to the presence of IgM-specific antibodies, which were not tested here because the horses did not have any history of viral encephalitis or other recent illness. All but two of these samples showed low PRNT90 titres against WNV (1/40) and only 38·5% of these samples were specific to WNV when tested by HI (Table 2). On the other hand, the single non-neutralizing IgG-positive sample detected could reflect infection with viruses other than WNV, SLEV and ILHV that were not tested here, but which may cross-react with WNV antibodies.
However, the lower seroprevalence found in Puebla could reflect lower WNV activity, which might be due to a more recent introduction of the virus there. In fact, WNV activity has been previously reported in locations relatively near to the Chiapas municipalities included in our study [Reference Morales5–Reference Blitvich8], but distant from the Puebla locations sampled here (Fig. 1). Although altitude, climate and rainfall are quite similar in the municipalities studied, other factors, such as the ecology of mosquitoes, could also play a role in the differences observed.
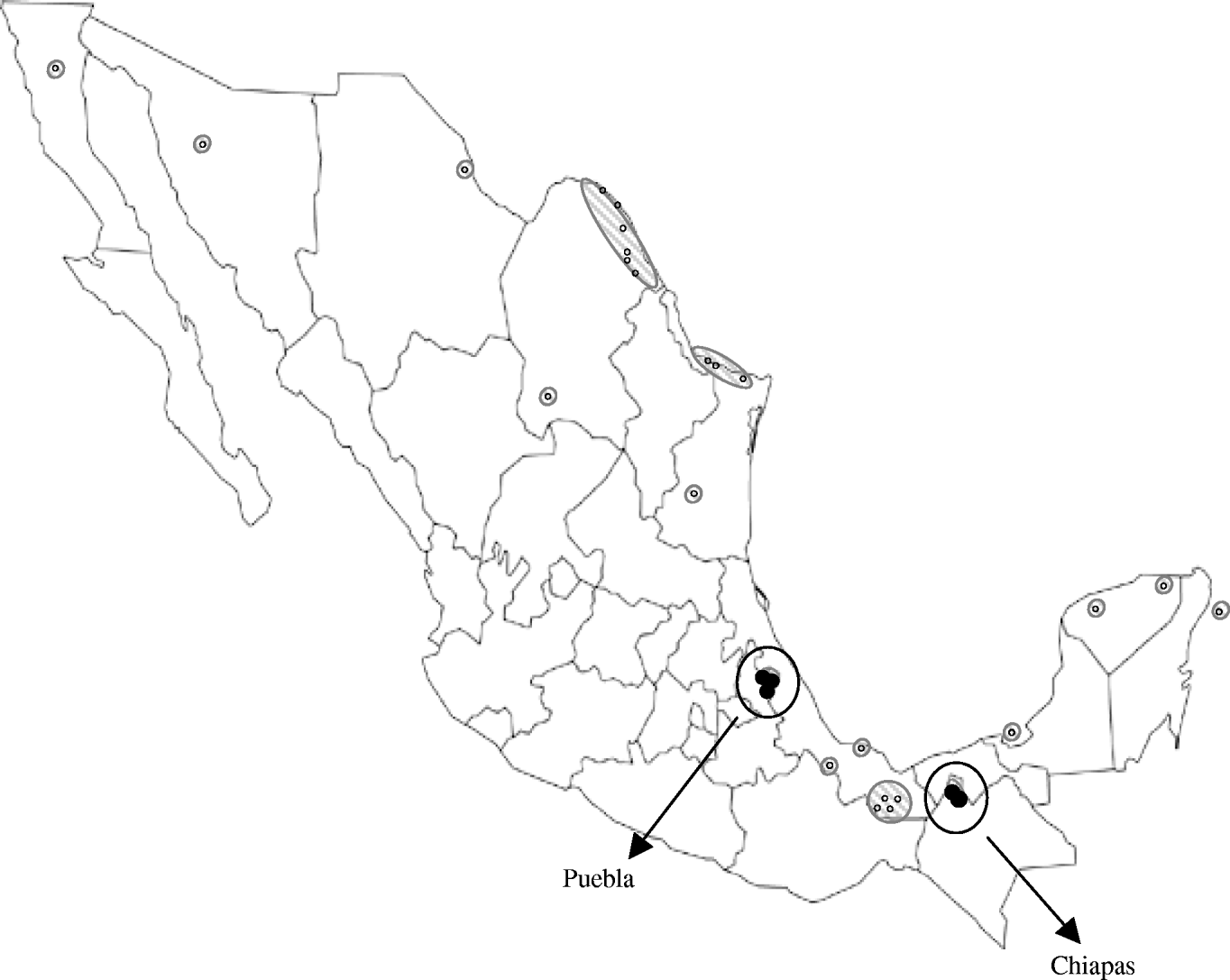
Fig. 1. Map showing the Mexican locations where West Nile virus activity had been detected prior to the present study [small white symbols (○) and hatched areas] and Puebla and Chiapas municipalities sampled here [circled black symbols (•)].
WNV appears to have been introduced into Mexico in two separate events: carried by birds migrating from southeastern USA to southern Mexico through the Caribbean Sea, and entering from southwestern USA into northern Mexico [Reference Deardorff9]. Isolation of WNV strains has been unexpectedly difficult in Mexico and the Caribbean [Reference Blitvich12], we performed several experiments to recover virus upon infection of susceptible cells with samples from this study but all attempts failed. Thus, no genomic analyses of the viral strains circulating in the studied areas could be conducted. However, the geographical location of the Chiapas municipalities sampled here suggests that the WNV activity detected there is probably due to the expansion of strains circulating in the Yucatan peninsula and neighbouring states, while the equidistant situation of the sampled Puebla municipalities from the two areas where WNV was introduced into Mexico makes it difficult to speculate about the origin of the strain colonizing this region.
The relatively high WNV seroprevalence found in Mexican horses in a previous study [Reference Estrada-Franco8] was initially suggested to be an overestimation due to the neurological disorders of the studied herd; nevertheless our data from asymptomatic, unvaccinated animals support these figures and the apparently lower WNV pathogenicity found in Mexico compared with that observed in the USA. The differences in clinical cases in horses and humans between the USA and Mexico are intriguing [Reference Blitvich12], particularly the lack of human cases in Mexico, where only seven WNV encephalitis cases have been described [Reference Elizondo-Quiroga11] (http://portal.salud.gob.mex). This disparity could be due to the wide distribution of other related and potentially cross-protective flaviviruses in the region, such as those analysed here (SLEV and ILHV), because individuals that had previously been in contact with these flaviviruses may have naturally acquired immunity against WNV. In fact, pre-existing immunity to Dengue virus (DENV) infection in humans provides partial protection to subsequent WNV infection [Reference Tesh17]; however, this is unlikely to happen in horses because DENV usually does not replicate in non-primate vertebrates and mosquito vectors and reservoir hosts for DENV and WNV are different [Reference Weaver and Barret18]. In addition, as previously suggested [Reference Blitvich12], a different virulence of the Mexican WNV strains could also contribute to the paucity of disease there.
The relatively high seropositivity found here in Mexican asymptomatic, unvaccinated horses suggests a continuous expansion of WNV activity throughout the country. Although the clinical consequences of viral infection in birds, horses and humans appear to be different than the worrisome situation in the USA [Reference Blitvich12], permanent surveillance of WNV activity in horses is important, as they are good sentinel candidates for enzootic viral activity.
ACKNOWLEDGEMENTS
The work was supported in part by grants (AGL2004-06071, AGL2007-61655, FIS-PI071310) from the Spanish Ministerios de Ciencia e Innovación (MICINN) and Sanidad to J.C.S., by grants C01-24 and 2003-025 form the Consejo Nacional de Ciencia y Tecnología, SAGARPA-CONACYT (Mexico) and by contract N01-AI25489 from the National Institutes of Health (U.S.). J.A. was supported by a scholarship from the Spanish MICINN, EER by the ‘Juan de la Cierva’ programme from the MICINN, and L.C. by a scholarship from INIA, Spain.
DECLARATION OF INTEREST
None.





