Type 2 diabetes mellitus (T2DM), an important global health problem, is becoming increasingly epidemic worldwide. In 2019, there are an estimated 4·63 diabetic patients worldwide and this number is projected to reach 700 million by 2045(1). However, in terms of the number of diabetics, China ranks first with a total number of about 116·4 million, which puts an immense strain on China healthcare system. In addition, T2DM and its serious complications have the characteristics of high mortality and disability rate and greatly increase the risk of CVD and cancer, resulting in the decline of life quality and life expectancy(Reference Okemah, Peng and Quiñones2). In the past decade, although great progress has been made in the clinical treatment of T2DM, long-term medications often bring some side effects such as hypoglycaemia, dyspepsia, nausea, etc. In this context, the unique advantages of natural medicine may provide favourable conditions for the treatment of diabetes.
Adipose tissue is a significant tissue that responds to changes in nutrient supply and environmental temperature(Reference Bartelt and Heeren3). Mammals possess two types of adipose tissue with different morphology and functions, namely white adipose tissue (WAT) and brown adipose tissue (BAT). WAT stores a large amount of nutrients in the form of lipids in monocytic white adipocytes. When food is scarce, the fat will be released in the form of fatty acids to provide energy for the body. BAT can both store nutrients in the form of lipids and dissipate energy in the form of heat energy, which is called non-shivering thermogenesis(Reference Yao, Shan and Zhang4). Classic brown adipocytes are rich in mitochondria that contain uncoupling protein-1 (UCP1) which is located in the inner mitochondrial membrane. UCP1 releases the coupling between electron transfer and phosphorylation in part of normal respiratory chain, which releases the energy generated by electrochemical gradient in the form of heat to maintain the body core temperature(Reference Lidell, Betz and Enerbäck5). In addition to the classic BAT, clusters of UCP1-expressing adipocytes in WAT with thermogenic capacity also response to various stimuli(Reference Harms and Seale6). This phenomenon is that ‘brown-like’ adipocytes (also known as beige adipocytes) accumulated in WAT are often referred to as ‘browning’ of WAT. There are numerous common characteristics in beige and brown adipocytes, such as from multilocular lipid droplet morphology to high mitochondrial content. Activating BAT and inducing browning of WAT have become novel underlying strategy for the treatment of obesity and T2DM. Indeed, brown and beige adipose tissues have been demonstrated to play a significant role in improving glucose homoeostasis, insulin sensitivity and lipid metabolism; all three factors are associated with the pathogenesis of T2DM(Reference Kaisanlahti and Glumoff7).
Mulberry (Morus alba L.) leaf is one of the commonly used traditional Chinese herbs and edible food, and it has been widely recognised for its good therapeutic effect on diabetes and its complications(Reference Ge, Chen and Tang8). There is evidence that mulberry leaf can decrease the body weight (BW), blood glucose, TAG, total cholesterol (TC) and LDL levels and improve insulin resistance through IRS-1/phosphatidylinositol 3 kinase/GLUT-4 signalling pathway in T2DM rats(Reference Cai, Sun and Fan9). Moreover, mulberry leaf ameliorates metabolic disorders in db/db mice by increasing the expression of adiponectin and reducing the expression of TNF-α, monocyte chemoattractant protein-1 and macrophage markers in WAT(Reference Sugimoto, Arai and Tamura10). The extract of mulberry leaf can reduce the levels of inflammatory mediators and NEFA in diabetic rats, reduce oxidative stress injury, improve the mitochondrial function of islet cells and protect islet beta cells(Reference Liu, Ma and Zhang11). The water extract of mulberry leaf can promote glucose uptake of skeletal muscle cells and improve hyperglycaemia, insulin sensitivity and hepatic steatosis in diabetic db/db mice through phosphatidylinositol 3 kinase/protein kinase B and AMP-activated protein kinase (AMPK) signalling pathways(Reference Bae, Jung and Jung12).
AMPK is a potentially important target for the treatment of T2DM. Once activated, AMPK regulates metabolism through phosphorylation of key metabolic proteins and transcription factors, promotes energy production pathway (catabolism) and inhibits energy storage pathway (anabolism)(Reference Desjardins and Steinberg13). In skeletal muscle and liver, the activation of AMPK promotes the uptake of glucose and fatty acids, enhances mitochondrial function and fatty acid oxidation, inhibits the synthesis of lipids and cholesterol and improves insulin resistance; all of these may be beneficial to patients with T2DM(Reference Esquejo, Salatto and Delmore14,Reference Cokorinos, Delmore and Reyes15) . Recently, it has been demonstrated that AMPK plays a vital role in regulating the metabolic activity of brown and beige adipose tissue. AMPK is essential for the activation of BAT and browning of WAT. AMPK in adipocytes is vital for maintaining mitochondrial integrity, responding to pharmacological agents and thermal stress and improving non-alcoholic fatty liver and insulin resistance(Reference Mottillo, Desjardins and Crane16).
Our previous study showed that the water extract of mulberry leaf inhibited inflammation and improved insulin resistance in T2DM mice through regulating toll-like receptors and insulin signalling pathways(Reference Tian, Wang and Liu17). In addition, the water extract of mulberry leaf can also regulate the balance of Ca and redox through parathormone/vitamin D receptor/Ca-binding protein and advanced glycation end products/receptor of advanced glycation end products/NADPH oxidase 4/NF-κB signalling pathway, improving diabetic osteoporosis(Reference Liu, Zhu and Liu18). However, it is not clear whether mulberry leaf can activate BAT and induce WAT browning in T2DM rats by regulating the AMPK signalling pathway to improve energy metabolism and insulin resistance. Therefore, to examine the possible application of mulberry leaf as a candidate anti-T2DM browning agent, this study focused on how mulberry leaf influences glucose and lipid metabolism and how it induces fat browning in T2DM rats.
Materials and methods
Preparation of mulberry leaf extracts
Mulberry leaf was purchased from Beijing Tong Ren Tang Co. Ltd (Beijing, China). The preparation of mulberry leaf extract follows the previous method(Reference Tian, Wang and Liu17). Briefly, 1 kg of grinded raw Mulberry leaf was soaked in 12 litre distilled water for 10 h at 85°C for twice, followed by filtering and concentrating the supernatants to 0·8 g crude drug/ml under vacuum. In our previous study, the main components of mulberry leaf were identified as isochlorogenic acid, 5,7-dihydroxycoumarin-7-O-β-D-glucopyranoside, scopolin, chlorogenic acid, kaempferol-3,7-di-O-D-glucopyranoside, 4-caffeoylquinic acid methyl ester, rutin, hyperoside, isoquercitrin, astragalin and isorhamnetin-3-O-glucopyranoside by HPLC-MS/MS(Reference Tang, Tian and Yang19).
Animals and treatments
Six-week-old male Sprague–Dawley rats were purchased from SPF (Beijing) Biotechnology Co. Ltd, Licence No. SCXK (Jing) 2016-0002. Rats were raised in a specific pathogen-free animal laboratory affiliated to the Experimental Animal Center of Beijing University of Chinese Medicine, Licence No. SYXK (Jing) 2016-0038. The animal protocol in this study was reviewed and approved by the medical and experimental animal ethics committee of Beijing University of Chinese medicine (No: BUCM-4-2019101202-4101).
Rats were acclimated at 22~24°C, 60~70% relative humidity and with a 12 h light–12 h dark cycle for 1 week prior to the experiments, provided with standard laboratory diet and water ad libitum. Then, they were randomly divided into two groups according to weight and fed with normal control diet (n 8) or a high-sugar/high-fat diet (The formula contained 63·6 % (w/w) basic feed, 15 % (w/w) lard, 20 % (w/w) sucrose, 1·2 % (w/w) cholesterol and 0·2 % (w/w) sodium cholate) for 4 weeks.
After 4 weeks of feeding, fasting and drinking for 12 h, 1 % streptozotocin (Sigma) was intraperitoneally injected with citric acid buffer solution (0·1 mmol/l, pH = 4·2–4·5, 4°C), with a dose of 35 mg/kg. Rats of control group were intraperitoneally injected with the same amount of citrate buffer solution. On the 7th day after injection, the random blood glucose of the high-sugar and high-fat diet group rats was detected for two successive days. The rats with random blood glucose higher than 11·1 mmol/l were used in the following experiments. The other rats were excluded for the subsequent analyses.
The included rats were randomly divided into four groups as follows (n 8/group): T2DM group, low-dose mulberry leaf extract (LMLE, 2·0 g crude drug/kg) treatment group, high-dose mulberry leaf extract (HMLE, 4·0 g crude drug/kg) treatment group and metformin (200 mg/kg) treatment group. Rats in normal group and T2DM group were administrated with equal amount of double distilled water. All rats were administered for 8 weeks through oral gavage once daily. BW, fasting blood glucose, food and water intake were measured once a week. The flow chart of the experiment is shown in Fig. 1(a).
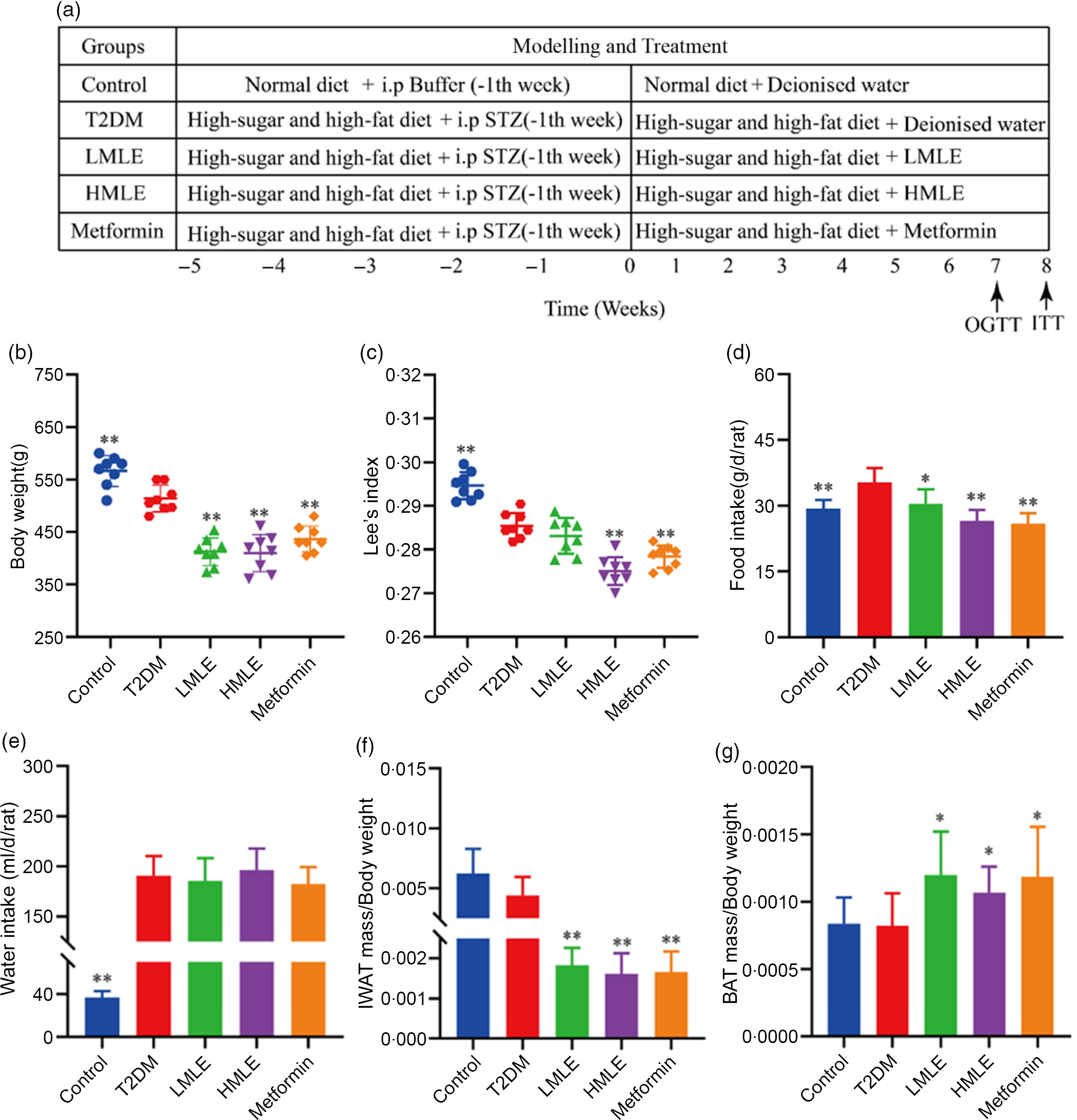
Fig. 1 Effects of mulberry leaf on body weight, food intake, water intake and adipose tissue mass in type 2 diabetes mellitus (T2DM) rats. (a) Flow chart of experiment. (b) Body weight. (c) Lee’s index. (d) Food intake. (e) Water intake. (f) The ratio of inguinal white adipose tissue (IWAT) mass:BW. (g) The ratio of BAT mass:BW. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 8). *P < 0·05, **P < 0·01 v. T2DM group.
Oral glucose tolerance test and insulin tolerance test
At the 7th week of administration, all rats were fasted and free of water overnight and then oral glucose tolerance test was performed by intragastric administration of glucose solution (2·0 g/kg). At the 8th week, all rats were fasted and free for water for 4 h and then insulin tolerance test (ITT) was carried out by intraperitoneally injected with insulin (0·75 U/kg). Blood glucose was, respectively, measured before (0 min) and at 15, 30, 60 and 120 min after oral glucose and insulin injection. The blood glucose curve of each group was drawn at different times in two experiments. The AUC was calculated by blood glucose level.
Lee’s index measurement
At the end of the treatment, the BW of the rats was accurately weighed and the body length (the distance from the tip of the nose to the anus) was accurately measured, and then the Lee’s index was calculated according to the reference(Reference Bernardis and Patterson20). Lee’s index = BW (g)^(1/3)/Nose–anal length (cm).
Adipose tissue mass/body weight measurement
Bilateral inguinal white adipose tissue (IWAT) and scapular BAT of all rats after kill were dissected, and IWAT and BAT were accurately weighed using an analytical balance with an accuracy of one thousandth. The ratios of IWAT mass:BW (IWAT:BW) and BAT mass:BW (BAT:BW) were calculated.
Biochemical analysis
Serum TC, TAG, LDL-cholesterol and HDL-cholesterol were measured by biochemical kits (Nanjing Jiancheng). Serum aspartate aminotransferase and alanine aminotransferase were measured by biochemical kits (Shanghai Yuanye). Serum NEFA and insulin were detected by ELISA kit (Kete). We accurately weighed the liver tissue, homogenised 0·1 g of liver tissue in 0·9 ml ethanol and centrifuged at 2500 rpm for 10 min at 4°C. The concentrations of TC and TAG in liver were determined by biochemical kits (Nanjing Jiancheng) and expressed as millimoles of lipids per g of tissue.
Histology and immunohistochemistry analysis
The frozen liver tissues were cut into 10 μm thick sections and mounted on slides, air-dried and then fixed in ice-cold 4 % paraformaldehyde solution for 15 min. Slides were rinsed with distilled water and then dried. Slides were stained in oil red O working solution for 8–10 min and then rinsed in distilled water. The nuclei were stained with haematoxylin. After rinsing the slides with distilled water, the slides were fixed with glycerin gelatin. The percentage of lipid droplets in the liver stained with oil red O was determined by using Image J.
Pancreas, IWAT and BAT fixed in 10 % formalin were embedded in paraffin and sectioned. Multiple sections were prepared and stained with haematoxylin–eosin (H&E) for morphological observation of pancreas and adipose tissues. Immunohistochemistry was performed to detect the expression of UCP1 (1:500, ab10983; Abcam) in IWAT and BAT of rats. Images were acquired using an inverted microscope (Olympus).
Quantitative real-time PCR analysis
Total RNA of IWAT and BAT was extracted with a Trizol® Reagent (Ambion). Reverse transcription of total RNA (1 μg) was performed with a Revert Aid First Stand cDNA Synthesis Kit (Thermo Scientific). Real-time quantitative PCR was performed with a SYBR Green Master Mix (Novoprotein). The PCR was run in triplicate for each sample using the Step One Real-Time PCR System (Applied Biosystems). After standardising the expression level of internal control actin in each sample, the data were expressed in arbitrary units. The sequences of primer in this study are shown in Table 1.
Table 1 Primer sequences were used for quantitative real-time reverse transcription PCR (qRT-PCR)
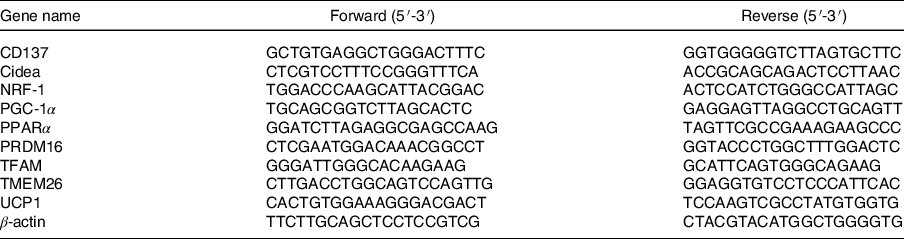
Cidea, cell death inducing DFFA like effector A; NRF-1, nuclear respiratory factor 1; PGC-1α, PPAR gamma coactivator 1 alpha; PRDM16, PRD1-BF-1-RIZ1 homologous domain containing protein 16; TFAM, mitochondrial transcription factor A; TMEM26, transmembrane protein 26; UCP1, uncoupling protein-1.
Western bolt analysis
The homogenised tissue was lysed in RIPA Lysis Buffer (Strong) containing protease and phosphatase inhibitors. BCA protein detection kit (Beyotime) was used to detect protein concentration. Total protein (10 μg) of each sample was added to SDS–PAGE gel to separate protein by electrophoresis and transferred to the PVDF membrane (Millipore). The PVDF membrane containing protein was incubated in a closed solution for 2 h. The PVDF membrane was incubated in the required primary antibodies, including AMPK antibody (1:1000, ab207442; Abcam), p-AMPK antibody (1:2000, ab23875; Abcam), PPAR gamma coactivator 1 alpha (PGC-1α) antibody (1:1000, ab72230; Abcam), Carnitine palmitoyl transferase 1 (CPT-1) antibody (1:1000, ab234111; Abcam), UCP1 antibody (1:1000, ab10983; Abcam) and α-tubulin antibody(1:5000, ab7291; Abcam) and incubated overnight at 4°C. After incubating with HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H + L) (1:5000, proteintech) for 2 h, the PVDF membrane was washed with washing solution TBST, treated with chemiluminescence reagent and exposed and photographed. Western blot bands were quantified using Image-Pro-Plus 6.0.
Statistical analysis
All data in this study were statistically analysed by using the SAS 8.2 software and expressed as the mean and standard deviation. For ITT or GTT, the results were analysed with one-way ANOVA repeated test. Other statistical analysis was performed using the one-way ANOVA with Tukey’s test. P-value < 0·05 was considered as statistically significant.
Results
Mulberry leaf reduces the body weight, Lee’s index, food intake and the ratio of inguinal white adipose tissue:body weight and increases the ratio of brown adipose tissue:body weight in type 2 diabetes mellitus rats
Initially, we analysed the effects of mulberry leaf on BW, water intake, food intake and adipose tissue content of T2DM rats. Compared with normal rats, the BW and Lee’s index of T2DM rats were significantly decreased (P < 0·01) and the food and water intake were notably increased (P < 0·01), which was consistent with the clinical symptoms of T2DM. When compared with T2DM rats, HMLE significantly reduced the BW (Fig. 1(b)), Lee’s index (Fig. 1(c)), food intake (Fig. 1(d)) and the ratio of IWAT:BW (Fig. 1(f)) to 20·9 %, 3·7 %, 24·6 % and 63·6 % (P < 0·01), respectively; LMLE treatment significantly reduced the BW, food intake and the ratio of IWAT:BW to 19·5 %, 13·9 % and 59·0 % (P < 0·05 or P < 0·01), respectively. HMLE, LMLE and metformin significantly increased the ratio of BAT:BW (P < 0·05) (Fig. 1(g)). However, HMLE, LMLE and metformin had no effect on water intake of T2DM rats (Fig. 1(e)).
Mulberry leaf ameliorates glucose metabolism disorder and insulin resistance in type 2 diabetes mellitus rats
To explore the effect of mulberry leaf on the pancreas of T2DM rats, we analysed the pancreatic tissue by H&E staining and detected the serum insulin level (Fig. 2(a) and 2(e)). H&E staining results showed that compared with normal control group, the islets of T2DM group were atrophied, the number of islet cells was significantly reduced and the islet cell arrangement was disordered. Compared with the T2DM group, the islet structure of the rats in the mulberry leaf treatment group was relatively complete, without obvious abnormalities, and only a few islet cells were necrotic. It suggested that mulberry leaf could protect pancreatic islet cells from injury. The results showed that the serum insulin level of T2DM group was significantly higher than that of normal control group and the serum insulin level of the rats in the mulberry leaf treatment group was significantly lower than that of the T2DM group.
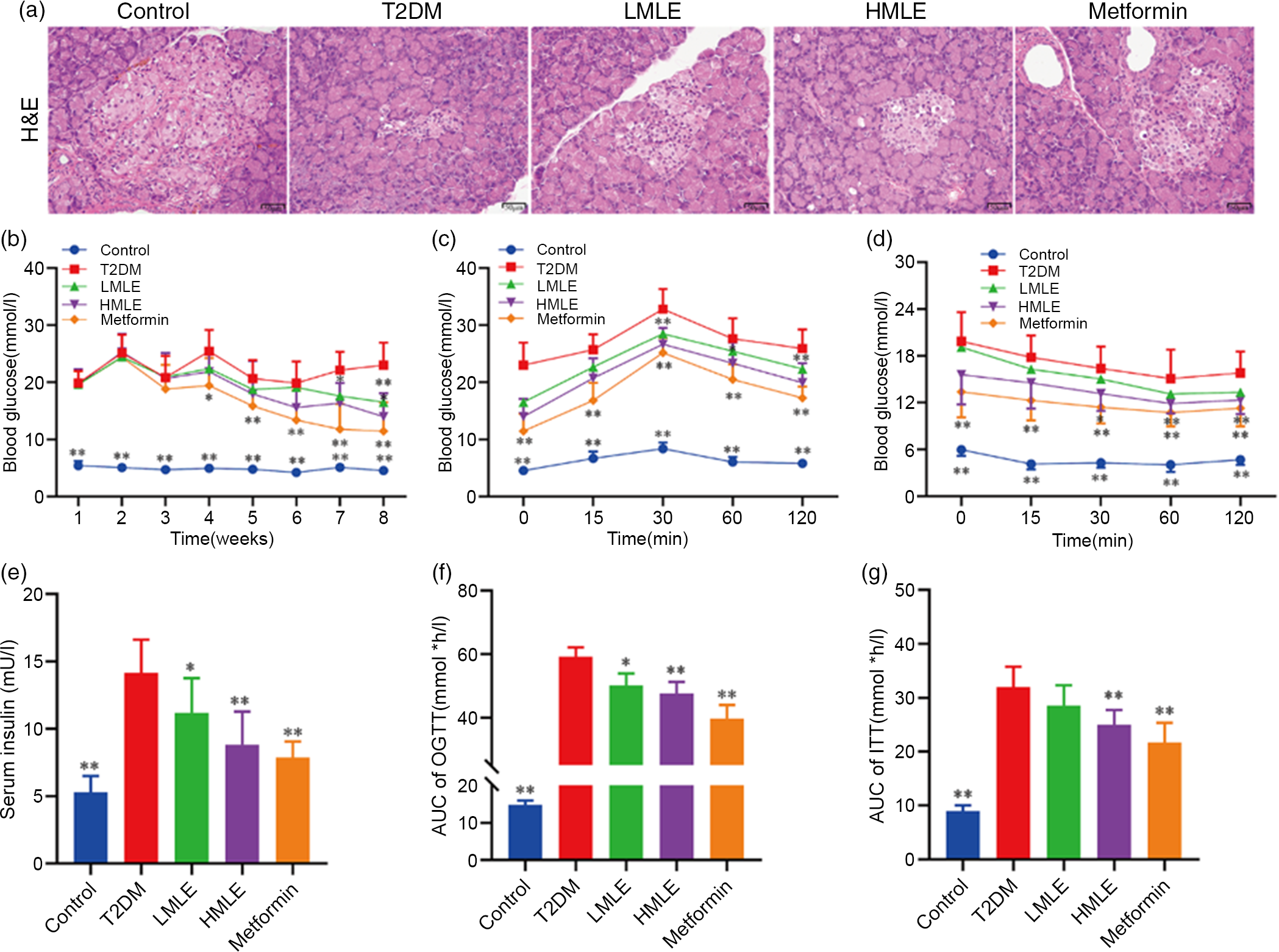
Fig. 2 Effects of mulberry leaf on blood glucose in type 2 diabetes mellitus (T2DM) rats. (a) Haematoxylin–eosin (H&E) staining of pancreas (40× magnification; scale bar = 50 μm). (b) Weekly blood glucose. (c) Oral glucose tolerance test (OGTT). Glucose levels were measured before (0) and at 15, 30, 60 and 120 min after fed glucose. (d) Insulin resistance test (ITT). Glucose levels were measured before (0) and at 15, 30, 60 and 120 min after insulin injection. (e) Serum insulin concentration. (f) Quantification of AUC from the OGTT. (g) Quantification of AUC from the ITT. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 8). *P < 0·05, **P < 0·01 v. T2DM group.
To test if mulberry leaf-treated rats exhibited blood glucose alterations, we measured the blood glucose of rats weekly in each group after administration (Fig. 2(b)). As compared with normal rats, the blood glucose of T2DM rats was notably increased (P < 0·01) and the average weekly blood glucose was much higher than 11·1 mmol/l, indicating that the model was successful and stable. When compared with T2DM rats, HMLE significantly reduced blood glucose of T2DM rats to 26·1 % and 39·2 % (P < 0·01 or P < 0·05) at 7th and 8th week, respectively, and LMLE significantly reduced blood glucose to 28·1 % (P < 0·01) at 8th week.
To examine whether mulberry leaf altered glucose metabolism in T2DM rats, the oral glucose tolerance test and ITT were investigated and the time–blood glucose curve of each group was drawn (Fig. 2(c), (d), (f) and (g)). The glucose levels in the T2DM group were significantly higher than those of the control group during the oral glucose tolerance test and ITT. Compared with normal rats, T2DM rats showed impaired glucose tolerance and increased insulin resistance and AUC index increased significantly (P < 0·01). After treatment with HMLE and metformin, AUC index of oral glucose tolerance test and ITT significantly decreased compared with T2DM rats. These results suggested that mulberry leaf could maintain glucose homoeostasis and improve the insulin sensitivity in T2DM rats.
Mulberry leaf improves the lipid levels of liver and serum in type 2 diabetes mellitus rats
We validated the effects of mulberry leaf on lipolysis and liver function in vivo. As we expected, the results of liver oil red O showed that compared with normal group, the intrahepatic lipid droplets of T2DM rats increased significantly; compared with T2DM rats, the lipid droplets in the liver of mulberry leaf-treated rats decreased significantly (Fig. 3(a) and (b)). Furthermore, we also detected the level of TC and TAG in the liver and the level of aspartate aminotransferase and alanine aminotransferase in the serum (Fig. 3(c), (d), (e) and (f)). Compared with normal group, the level of TC and TAG in the liver and the level of aspartate aminotransferase and alanine aminotransferase in the serum of rats in T2DM group were significantly increased. Compared with T2DM rats, the level of TC and TAG in the liver and the level of aspartate aminotransferase and alanine aminotransferase in the serum of rats in mulberry leaf treatment groups were significantly decreased (P < 0·01 or P < 0·05).
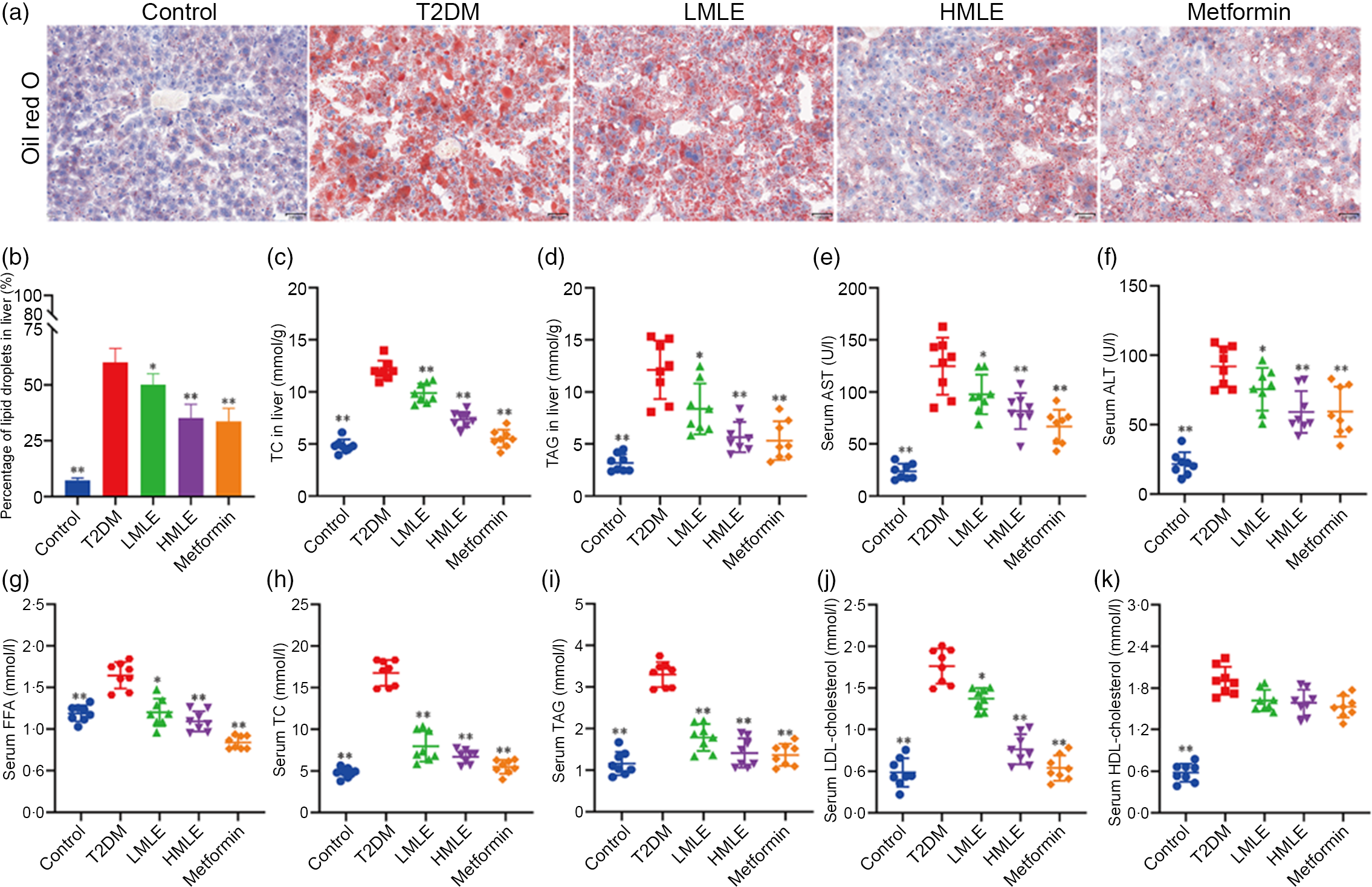
Fig. 3 Effects of mulberry leaf on lipid level in type 2 diabetes mellitus (T2DM) rats. (a) Oil red O staining of liver tissues (20× magnification; scale bar = 100 μm). (b) The proportion of lipid droplets in the liver. (c) Liver total cholesterol (TC) concentration. (d) Liver TAG concentration. (e) Serum aspartate aminotransferase (AST) concentration. (f) Serum alanine aminotransferase (ALT) concentration. (g) Serum NEFA concentration. (h) Serum TC concentration. (i) Serum TAG concentration. (j) Serum LDL-cholesterol concentration. (k) Serum HDL-cholesterol concentration. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 8). *P < 0·05, **P < 0·01 v. T2DM group.
Next, we investigated the effect of mulberry leaf on blood lipid metabolism in T2DM rats by measuring the concentrations of NEFA, TC, TAG, LDL-cholesterol and HDL-cholesterol in serum (Fig. 3(g), (h), (i), (j) and (k)). Lipid examination showed that the levels of NEFA, TC, TAG, LDL-cholesterol and HDL-cholesterol in the T2DM group were markedly higher than those in the control group (P < 0·01). When compared with T2DM rats, HMLE, LMLE and metformin significantly reduced the levels of NEFA, TC, TAG and LDL-cholesterol in serum (P < 0·01 or P < 0·05). However, no obvious difference was found in serum HDL-cholesterol in the treatment groups and T2DM group rats. In summary, these results suggested that mulberry leaf could improve the lipid metabolism disorder and liver function by increasing the lipolysis of T2DM rats.
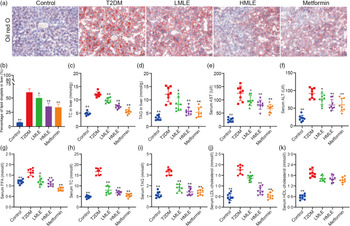
Mulberry leaf induces browning of inguinal white adipose tissue in type 2 diabetes mellitus rats
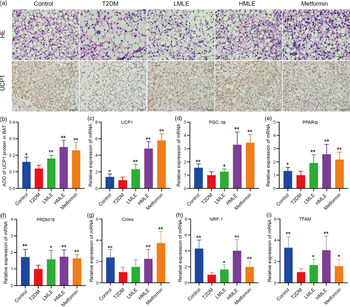
To confirm the effect of mulberry leaf on the morphology and function of IWAT, the H&E staining and immunohistochemical analysis of IWAT in rats were performed (Fig. 4(a)). The H&E staining and immunohistochemistry showed that rats in T2DM group had larger lipid droplets and the low average optical density of UCP1 protein in IWAT after several months of high-sugar and high-fat diet challenge (Fig. 4(b)). Consistent with the reduction in IWAT mass, the T2DM rats treated by mulberry leaf had smaller adipocytes in IWAT, which suggested that mulberry leaf significantly reduced the lipid accumulation. The IWAT of mulberry leaf-treated rats showed great amount clusters of UCP1-expressing multilocular adipocytes by UCP1 immunohistochemistry.
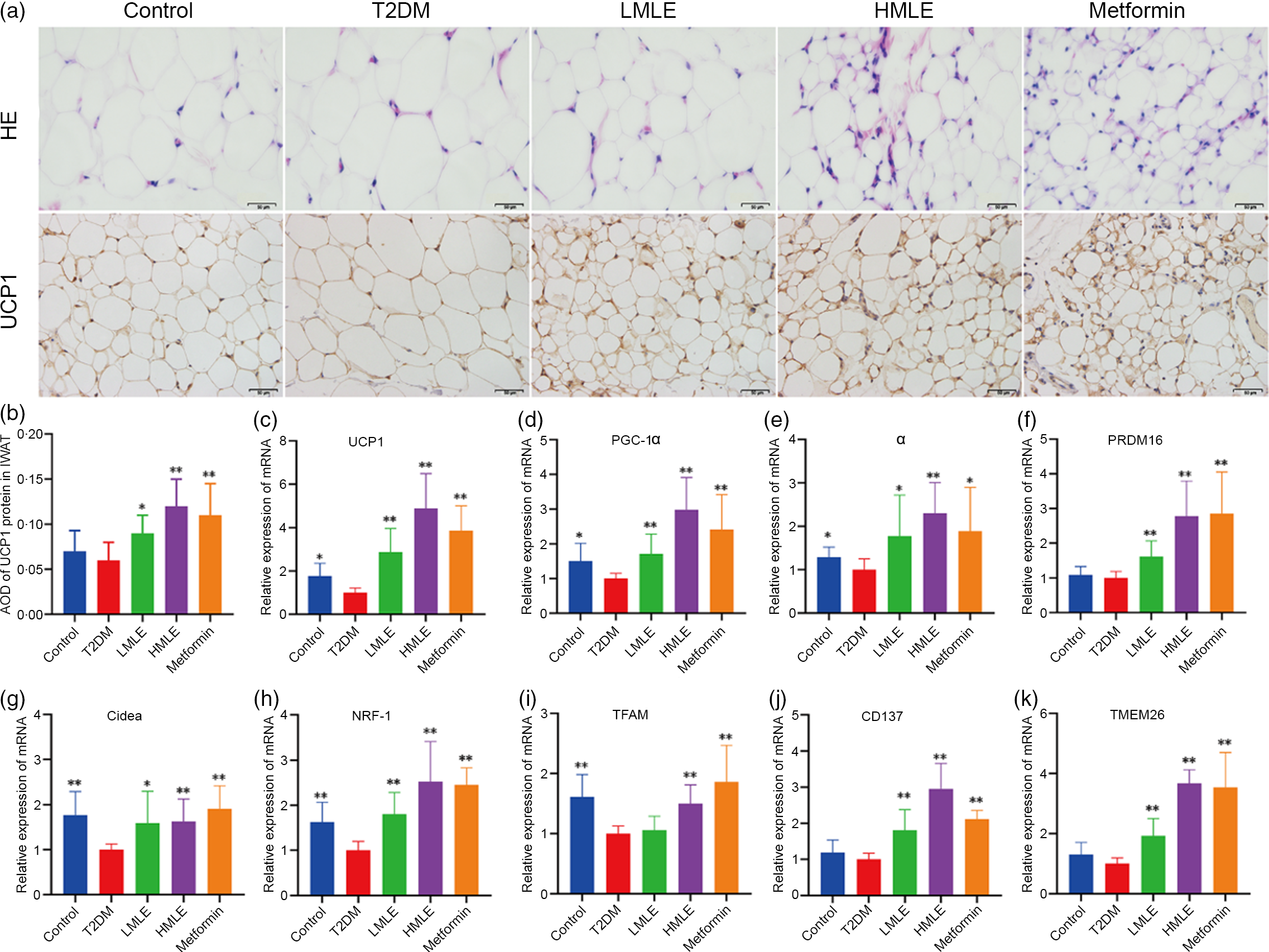
Fig. 4 Effects of mulberry leaf on the morphology and function of inguinal white adipose tissue (IWAT) in type 2 diabetes mellitus (T2DM) rats. (a) Haematoxylin–eosin (H&E) staining in IWAT and immunohistochemical analysis of uncoupling protein-1 (UCP1) in IWAT (40× magnification; scale bar = 50 μm). (b) Average optical density (AOD) of UCP1 protein in IWAT. (c) Relative expression of UCP1 mRNA in IWAT. (d) Relative expression of PPAR gamma coactivator 1 alpha (PGC-1α) mRNA in IWAT. (e) Relative expression of PPARα mRNA in IWAT. (f) Relative expression of PRD1-BF-1-RIZ1 homologous domain containing protein 16 (PRDM16) mRNA in IWAT. (g) Relative expression of cell death inducing DFFA like effector A (Cidea) mRNA in IWAT. (h) Relative expression of NRF-1 mRNA in IWAT. (i) Relative expression of mitochondrial transcription factor A (TFAM) mRNA in IWAT. (j) Relative expression of CD137 mRNA in IWAT. (k) Relative expression of transmembrane protein 26 (TMEM26) mRNA in IWAT. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 3). *P < 0·05, **P < 0·01 v. T2DM group.
To investigate the effect of mulberry leaf on adaptive thermogenesis of IWAT in T2DM rats, we first evaluated the mRNA expression of brown/beige adipocyte marker genes, such as UCP1, PGC-1α, PPARα, PRD1-BF-1-RIZ1 homologous domain containing protein 16 (PRDM16), cell death inducing DFFA-like effector A (Cidea), CD137 and transmembrane protein 26 (TMEM26). As expected, the expression of several brown adipocyte marker genes, including UCP1, PGC-1α, PPARα, PRDM16 and Cidea, was significantly up-regulated in IWAT after mulberry leaf treatment (Fig. 4(c), (d), (e), (f) and (g)). The expression of beige adipocyte marker genes, such as CD137 and TMEM26, was also markedly increased in IWAT from mulberry leaf-treated rats (Fig. 4(j) and (k)). Importantly, we next investigated the expression of Nuclear respiratory factor 1 (NRF-1) and mitochondrial transcription factor A (TFAM) of IWAT in mulberry leaf treated and found that it was notably enhanced after HMLE treatment (Fig. 4(h) and 4(i)), which suggested that mulberry leaf increased mitochondrial biogenesis of IWAT.
Mulberry leaf activates brown adipose tissue in type 2 diabetes mellitus rats
Meanwhile, we also investigated the effects of mulberry leaf on BAT morphology and function by H&E staining and immunohistochemical analysis (Fig. 5(a)). As indicated in H&E staining, the sizes of brown adipocytes in the T2DM group rats were markedly larger than those in the control group rats and brown adipocytes in the T2DM group rats have a tendency to transform from multilocular adipocytes to unilocular ones. Consistent with the reduction of adipocyte diameter in IWAT, the T2DM rats treated by mulberry leaf had smaller brown adipocytes compared with T2DM group rats. As in Fig. 5(b), the results of immunohistochemistry showed that the expression of UCP1 protein in BAT of T2DM group rats was significantly decreased compared with the control rats (P < 0·05). Consistently, immunohistochemical analysis revealed that the expression of UCP1 protein in BAT of mulberry leaf-treated rats was notably higher than those of T2DM group rats (P < 0·01).
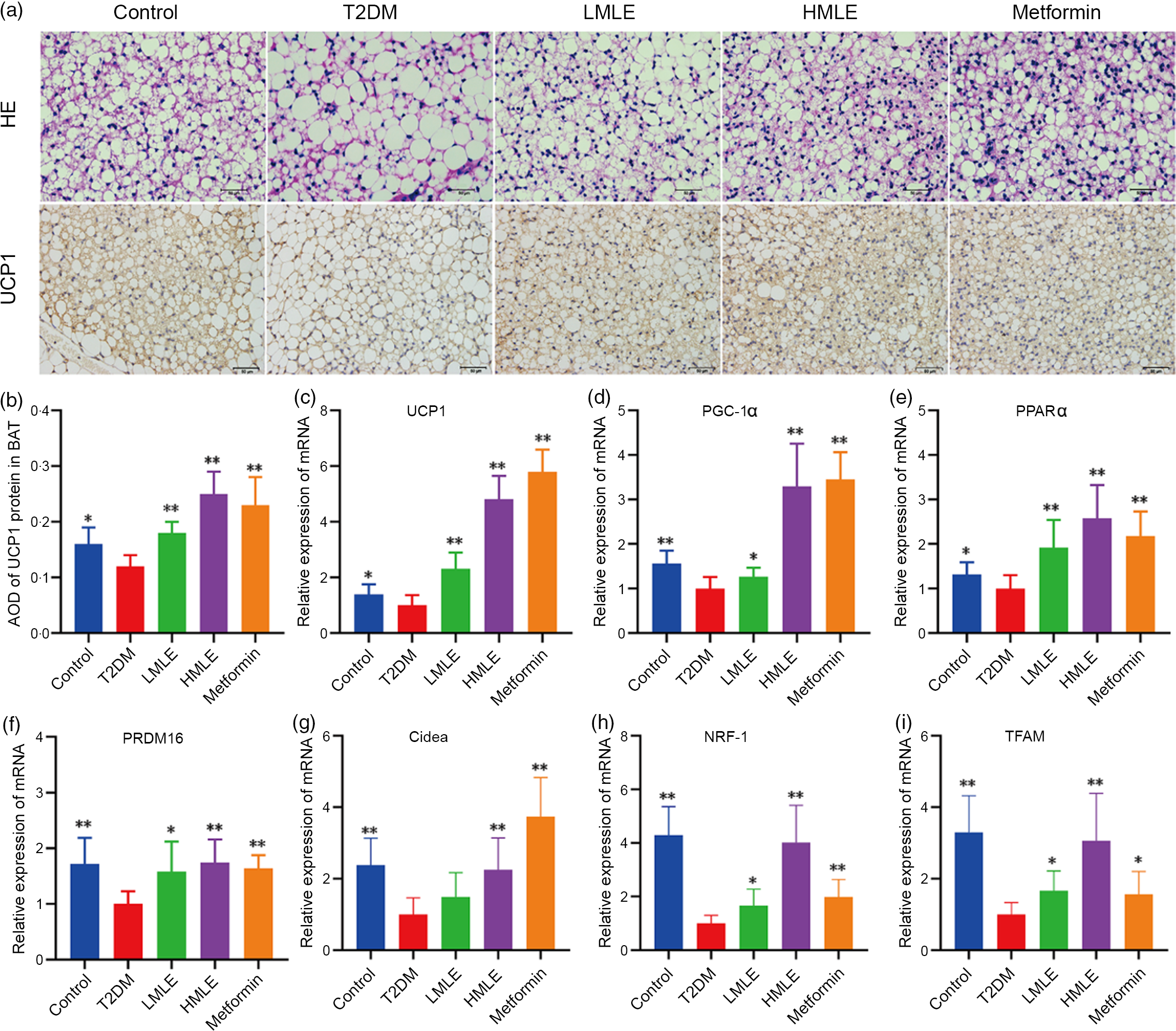
Fig. 5 Effects of mulberry leaf on the morphology and function of brown adipose tissue (BAT) in type 2 diabetes mellitus (T2DM) rats. (a) Haematoxylin–eosin (H&E) staining in BAT and immunohistochemical analysis of uncoupling protein-1 (UCP1) in BAT (40× magnification; scale bar = 50 μm). (b) Average optical density (AOD) of UCP1 protein in BAT. (c) Relative expression of UCP1 mRNA in BAT. (d) Relative expression of PPAR gamma coactivator 1 alpha (PGC-1α) mRNA in BAT. (e) Relative expression of PPARα mRNA in BAT. (f) Relative expression of PRD1-BF-1-RIZ1 homologous domain containing protein 16 (PRDM16) mRNA in BAT. (g) Relative expression of cell death inducing DFFA like effector A (Cidea) mRNA in BAT. (h) Relative expression of NRF-1 mRNA in BAT. (i) Relative expression of mitochondrial transcription factor A (TFAM) mRNA in BAT. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 3). *P < 0·05, **P < 0·01 v. T2DM group.
Based on the above findings, we next investigated the influences of mulberry leaf on BAT activity by the expression of brown adipocyte-specific marker genes (UCP1, PGC-1α, PPARα, PRDM16 and Cidea). Consistent with the activation of thermogenic genes in IWAT, mulberry leaf induced the activation of thermogenesis-regulating genes in BAT. As shown in Fig. 5(c), (d), (e), (f) and (g), mulberry leaf apparently increased the expression of brown adipocyte marker genes (UCP1, PGC-1α, PPARα, PRDM16 and Cidea) in BAT compared with the T2DM group rats (P < 0·01 or P < 0·05). Moreover, the expression of mitochondrial biosynthesis-related genes, including NRF-1 and TFAM in BAT, was strongly activated by mulberry leaf (Fig. 5(h) and (i)).
Mulberry leaf induces browning of inguinal white adipose tissue through AMP-activated protein kinase signalling pathway in type 2 diabetes mellitus rats
As a sensor or gauge of cellular energy, AMPK plays a critical role in modulating energy homoeostasis by regulation of critical metabolic and signalling pathways(Reference Chung, Ahmad and Tang21). Current evidence has implicated AMPK in the hypothalamus, and hindbrain is related to feeding, BAT thermogenesis and browning of WAT through modulation of the sympathetic nervous system and glucose homoeostasis(Reference López, Nogueiras and Tena-Sempere22). We attempted to investigate whether mulberry leaf could regulate AMPK signalling pathway to induce browning of IWAT in T2DM rats. Further western blot assays were performed to evaluate the effect of mulberry leaf on regulating AMPK signalling pathway (Fig. 6(a)). As expected, protein levels of the p-AMPK, PGC-1α, CPT-1 and UCP1 in IWAT were simultaneously up-regulated by mulberry leaf (Fig. 6(b), (c), (d) and (e)), indicating that a browning effect was induced by mulberry leaf.
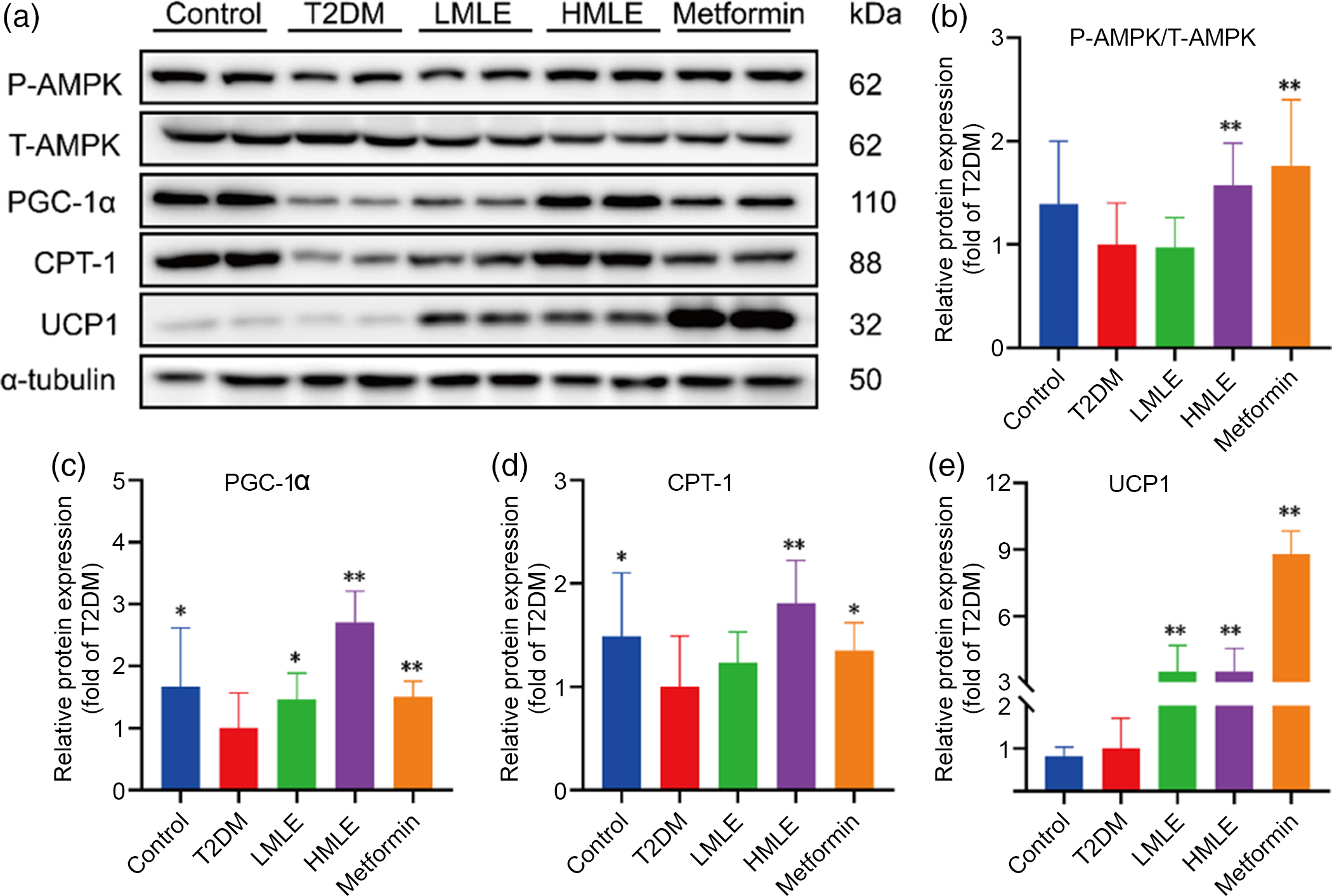
Fig. 6 Effects of mulberry leaf on AMP-activated protein kinase (AMPK) signalling pathway of inguinal white adipose tissue (IWAT) in type 2 diabetes mellitus (T2DM) rats. (a) Western blotting assays were performed to measure the changes of p-AMPK, T-AMPK, PPAR gamma coactivator 1 alpha (PGC-1α), carnitine palmitoyl transferase 1 (CPT-1) and uncoupling protein-1 (UCP1) proteins in IWAT. (b) Relative expression of p-AMPK/T-AMPK protein in IWAT. (c) Relative expression of PGC-1α protein in IWAT. (d) Relative expression of CPT-1 protein in IWAT. (e) Relative expression of UCP1 protein in IWAT. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 3). *P < 0·05, **P < 0·01 v. T2DM group.
Mulberry leaf activates brown adipose tissue through AMP-activated protein kinase signalling pathway in type 2 diabetes mellitus rats
In accordance with above findings, we hypothesised that AMPK might also play a role in brown adipogenesis under mulberry leaf treatment. Next, we further evaluated the mulberry leaf-induced protein changes of AMPK signalling pathway in BAT (Fig. 7(a)). Consistent with the IWAT, mulberry leaf also enhanced the protein levels of p-AMPK, PGC-1α, CPT-1 and UCP1 in BAT of T2DM rats (Fig. 7(b), (c), (d) and (e)), suggesting that the activation of mulberry leaf on AMPK signalling pathway is essential to increase BAT activity.
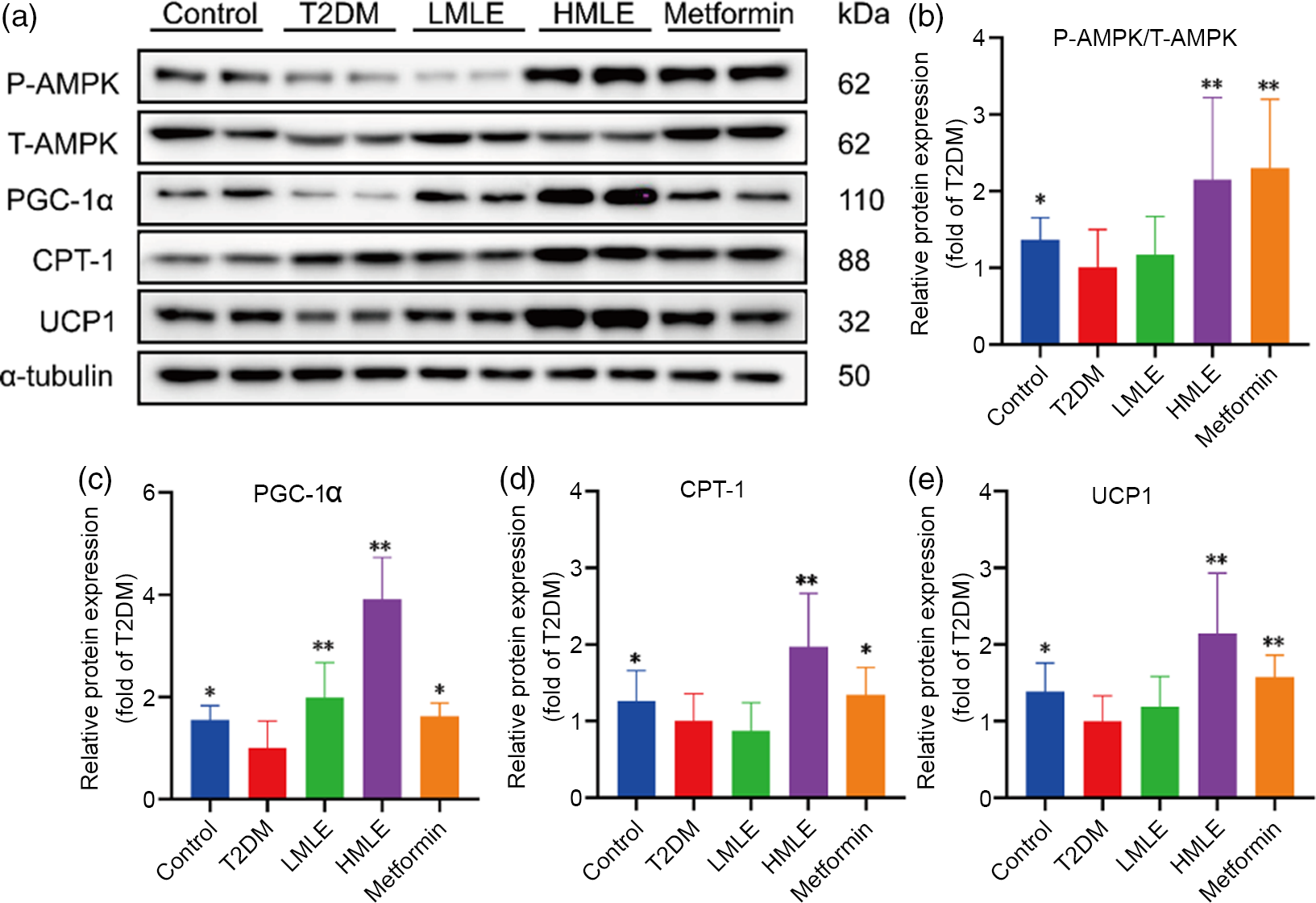
Fig. 7 Effects of mulberry leaf on AMP-activated protein kinase (AMPK) signalling pathway of brown adipose tissue (BAT) in type 2 diabetes mellitus (T2DM) rats. (a) Western blotting assays were performed to measure the changes of p-AMPK, T-AMPK, PPAR gamma coactivator 1 alpha (PGC-1α), carnitine palmitoyl transferase 1 (CPT-1) and uncoupling protein-1 (UCP1) proteins in BAT. (b) Relative expression of p-AMPK/T-AMPK protein in BAT. (c) Relative expression of PGC-1α protein in BAT. (d) Relative expression of CPT-1 protein in BAT. (e) Relative expression of UCP1 protein in BAT. LMLE, mulberry leaf extract (2·0 g crude drug/kg); HMLE, mulberry leaf extract (4·0 g crude drug/kg). Data were shown as mean and standard deviation (n 3). *P < 0·05, **P < 0·01 v. T2DM group.
Discussion
T2DM is a common chronic disease that is closely related to overweight and obesity, ageing, ethnicity and family history(1). In recent years, activating BAT and inducing WAT of browning have generated growing interest as a strategy against T2DM, obesity and related metabolic diseases. Generally, classic brown adipocytes and beige adipocytes enhance energy consumption by thermogenesis, thereby preventing the development of obesity and type 2 diabetes. Recently, it was reported that activation of thermogenic beige adipocytes can also take place in human adults pointed to browning of WAT as a promising therapeutic target in obesity, T2DM and related metabolic diseases(Reference Min, Kady and Nam23,Reference Guénantin, Briand and Capel24) . In the present study, we explored that mulberry leaf improved glucose and lipid metabolism disorders in T2DM rats by induced adipose browning, in order to determine the potential of mulberry leaf as a candidate anti-T2DM browning agent.
Generally, the important characteristics of T2DM are hyperglycaemia, hyperlipidaemia, impaired glucose tolerance and insulin resistance. In the current study, the T2DM rats showed evident hyperglycaemia, hypertriacylglycerolaemia, glucose intolerance, insulin resistance, islet cell atrophy and abnormal liver function. However, these changes were significantly ameliorated after mulberry leaf treatment. These are consistent with the previous studies(Reference Cai, Sun and Fan9,Reference Liu, Ma and Zhang11) . Lee’s index can be used as an index to evaluate the obesity degree of adult obese rats(Reference Bernardis and Patterson20). The present work revealed that mulberry leaf reduced BW and Lee’s index, which may result from inhibited the food intake of T2DM rats. It has been found that gut hormone secretin as a non-sympathetic BAT activator mediating prandial thermogenesis, which consequentially induces satiation(Reference Li, Schnabl and Gabler25). Mechanistically, meal-associated rise in circulating secretin activates BAT thermogenesis by stimulating lipolysis upon binding to secretin receptors in brown adipocytes, which is sensed in the brain and promotes satiation(Reference Li, Schnabl and Gabler25). Therefore, BAT can be considered as the tissue that causes satiety, which may be the reason for the decreased food intake of rats in the HMLE treatment group.
It has been previously shown that the reduced WAT mass was associated with smaller adipocyte size, a feature that correlates with insulin sensitivity(Reference Zhang, Zhang and Li26). In this study, the results unravelled the potential improvement role of mulberry leaf on insulin sensitivity by evident reductions in the ratio of IWAT mass:BW and sizes of adipocytes in T2DM rats. Moreover, the ratio of BAT mass:BW was significantly increased in T2DM rats after mulberry leaf treatment. These results are consistent with previous studies that cold exposure may be a potential therapy for diabetes by increasing BAT mass(Reference Hanssen, Hoeks and Brans27).
It has been reported that brown-like adipocytes within WAT differ from classic white adipocytes both by morphology and function(Reference Wu, Boström and Sparks28,Reference Sidossis and Kajimura29) . Beige adipocytes resemble brown adipocytes in having extremely high expression of UCP1 and thermogenesis by certain stimulation, such as cold induction(Reference Lim, Honek and Xue30,Reference Yao, Cui and Chen31) or pharmacological activation(Reference Merlin, Sato and Chia32). An important finding from the present study is the appearance of brown-like cells with multilocular lipid droplets and high expression of UCP1 protein in IWAT by mulberry leaf treatment. In parallel, the T2DM rats treated by mulberry leaf had smaller brown adipocytes and more expression of UCP1 protein in BAT. From the findings above, we speculated that mulberry leaf plays a significant role in activating BAT and inducing browning of WAT in vivo. Consistent with our hypothesis, the results demonstrated that the expression of several brown adipocyte marker genes, including UCP1, PGC-1α, PPARα, PRDM16 and Cidea, was significantly up-regulated in both IWAT and BAT after mulberry leaf treatment. As a hub linking nutritional signal, hormonal signal and energy metabolism, PGC-1α regulates mitochondrial biogenesis, adaptive thermogenesis and oxidative metabolism in BAT, where it is typically expressed, and binds to complexes of PPARα, which activates UCP1 expression by binding to a PPAR response element in the UCP1 promoter(Reference Bartelt and Heeren3,Reference Liu and Lin33) .
In this study, the results demonstrated that the expression of PGC-1α of IWAT increased in T2DM rats treated with mulberry leaf indicating the transformation of white adipocytes into Beige cells, which confirmed that PGC-1α, a transcription regulator of mitochondrial biogenesis, plays a significant role in fat browning. Moreover, PGC-1α interacts with other nuclear transcription factors such as PRDM16, NRF-1 and NRF-2 to enhance UCP1 expression; subsequently, the induction of NRF-1 and NRF-2 leads to the increased expression of TFAM(Reference Wu, Puigserver and Andersson34). Consistent with the previous report, the results demonstrated that mulberry leaf could increase the expression of mitochondrial-related genes NRF-1 and TFAM in both IWAT and BAT. It has been previously shown that the UCP1-positive cells contained CD137 and TMEM26 from the inguinal depot, but not from the interscapular brown adipose depot, indicating that CD137 and TMEM26 are marker genes of beige adipocytes(Reference Wu, Boström and Sparks28). Importantly, the study presented here demonstrated that mulberry leaf-mediated induction of beige mark genes, including CD137 and TMEM26, was all robustly increased in IWAT compared with the T2DM group rats.
AMPK is considered as a well-known sensor and regulator of energy metabolism and mitochondrial biogenesis(Reference Steinberg and Carling35). In skeletal muscle, AMPK can also directly phosphorylate PGC1α, increasing glucose uptake, fatty acid oxidation and mitochondrial biogenesis(Reference Jäger, Handschin and St-Pierre36). It has been found that an involvement of the AMPK pathway in browning, for example, berberine(Reference Zhang, Zhang and Li26), resveratrol(Reference Wang, Liang and Yang37) and 6-Gingerol(Reference Wang, Zhang and Dong38), promotes browning via an AMPK-dependent pathway. Consistent with previous studies, our results demonstrated that AMPK plays a key role in inducing browning induced by mulberry leaf. Mulberry leaf simultaneously enhanced the protein levels of p-AMPK, PGC-1α, CPT-1 and UCP1 in both IWAT and BAT of T2DM rats. Recently, studies demonstrate that AMPK is a target for mulberry leaf in the regulation of metabolism(Reference Gao, Zhang and Wang39). Flavonoids extracted from mulberry leaf may improve skeletal muscle insulin resistance and mitochondrial function in db/db mice and L6 myocytes through AMPK-PGC-1α signalling pathway(Reference Meng, Qi and Fu40). Mulberry leaf can also increase the expression of HO-1 via activating the AMPK/NRF-2 signalling pathway to attenuate the inflammatory response(Reference Gao, Zhang and Wang39).
In this study, we found that mulberry leaf, a traditional Chinese medicine, could reduce BW, blood glucose and lipid, enhance insulin sensitivity, improve liver function and induce brown-like adipocytes in IWAT of T2DM rats. These effects can be in part attributed to mulberry leaf capacity to activate BAT and induce browning of IWAT via AMPK pathway. In our previous study, the main components of mulberry leaf were identified as isochlorogenic acid, 5,7-dihydroxycoumarin-7-O-β-D-glucopyranoside, scopolin, chlorogenic acid, kaempferol-3,7-di-O-D-glucopyranoside, 4-caffeoylquinic acid methyl ester, rutin, hyperoside, isoquercitrin, astragalin and isorhamnetin-3-O-glucopyranoside by HPLC-MS/MS(Reference Tang, Tian and Yang19). We read references and found that rutin, chlorogenic acid and quercetin in mulberry leaf may be the main molecular components to activate BAT and induce browning of WAT. Yuan et al. reported that rutin increased energy expenditure and improved glucose homoeostasis in obese mice by enhancing BAT activity and inducing brown-like adipocyte (beige) formation in subcutaneous adipose tissue(Reference Yuan, Wei and You41). It has been found that chlorogenic acid can block the development of obesity by targeting the liver PPARγ pathway, thereby improving other metabolic parameters such as blood glucose and insulin sensitivity in mice(Reference Ma, Gao and Liu42). Interestingly, it has been recently demonstrated that chlorogenic acid attenuated obesity as a non-stimulant thermogenic substance, which results in up-regulation of AMPK, a key sensor of energy metabolism(Reference Li, Qi and Li43). It has been reported that quercetin can induce the transformation of white adipocytes into brown adipocytes in mice and 3T3-L1 cells. Mechanistically, quercetin increased the expression of related thermogenic genes (PRDM 16, PGC-1α, UCP1 and Cidea) in white adipocytes by regulating the AMPK/SIRT1/PGC-1α signalling pathway, thereby inducing browning of WAT(Reference Lee, Parks and Kang44).
Conclusions
In summary, this study demonstrated that mulberry leaf could activate AMPK, increase PGC-1α, UCP1 and CPT-1 protein activity and enhance mitochondrial biogenesis and fatty acid β oxidation, resulting in increasing energy consumption and insulin sensitivity, and reducing blood glucose and lipid (Fig. 8). These findings establish a significant role for mulberry leaf in regulating BAT thermogenesis and browning of IWAT, and we identify mulberry leaf as a new potential browning agent for treating patients with T2DM.
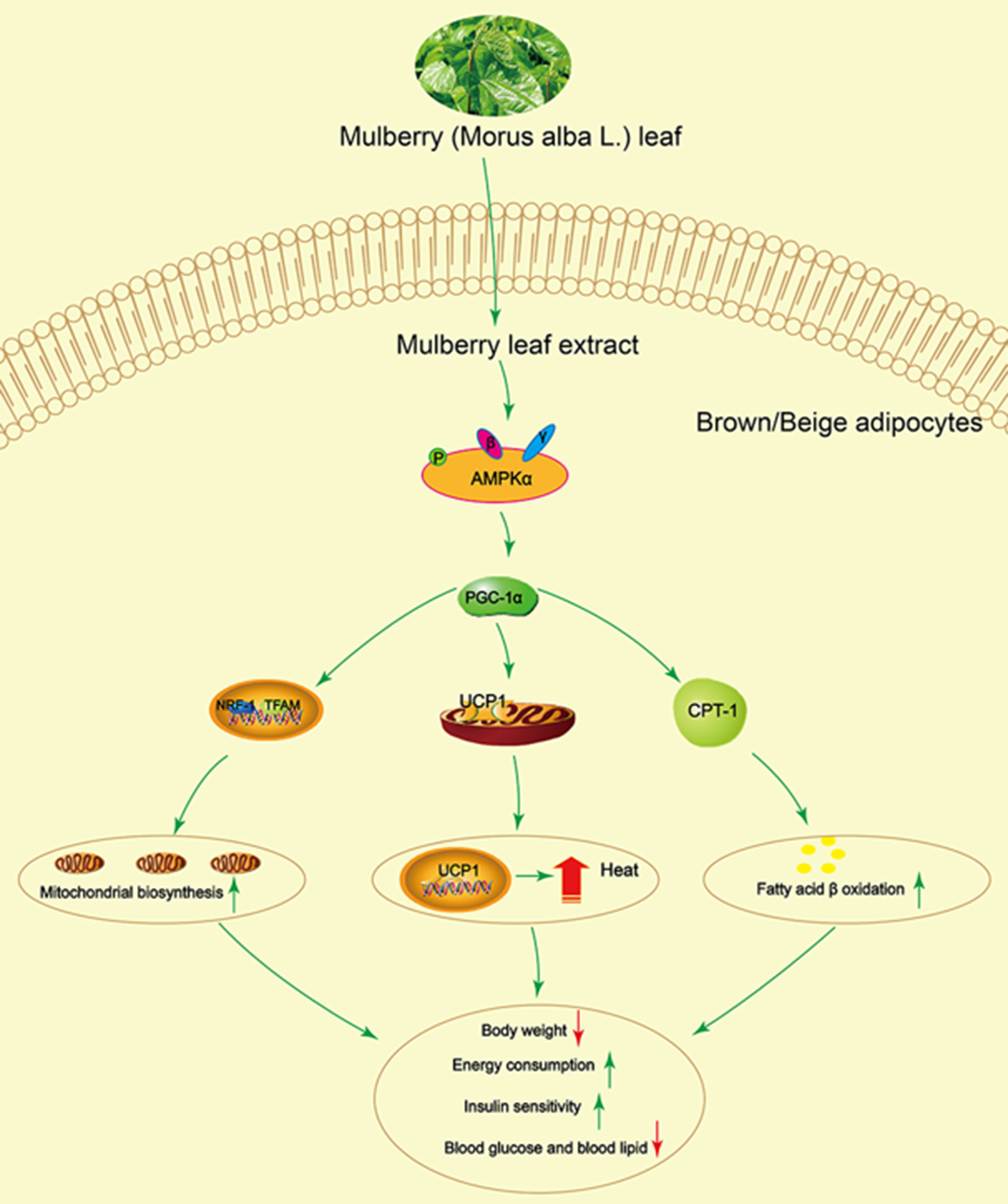
Fig. 8 Mulberry leaf activates brown adipose tissue (BAT) and induces browning of inguinal white adipose tissue (IWAT) through AMP-activated protein kinase (AMPK) signalling pathway. Mulberry leaf can activate AMPK, increase PPAR gamma coactivator 1 alpha (PGC-1α), uncoupling protein-1 (UCP1) and carnitine palmitoyl transferase 1 (CPT-1) protein activity and enhance mitochondrial biogenesis and fatty acid β oxidation, resulting in increasing energy consumption and insulin sensitivity, and reducing blood glucose and lipid. Note: The green arrow indicates activation or promotion, and the red arrow indicates reduction.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81773960 and 81973535).
L. C., J. W., Y. A., H. Dai, Y. D., L. S., Y. L., H. L., C. W., H. Du, X. Z. and B. Z. participated in drafting, editing and writing the manuscript. X. Z. completed pathological experiment and diagnosis. L. C. and B. Z. approved the final version of the manuscript. L. C. and B. Z. designed the table and figures.
The authors declare that they have no competing interest.