Digital Mental Health Interventions (DMHIs) are interventions that use technologies such as apps, computers or virtual reality to support people living with mental health conditions.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1,Reference Torous, Firth, Mueller, Onnela and Baker2 Such interventions have the potential to reach a large number of individuals, significantly widen access to care and effectively scale evidence-based support.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1 There is increasing evidence for the effectiveness of DMHIs in improving mental health conditions; however, evaluating their safety remains a key challenge for professionals in the field.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1,Reference Martinez-Martin and Kreitmair3,Reference Taher, Hsu, Hampshire, Fialho, Heaysman and Stahl4
DMHIs that meet the definition of a ‘medical device’ are regulated by various medical regulatory bodies across the world.5,Reference Carl, Jones, Lindhiem, Doss, Weingardt and Timmons6 A medical device, as defined by the EU Directive 93/42/EEC.11, is ‘any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease’.Reference Parvizi and Woods7 In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for regulating both pharmaceuticals and medical devices, and thus all DMHIs are classified as such.Reference Carl, Jones, Lindhiem, Doss, Weingardt and Timmons6 Therefore the UK's regulatory framework currently applies only to DMHIs intended to treat or alleviate mental ill health, for example those designed for specific, clinically recognised conditions like depression, paranoia or insomnia and specifically intended for use only in those predefined populations. In contrast there is a wide range of similar products that are intended to improve mental well-being but which fall outside the medical regulatory framework and therefore the remit of this paper.Reference Carl, Jones, Lindhiem, Doss, Weingardt and Timmons6,Reference Espie and Henry8 This includes anything designed for general public use, for example wellness, exercise, healthy eating, meditation/mindfulness or motivational apps.
From a safety perspective, a medical device's life cycle can be divided into two phases: the pre- and post-market phase. The pre-market phase is when the product's effectiveness and safety is still being tested. The post-market phase is when the product has been approved by the appropriate regulators to be placed on the market and used publicly.Reference Carl, Jones, Lindhiem, Doss, Weingardt and Timmons6
Assessing the safety of DMHIs in the pre-market phase
In the pre-market phase, the safety of DMHIs is assessed mainly in clinical trials by collecting and analysing Adverse Events and Serious Adverse Events (SAEs) data.Reference Wykes, Lipshitz and Schueller9 According to the Good Clinical Practice guidelines of the International Council for Harmonization, an adverse event is an ‘untoward medical occurrence’, and an SAE is an adverse event that results in hospitalisation, death, disability or a birth defect, is a medically important event or would have resulted in any of these outcomes if preventative action had not been taken.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1
Negative effects or adverse events relevant to mental health interventions include deterioration of symptoms (the ‘deterioration effect’), novel symptoms and/or non-response.Reference Rozental, Kottorp, Boettcher, Andersson and Carlbring10 The deterioration effect occurs when people's symptoms worsen during therapy.Reference Berk and Parker11 Approximately 3–10% of psychotherapy patients experience deterioration.Reference Berk and Parker11 There is division in the literature on whether symptom deterioration is an integral and necessary part of therapy or whether it is an unwanted side-effect.Reference Linden12 Novel symptoms are when people experience new mental health symptoms during treatment, ones that they did not present with prior to the onset of the treatment.Reference Rozental, Andersson, Boettcher, Ebert, Cuijpers and Knaevelsrud13 Non-response is when people's target symptoms do not improve. This is seen as a negative effect because the treatment might have prevented the person from receiving an effective therapy.Reference Rozental, Andersson, Boettcher, Ebert, Cuijpers and Knaevelsrud13 Furthermore, the digital aspect of DMHIs introduces new risks to mental health therapies such as technical issues and privacy concerns.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1,Reference Bradstreet, Allan and Gumley14
Despite efforts to adequately assess the safety of mental health interventions, numerous research studies have found that the collection, monitoring and analysis of adverse events and SAEs data in face-to-face/digital psychological therapy trials are still in a premature phase.Reference Taher, Hsu, Hampshire, Fialho, Heaysman and Stahl4,Reference Linden12–Reference Terry and Gunter15 A recent review on the identification and categorisation of adverse events in DMHI trials found that only six out of 23 of the included trials (26%) reported adverse events data within their primary results.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1 The lack of consensus on the definition of what constitutes an adverse event and SAE in a trial for a mental health intervention and what data needs to be collected, and how, have been cited as the main reasons for the current inferior standard of adverse events monitoring and reporting in DMHIs.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1,Reference Taher, Hsu, Hampshire, Fialho, Heaysman and Stahl4,Reference Papaioannou, Cooper, Mooney, Glover and Coates16 Furthermore, reliance on guidelines that were originally developed for assessing the safety of pharmacotherapies can be a further hindrance.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1
Assessing the safety of DMHIs in the post-market phase
In the post-market phase, the MHRA uses Post Market Surveillance (PMS) to monitor the safety of drugs and medical devices that reach the market.Reference Raj, Fernandes, Charyulu, Dubey, Ravi and Hebbar17 As part of PMS, the manufacturer is responsible for continuously monitoring the safety of their product(s), implementing strategies to minimise risk(s) and reporting their findings to the regulatory body.Reference Parvizi and Woods7 Additionally, the MHRA uses a spontaneous recording system called the ‘Yellow Card Scheme’ which is a safety monitoring system.Reference Raj, Fernandes, Charyulu, Dubey, Ravi and Hebbar17 Its purpose is to identify new side-effects that might not have been previously known and to monitor the safety of products on the market. It allows health professionals, as well as patients and caregivers, to report any suspected adverse reactions directly to the MHRA.Reference Raj, Fernandes, Charyulu, Dubey, Ravi and Hebbar17 The Yellow Card Scheme has shown effectiveness in helping the MHRA assess the safety of drugs and medical devices.Reference Raj, Fernandes, Charyulu, Dubey, Ravi and Hebbar17 A review of Yellow Card data found that, compared with health professionals, patients are more likely to report adverse reactions that fall under ‘psychiatric disorders’ – a categorisation that groups all psychiatric, psychological and mental health reactions under the Yellow Card Scheme.Reference Hazell, Cornelius, Hannaford, Shakir and Avery18 It is speculated that this might be because health professionals may give less importance to such reactions (mental health reactions), or they do not acknowledge such reactions as ‘adverse’.Reference Hazell, Cornelius, Hannaford, Shakir and Avery18 This further highlights the importance of adapting and promoting the use of the Yellow Card Scheme to assess the safety of DMHIs.
The regulation of DMHIs using medical regulatory methods has some benefits, such as integrating mental health interventions into an established and robust regulatory framework used across the healthcare sector.5 However, using a regulatory process for DMHIs that was originally developed for other purposes could have several undesirable consequences, such as missing important harms or overemphasising less significant harms.Reference Taher, Hsu, Hampshire, Fialho, Heaysman and Stahl4 There is already an awareness of the need for better regulatory methods for DMHIs; in the UK, this is evident from the £1.8 million received by the MHRA and National Institute for Health and Care Excellence to specifically explore the regulation of DMHIs.19 To contribute towards this objective, we employed the Delphi technique to reach an experts’ consensus on ‘What are the adaptations needed for the existing medical based model that is used to assess safety of DMHIs?’
Method
Study design
An online Delphi technique was used to reach an experts’ consensus on how the medical methods used for assessing safety can be adapted to better meet the objectives of DMHIs. The Delphi technique is a tool used to reach a consensus, through controlled feedback on topics with limited evidence or where a consensus does not exist.Reference Taylor20,Reference Santaguida, Dolovich, Oliver, Lamarche, Gilsing and Griffith21 The main components of a Delphi technique are anonymity, iteration, controlled feedback and statistical stability.Reference Rozental, Andersson, Boettcher, Ebert, Cuijpers and Knaevelsrud13,Reference Santaguida, Dolovich, Oliver, Lamarche, Gilsing and Griffith21,Reference Nasa, Jain and Juneja22
Recruitment and participants
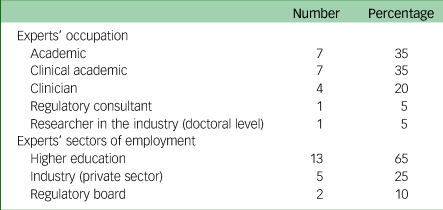
Purposive sampling was used to invite participants to participate in this Delphi study. Individuals were eligible to participate if they were professionals (researchers, clinicians or regulatory consultants) with experience/knowledge in the field of digital mental health in the UK. There were no exclusion criteria. Experts who met the eligibility criteria were contacted via email or LinkedIn. A snowball technique was also used to recruit participants. Thirty-six professionals were invited to join the panel, of whom 25 consented (69%) and 20 participated (55%). Thus, the sample size was in the average range for Delphi studies.Reference Taylor20 Of the 20 participants, 11 (55%) were females. The majority of the participants (17/20, 85%) were based in the UK, with three living in Canada, Australia and the Netherlands. Participants had an average of 19 years’ (range 5–37, s.d. = 7.45) general working experience and 8 years (range 2–15, s.d. = 4.51) in the field of digital mental health. See Table 1 for further details on the participants’ occupations. As common in expert Delphi studies, participating panel members were offered authorship on any resulting publications.Reference Smoktunowicz, Barak, Andersson, Banos, Berger and Botella23
Table 1 Experts’ occupations and employment sector
Data collection and analysis
Three rounds were conducted to reach a consensus in this study. Participants were given 2 weeks to complete each round.
Round 1: item generation
Participants were sent a link to an 11-question qualitative questionnaire to collect their opinion on how the safety of DMHIs is currently assessed (see Supplementary Appendix A available at https://doi.org/10.1192/bjo.2024.713 for the list of questions). Open-ended qualitative questionnaires are usually used in Delphi studies to generate items using the panel's opinions.Reference Taylor20 Twenty participants completed round 1. The qualitative data collected were analysed using open coding to generate items. Open coding is ‘the process of identifying, naming, and categorising concepts in the data’.Reference Cascio, Lee, Vaudrin and Freedman24 The authors R.T. and P.B. analysed the data separately and generated items based on the data. Afterwards, they compared and discussed their individual items and agreed on a final list of 64 items. This process was overseen by a third author J.Y.
Round 2: collecting controlled feedback
A quantitative questionnaire comprising the 64 final items was shared with all 20 participants who participated in round 1. Participants were asked to rate their agreement with each item on a 5-point Likert scale (strongly disagree, disagree, neutral, agree or strongly agree). Twenty participants completed round 2 (100%).
Round 3: reaching a quantitively predefined consensus
Twenty individual questionnaires were developed. Each questionnaire contained the same items and rating options from round 2, the group's answers, plus the individual's rating. Considering the group's ratings, participants were given an opportunity to reappraise their previous ratings (see Fig. 1). Eighteen participants (90%) completed round 3. The percentage of people who agreed (agree/strongly agree) and disagreed (disagree/strongly disagree) with each item was calculated. Consensus was predefined as any item that reached 80% agreement (the most common method used to define consensus.Reference Diamond, Grant, Feldman, Pencharz, Ling and Moore25,Reference Seidler, Rice, Ogrodniczuk, Oliffe, Shaw and Dhillon26
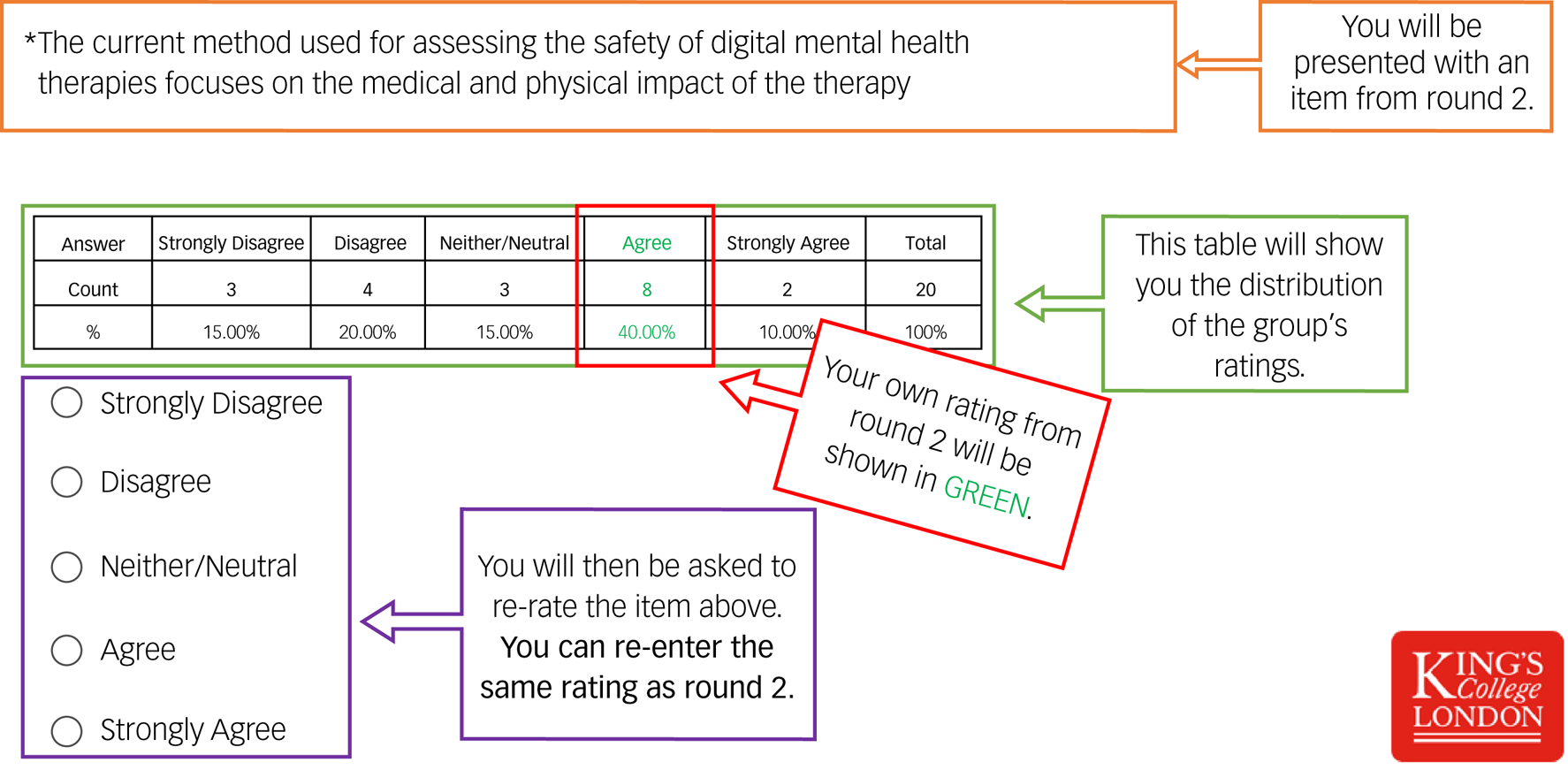
Fig. 1 A screenshot of the guide used to assist participants in round 3.
Ethical clearance
This study was registered as a minimal risk study at King's College London under the reference number MRSP-22/23-34777. Under the above approval, consent was implied by participants’ email agreement to take part and completion of the various requested survey activities (Round 1–3). Furthermore, at the end of each online questionnaire, the following message was presented to participants: ‘Please be aware that submitting this form implies consent to participate in this research’.
Results
Forty-one out of 64 items reached consensus (64%). The items that reached consensus in this study can be grouped under five main themes. Table 2 shows percent agreement for the 41 items reaching consensus, organised by theme. Supplementary Appendix B shows all 64 items with corresponding agreement and disagreement rates.
Table 2 Items from round 1 reaching consensus (≧80 agreement) organised by theme. Bold text denotes items scoring >90% agreement

Enhancing the quality of adverse events data in DMHIs
Under this theme, experts echoed researchers’ findings on the poor quality of adverse events data in DMHI studies.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1 A total of 94% of experts agreed that ‘The digital mental health field lacks clear guidance on what adverse events data should be collected, when and how this should happen, and how these should be categorised, analysed and reported’. All experts agreed that ‘Generating publication guidelines for reporting adverse events data that are required by journals can help ensure that adverse events data are collected and reported consistently’. Furthermore, under this theme experts concluded that ‘Determining the relatedness of the adverse event to the therapy is paramount for assessing safety’ (94%) and that ‘When assessing adverse events in digital mental health interventions, it is very important to differentiate between self-guided and clinician-guided therapies’ (89%).
Redefining SAEs for DMHIs
One expert said in round 1: ‘The current definition of serious adverse events (hospitalisation, death, congenital abnormalities … ) is not fit for purpose for mental health interventions compared to pharmaceutical, surgical etc.’. When this item was later shared with the panel of experts in round 3, 94% concurred with it. In all, 83% of experts agreed that ‘Congenital abnormalities as a serious adverse event of digital mental health interventions is not plausible’. Experts also expressed that the traditional definition of an SAE is not comprehensive of what should be considered an SAE for a DMHI. All experts agreed that ‘In the context of mental health therapies, it is important for “serious” to be inclusive of serious effects on mood, behaviour, general well-being and functioning, not just physical harms’. In addition to loss of life, hospitalisation and disability, suicide attempts (94%), increase in harm towards others (100%), increase in intentional self-harm (100%) and seizures that are induced because of on-screen flashes (100%) should be considered SAEs even if they do not lead to hospitalisation. Experts added that in the case of a DMHI, any harm that is sustained for longer than 6 months should be considered an SAE (89%).
Reassessing short-term deterioration in face-to-face and DMHIs as a therapeutic risk
Experts reached a consensus that ‘the short-term distress generated by focusing on the emotional experience (perhaps discussing painful or traumatic experiences or going into new or anxiety-provoking situations) is a part of the therapy process and should not be considered an adverse event or safety concern’ (89%). However, they also acknowledged that such a deterioration could lead to negative outcome/harm; thus, such deterioration needs to be monitored and not ignored (83%). For that, experts encouraged long-term follow-ups to differentiate between short-term and long-term deterioration (89%). They also suggested several ways to support individuals experiencing deterioration; eight of these reached consensus (see Supplementary Appendix C).
Maximising the benefit of the Yellow Card Scheme in DMHIs
All experts agreed that ‘There is not enough awareness among users that the Yellow Card Scheme is used for reporting adverse reactions in digital mental health therapies’. Furthermore, 83% of experts agreed with the item ‘I was not aware that the Yellow Card Scheme is used to report the adverse reactions of digital mental health therapies’. Experts acknowledged that one drawback of the Yellow Card Scheme for collecting adverse reactions is that it relies on individuals to report risks, which they may not be willing or able to do; for that, they suggested that Patient Reported Outcomes could be automatically collected within the digital therapy itself to help identify risks (94%).
Acknowledging the benefit of the Yellow Card Scheme, experts determined a few improvements that would make it more useful for DMHIs. First, experts noted that grouping all mental health adverse reactions under ‘psychiatric disorders’ risks losing useful and important detail on the different mental health adverse reactions experienced by individuals and may discourage reporting milder mental health adverse reactions (89% and 94% respectively). Instead, they suggested having a broader and more inclusive category such as ‘mental health adverse reactions’, with subtypes that relate to the different adverse reactions that could occur under that category (94% and 83% respectively). Experts settled on four proposed subtypes: intentional self-harm (100%), threat to others (100%), suicidal ideation/behaviours (94%) and new mental health symptoms (89%). Additionally, experts agreed that having the following information about every mental health adverse reaction reported on the Yellow Card website under the DMHI's profile would be helpful: severity/impact on the individual (94%), duration of adverse reaction (89%), whether the event led to the individual terminating therapy (89%) and a description of the adverse reaction (free text) (89%).
Developing a harmonised approach for assessing the safety of psychological interventions generally
Experts agreed that there should be a single harmonised approach for assessing and reporting the safety of face-to-face and DMHIs. Experts assented that such an approach would allow professionals to compare the safety of face-to-face and DMHIs (89%), and thus better understand the risks of mental health therapies, and contribute to stronger safety standards and safer interventions (94%). Moreover, experts speculated that ‘If there was one structured way to report adverse reactions in face-to-face and digital mental health therapies, clinicians and mental health professionals will be more likely to report them’ (83%). At the very least, the safety issues related to clinical support that are common to face-to-face and digital therapy such as the deterioration effect need to be harmonised, and the safety issues that arise from the use of a digital tool such as technical issues could be added to that (83%).
Items with high disagreement rates
We also explored the items that had high rates of disagreement. See Supplementary Appendix B for the percentage of disagreement (i.e. rating disagree/strongly disagree) for all items. No items reached consensus disagreement (80% or higher). However, two items equalled or exceeded 50%. A total of 61% of experts disagreed that ‘triggering trauma-related flashbacks’ should be considered an SAE. Half of the experts disagreed that ‘triggering a panic attack’ should be considered an SAE. Although these items arose directly at the suggestion of some experts, more than half the group disagreed. These disagreement rates illustrate the difficulty defining what constitutes an SAE for DMHIs and reveal that some areas divide opinion.
Discussion
This study successfully reached an experts’ consensus on ten recommendations for how to adapt the medical method used for assessing the safety of DMHIs. Our panel's consensus suggestions have the potential to improve the process of safety assessment, enhancing the field's approach to the safety of DMHIs. Table 3 provides a summary of the recommendations.
Table 3 List of recommendations on how to adapt the medical regulatory safety assessment model to meet the needs of the digital mental health field

Improving adverse events data in DMHI trials
The Delphi study showed a consensus on the lack of clear guidelines on how to collect, analyse and report adverse events data in DMHIs. Using guidelines that were originally developed for pharmacological trials is adversely affecting the quality of adverse events data in DMHIs.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1,Reference Papaioannou, Cooper, Mooney, Glover and Coates16 Consequently, experts unanimously agreed that generating publication guidelines to support professionals on what adverse events data need to be collected, how often and how they need to be analysed, will help standardise adverse events reporting in the field and allow for more comprehensive and systematic analysis of adverse events data (recommendation 1).Reference Rozental, Andersson, Boettcher, Ebert, Cuijpers and Knaevelsrud13
To effectively assess the safety of DMHIs, determining the relatedness of an adverse event to the treatment is key (recommendation 2), which can be less straightforward in a DMHI compared with a pharmacological intervention.Reference Rozental, Kottorp, Boettcher, Andersson and Carlbring10 Additional data collection to support this judgement could include onset, previous similar instances, and participant's and clinician's opinions on relatedness. This could improve the robustness of relatedness decision-making.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1
When assessing or comparing the safety of DMHIs, it is important to differentiate between self-guided and clinician-guided DMHIs (recommendation 3). The concern is that self-guided interventions can pose more risk in the absence of a professional guiding the individual, explaining content to them, assessing risk and providing emotional support or encouragement to people when they need it.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1 However, a review of the state of adverse events data in DMHIs found that studies of clinician-guided interventions were more likely to address adverse events in their publication compared with self-guided interventions.Reference Gómez Bergin, Valentine, Rennick-Egglestone, Slade, Hollis and Hall1 Adverse events in self-guided interventions are more likely to be missed because of the lack of human contact, and thus might be underreported. Thus, it is crucial for self-guided interventions to integrate within the product a way of collecting and monitoring adverse event and safety data, such as collecting pre/post data to detect deterioration or using automated triggers to alert clinicians to a worsening condition.Reference Seidler, Rice, Ogrodniczuk, Oliffe, Shaw and Dhillon26 In such cases, individuals would need to be informed and provide consent for such data to be collected and shared with the clinical team.
Broadening the definition of SAE for DMHIs
Experts in this study agreed that the pharmacological frame of reference for SAEs is not ‘fit for purpose’ for DMHIs. They argued that the definition of an SAE in the digital mental health field is too narrow and needs to be inclusive of any potentially life-threatening effects on mood, behaviour, general well-being and functioning. They concurred that adverse events such as increase in harm towards others, increase in intentional self-harm and any harm that is sustained for longer than 6 months should be considered ‘serious’, regardless of whether it led to hospitalisation or not. An MHRA-led consultation on the proposed changes to the regulatory framework for medical devices in 2021 (891 respondents) found that professionals requested mental health impacts to be included under ‘serious’ adverse events.27 The UK government's response was that at that time ‘appropriate mechanisms were not in place to sufficiently regulate the inclusion of these impacts’.27 We therefore conclude that further consultation is needed to identify and operationalise how the existing SAE definition might best be broadened to capture serious impacts on mental health (recommendation 4).
Short-term deterioration is not a safety concern in mental health therapy
Experts in this study reached a consensus that short-term deterioration of psychological symptoms occurring in the course of a psychological treatment can be an integral part of therapeutic change and is therefore not inevitably a safety concern. Nevertheless, experts stressed that these symptoms could amount to an adverse reaction, for example if the person drops out of therapy as a result. Thus, professionals need to monitor such deterioration and support individuals through it (recommendation 5). Similar to other negative effects, individuals need to be informed of the possibility of experiencing deterioration during therapy, and such deterioration needs to be normalised as part of the process.Reference Linden and Schermuly-Haupt28 Additionally, guidelines for professionals and patients are needed on the management of short-term deterioration.Reference Curran, Parry, Hardy, Darling, Mason and Chambers29 Our experts provided a list of suggestions (see Supplementary Appendix C) for supporting people experiencing therapy-related deterioration.
Adapting the Yellow Card Scheme for DMHIs
The medical field has already established that individuals are more likely than healthcare professionals to report adverse reactions that fall under the category ‘psychiatric conditions’.Reference Hazell, Cornelius, Hannaford, Shakir and Avery18 Experts in our study reached consensus on several ways to adapt the Yellow Card data collection and reporting process for DMHIs that are classified as medical devices, to maximise their benefit. First, there is a need for a campaign to raise awareness around the use of the Yellow Card Scheme for reporting the adverse reactions to DMHIs among both patients and professionals (recommendation 7). One suggestion is to make it a requirement for all DMHIs (including pre-market) to inform and direct people to the Yellow Card reporting website. Second, there was a consensus to use more appropriate, less stigmatising language by replacing ‘psychiatric disorders’ with ‘mental health adverse reactions’ (recommendation 8). Third, given that these are very general umbrella terms, it would be helpful to include further subcategories. Suggestions from our experts included ‘intentional self-harm’, ‘threat to others’, ‘suicidal ideation/behaviours’ and ‘new mental health symptoms’ (recommendation 9a). Fourth, it would be helpful to record the following contextual information for every reported adverse reaction: severity/impact on the individual, duration of adverse event, whether therapy was terminated as a consequence and a free text description of the adverse reaction (recommendation 9b).
Using a harmonised approach to assess the safety of psychological therapies generally
Perhaps one of the reasons why it has been difficult to adapt the medical model for DMHIs is that this model was not developed to assess the safety of face-to-face psychological therapies. The current quality of adverse event reporting in trials of face-to-face interventions is poor and is not subject to the same regulation as medical devices.Reference Linden12 Face-to-face interventions would also benefit from a standardised safety assessment method. Currently there is insufficient information about how the negative effects or adverse events data of face-to-face and DMHIs compare.Reference Rozental, Andersson, Boettcher, Ebert, Cuijpers and Knaevelsrud13 An ideal solution would be to develop one harmonised approach to assess the safety of face-to-face and DMHIs (recommendation 10). One harmonised approach could enhance the quality of safety assessments in traditional face-to-face interventions and ensure that safety evaluations of DMHIs are appropriate, proportionate and effective. Whether it is plausible to meet the needs of statutory regulators, clinicians, researchers, manufacturers and other stakeholders within a single overarching framework remains to be seen. Nevertheless, possible benefits our experts highlighted included better quality safety data, a more accessible method for non-medical mental health practitioners and better safety standards for both face-to-face and DMHIs.
Limitations
There are several limitations of this Delphi study. First, patients’ and their carers’ perspectives were absent in the results. Second, 65% of experts who participated work in higher education, leading to a likely overrepresentation of views from within this sector. It is important to note that 9 out of the 16 invitees (56.25%) who were invited but did not participate in the study came from non-academic backgrounds, primarily clinicians and co-founders. Third, evidence reliability is not possible in Delphi studies as the results might have differed if a different group of experts had been selected.
Conclusion
This Delphi study reached expert consensus on ten recommendations that can be used to adapt the medical regulatory safety assessment model to meet the needs of the digital mental health field. These recommendations, if implemented, have the potential to improve the process of safety assessment of psychological therapies in general and DMHIs in particular. In turn this may result in better quality of mental health safety data, a more effective risk mitigation process in DMHIs and more accurate risk–benefit analysis of DMHIs.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2024.713
Data availability
The data that support the findings of this study are available on request from the corresponding author, J.Y.
Acknowledgements
This work was supported by the Medical Research Council (MRC) Biomedical Catalyst: Developmental Pathway Funding Scheme, MRC Reference: MR/V027484/1. We would like to express our gratitude to all the experts who participated in this study, helping us contribute to the development of the field. We also would like to express our gratitude to the National Institute for Health and Care Research (NIHR) Biomedical Research Centre hosted at South London and Maudsley NHS Foundation Trust in partnership with King's College London. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, Department of Health and Social Care, the ESRC or King's College London. For the purposes of open access, the corresponding author has applied a Creative Commons Attribution (CC BY) licence to any Accepted Author Manuscript version arising from this submission.
Author contributions
R.T., D.S., S.S. and J.Y. designed the study. R.T. applied for ethics, collected data for the study, analysed it and wrote the first draft of the study under the supervision of D.S., S.S. and J.Y. P.B. was involved in the data analysis. S.A., M.A.-J., H.B., L.D., B.E.W., H.D.H., C.L.H., A.H., A.L.H., S.L., T.M., A.M., T.R.M., E.P.V., G.R.T., L.T., D.v.d.B., N.V., B.W.S. and K.S.Y. were all participants in the study and were involved in the write-up process.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None of the authors have any interests to declare, except A.L.H. who reports being employed by Big Health Ltd and is a shareholder in the company.








eLetters
No eLetters have been published for this article.