INTRODUCTION
Healthcare-associated infections (HAIs) have been recognized as a major public health priority for the past five decades, and therefore, many countries have established national surveillance systems [Reference Harbarth, Sax and Gastmeier1–Reference Agodi5]. According to Harbarth et al. [Reference Harbarth, Sax and Gastmeier1], at least 20% of all nosocomial infections are probably preventable and several studies have shown that surveillance and subsequent policy change can decrease HAI rates [Reference Gastmeier6–Reference Srinivasan, Craig and Cardo9]. From an epidemiological viewpoint, the mortality rate of HAIs is determined by both the incidence rate and the case-fatality rate. Similarly, the incidence rate is frequently affected by environmental factors, such as disinfection, sterilization, and isolation, and patient characteristics such as age, hygiene, comorbidities, immunocompromised status, and length of hospital stay. The case-fatality rate can be dependent on early detection of infection, timing of treatment, medical technology, patient comorbidities, patient age, treating physician, medical institution, intensive care, catheter use, and antibiotic resistance [Reference Luzzati10–Reference Kaech12].
In the presence of so many contributory factors it is therefore of great interest to develop a new method to deconstruct HAI mortality rate as a function of its incidence and case-fatality rates. This study attempts to identify and differentiate the contribution of several risk factors to the incidence and case-fatality rates, and through a retrospective study of data gathered over a 20-year time period.
METHODS
Study population and design
The study was conducted in a 921-bed urban tertiary medical centre with an intensive-care unit (ICU) bed capacity of 91 and ~27 000 inpatient admissions annually. It was designed as an incidence-death follow-up cohort study (Fig. 1) and patients who fulfilled the criteria for HAI between January 1994 and December 2013 were enrolled. A nationwide health insurance scheme has been in operation since March 1995 in Taiwan but prior to this, the hospital information system, including inpatient, outpatient, emergency, prescription, bio-laboratory, etc., was used for automatic insurance reimbursement and payment.
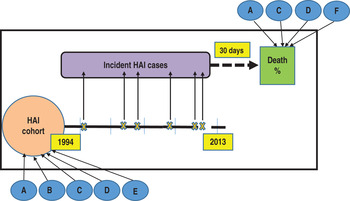
Fig. 1. Study design: incidence-death follow-up cohort. Healthcare-associated infections (HAIs) incident cases, subsequent deaths and factors affecting both. A, age; B, gender; C, department of admission; D, site of infection; E, admission type; F, intensive unit care.
From 1993, an HAI registry system was also set up independently from the insurance payment system by the infection control team in Shin Kong Wu Ho-Su Memorial Hospital which was regulated by hospital auditing and accreditation. Since then, a central infection committee in the hospital has coordinated the infection control and confirmed HAI cases on a weekly basis, and patients who developed a new episode of infection at least 48 h after admission were reported to the HAI registry.
Infections were classified and defined according to the U.S. Centers for Disease Control and Prevention/National Healthcare Safety Network as urinary tract infection (UTI), surgical site infection (SSI), pneumonia (PNEU), bloodstream (Bacteraemia), skin and soft tissue (SST), gastrointestinal system (GI), eye, ear, nose, throat, or mouth (EENT), and other (central nervous, reproductive tract, bone and joint, and cardiovascular system infections) [Reference Horan, Andrus and Dudeck13, Reference Garner14]. The study was reviewed by the Ethics Committee of Shin Kong Wu Ho-Su Memorial Hospital and was approved by the Institutional Review Board with Issue Number 20100502R.
Incidence and case-fatality of HAIs
Incident cases of HAI were identified as hospitalized patients free of infection who developed an HAI at least 48 h after admission and these cases were followed for 30 days. The incidence rate was expressed as the number of HAI episodes/1000 patient-days and HAI-related 30-day case-fatality rates were as for previously published studies [Reference Freire15–Reference Poikonen17] and expressed per 100 HAI patients. The mortality rate, i.e. the number of deaths/1000 patient-days, was calculated from the product of the incidence and case-fatality rates. The incidence-death cohort was further used to analyse the secular time trends of HAIs and their corresponding risk factors (Fig. 1).
Data for all confirmed cases and related factors were prospectively collected in the database. For post-discharge surveillance, outcomes were ascertained through the hospital system (date and reason of discharge) and the Taiwanese National Mortality Registry (date and cause of death by individual). The database contained comprehensive information regarding patient age, gender, date of admission, discharge and transfer, infection site, culture sample date, indwelling bladder catheter use, central venous catheter use, International Classification of Diseases (Ninth Revision) Clinical Modification (ICD-9-CM) diagnostic codes, admission type (from emergency or outpatient department), admitting medical department, and number of HAI episodes/admission.
Statistical analysis
Descriptive analyses of fatal outcomes were based on the mortality rates of all admitted patients and the case-fatality rates of patients with HAI and time trends depicted graphically for incidence rate, case-fatality rate, and mortality rate. Time trends for the incidence and case-fatality rates of HAIs were analysed separately for each of the major infection sites annually from 1994 to 2013.
To evaluate the independent effects of the incidence and case-fatality rates on the time trend for HAI-related mortality, we deconstructed the 30-day mortality rate into the incidence rate (annual number of HAIs 1994–2013), and the 30-day case-fatality rate, the latter being defined as number of HAI patients who died within 30 days divided by number of HAI cases. This is expressed as follows:
 $$\eqalign{& {\rm 30-day}\; \hbox{HAI mortality rate} \cr &= \displaystyle{{\hbox{number of HAI incident cases}} \over {\hbox{at risk population} \left( {\hbox{patient-days}} \right)}} \cr &= \displaystyle{{{\rm number\; of\; HAI\; incident\; cases}} \over {{\rm at\; risk\; population\;} \left( {{\rm patient-days}} \right)}} \cr & \times \displaystyle{{\; \rm number\; of\; {\rm HAI - related\; 30-day\; death}} \over {{\rm number\; of\; HAIs}}}\left( \% \right) \cr & \propto \; \left( {{\rm incidence\; rate}} \right) \times {\rm \;} \left( {{\rm 30-day\; case-fatality\; rate}} \right).} $$
$$\eqalign{& {\rm 30-day}\; \hbox{HAI mortality rate} \cr &= \displaystyle{{\hbox{number of HAI incident cases}} \over {\hbox{at risk population} \left( {\hbox{patient-days}} \right)}} \cr &= \displaystyle{{{\rm number\; of\; HAI\; incident\; cases}} \over {{\rm at\; risk\; population\;} \left( {{\rm patient-days}} \right)}} \cr & \times \displaystyle{{\; \rm number\; of\; {\rm HAI - related\; 30-day\; death}} \over {{\rm number\; of\; HAIs}}}\left( \% \right) \cr & \propto \; \left( {{\rm incidence\; rate}} \right) \times {\rm \;} \left( {{\rm 30-day\; case-fatality\; rate}} \right).} $$
Poisson regression models were used to calculate the relative risk of developing an HAI. Episodes of HAI and associated covariates were of interest; therefore, the model form was expressed as
![]() ${\rm log}\left\{ {n_i} \right\} = {\rm log}\left\{ {N_i} \right\} + {\rm x}_i^{\prime} $
I
2
, where n
i
denotes the number of events of interest (episodes of HAI), N
i
denotes patient-days (offset) in each level, x is the vector with covariates, and
I
2
is the corresponding regression coefficient. In the condition of over-dispersion, the negative binomial distribution and scale adjustment were applied in the estimation procedure. Those HAI cases that died within 30 days of the date of infection were treated as uncensored events (HAI-related case-fatality) whereas those who were still alive at 30-day follow-up were regarded as censored cases. Cox proportional hazards models were used to estimate hazard ratios (HRs) for HAI-related death. Both univariable and multivariable analyses were performed using these models. To investigate the risk of being an HAI death taking other covariates into account simultaneously, the multivariable Cox regression analysis was performed to estimate the adjusted hazard ratio (aHR). Data analysis was performed using SAS statistical analysis software, version 9.3 (SAS Institute Inc., USA). The statistical significance level was set as α = 0·05.
${\rm log}\left\{ {n_i} \right\} = {\rm log}\left\{ {N_i} \right\} + {\rm x}_i^{\prime} $
I
2
, where n
i
denotes the number of events of interest (episodes of HAI), N
i
denotes patient-days (offset) in each level, x is the vector with covariates, and
I
2
is the corresponding regression coefficient. In the condition of over-dispersion, the negative binomial distribution and scale adjustment were applied in the estimation procedure. Those HAI cases that died within 30 days of the date of infection were treated as uncensored events (HAI-related case-fatality) whereas those who were still alive at 30-day follow-up were regarded as censored cases. Cox proportional hazards models were used to estimate hazard ratios (HRs) for HAI-related death. Both univariable and multivariable analyses were performed using these models. To investigate the risk of being an HAI death taking other covariates into account simultaneously, the multivariable Cox regression analysis was performed to estimate the adjusted hazard ratio (aHR). Data analysis was performed using SAS statistical analysis software, version 9.3 (SAS Institute Inc., USA). The statistical significance level was set as α = 0·05.
RESULTS
A total of 552 233 admissions were registered during the period from 1 January 1994 to 31 December 2013. Altogether, 6776 (1·2%) admissions were excluded either because the date of birth or department were missing (n = 254) or the admission was indicated for a health check-up only (n = 6522). Therefore, 545 457 admissions from 315 209 patients with 4 210 609 patient-days were available for analysis. From this set, 10 117 patients developed HAIs and 20 221 cultures were obtained. There were 2965 deaths within 30 days of HAI onset (Fig. 2).
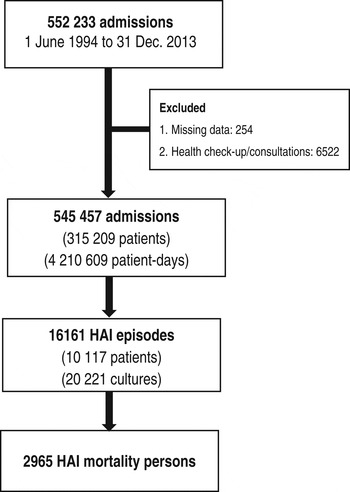
Fig. 2. Number of admission, patients, episodes of healthcare-associated infections (HAIs), and deaths between 1994 and 2013 in Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan.
The median length of hospital stay for HAI patients was 35 days [inter-quartile range (IQR) 22–58 days], and 5 days (IQR 3–8) for non-HAI patients. Males accounted for 52·29% of cases (8450 episodes) with a median age of 68·00 years (IQR 54·73–76·94), and females for 47·71% of cases (7711 episodes) with a median age of 71·94 years (IQR 60·92–80·25). A total of 9997 (61·86%) episodes occurred in subjects aged ⩾65 years, and the time trend for these patients was increasing during the study period. The incidence rate was highest for urinary tract infections (1·59 episodes/1000 patient-days), followed by bacteraemia (1·09), pneumonia (0·72), and surgical site (0·45). Bacteraemia had the highest (34·4%) case-fatality rate, followed by pneumonia (28·1%) and gastrointestinal tract infections (25·1%). Bacteraemia and urinary tract infections accounted for the majority of deaths.
Time trends of incidence rate and case-fatality rate
Figure 3a shows the mortality rate of HAIs according to the annual incidence and case-fatality rates. The overall incidence rate decreased from 2008 whereas the case-fatality rate increased initially and then decreased. Over the 20 years of longitudinal follow-up, the incidence rate fell by 32·4% from 3·41 to 2·31/1000 patient-days with a marked 14·5% reduction since 2008. By contrast, the case-fatality rate rose marginally by 3·7%, from 13·5% in 1994 to 14·0% in 2013. The net balance of these opposing trends yielded a decrease (29·8%) in mortality, from 0·46 to 0·32 deaths/1000 patient-days.
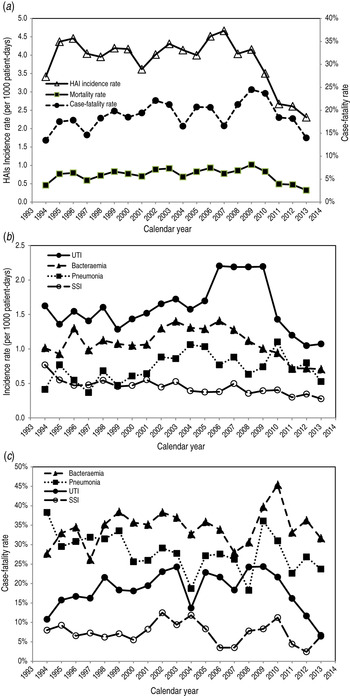
Fig. 3. (a) Annual healthcare-associated infection (HAI) incidence rate, case-fatality rate, and mortality rate. (b) Annual incidence rate and (c) annual case-fatality rate deconstructed by infection sites, 1994–2013 in Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan. Reported annual incidence rate is based on the events/1000 patient-days per year, case-fatality rate is number of deaths/100 HAI episodes and annual mortality rate is number of HAI-related deaths/1000 patient-days per year. UTI, urinary tract infection; SSI, surgical site infection.
The corresponding time trends for specific sites of infection are shown in Figure 3(b, c). Urinary tract infections accounted for most of the HAIs and both the urinary tract infection incidence and case-fatality rates declined since 2010. Similar incidence trends were observed for bacteraemia and surgical site infections but these began to decline prior to 2008, and their case-fatality rates declined since 2011.
Patient age was found to be a significant factor in the multivariable Poisson model used to evaluate contributing factors to the incidence of HAIs with the highest relative risk for those aged >80 years; the lowest risk age group was 20–29 years. Females had a 5·9% higher relative risk of contracting HAIs compared to males, and similarly oncology department patients showed an increased risk compared to other departments. Moreover, admissions from the emergency department had a 59·8% higher risk of HAI compared to outpatient departments. After adjusting for age, gender, and department of hospitalization, the patient admission type (outpatient or emergency) and site of infection remained associated with increased HAI incidence (Table 1).
Table 1. Univariable and multivariable analysis for HAI incidence
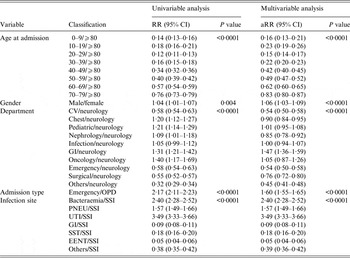
aRR, Adjusted relative risk; CI, confidence interval; CV, cardiovascular; GI, Gastrointestinal unit; PNEU, pneumonia; SSI, surgical site infection; UTI, urinary tract infection; SST, skin and soft tissue; EENT, eye, ear, nose, throat, or mouth. Other infection site: central nervous, reproductive tract, bone and joint, and cardiovascular system infections; OPD, outpatient department.
The multivariable Cox regression model also found age to be a significant risk factor for case-fatality. The aHR for HAI case-fatality fell in the 20–29 years age group and subsequently increased with advancing age. Gender was not an independent predictor for case-fatality. Residence in the oncology department was most associated with death from HAI compared to neurology and admission from other departments except paediatrics. The significant effects continued after adjusting for other covariates. Compared with surgical site infection, all infection sites demonstrated significant risk of fatality with 30 days with bacteraemia posing the highest hazard (Table 2).
Table 2. Univariable and multivariable analysis for HAI case-fatality rate
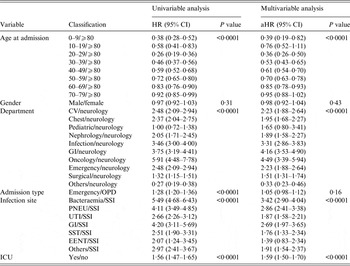
aHR, Adjusted hazard ratio; CI, confidence interval; CV, cardiovascular; GI, Gastrointestinal unit; PNEU, pneumonia; SSI, surgical site infection; UTI, urinary tract infection; SST, skin and soft tissue; EENT, eye, ear, nose, throat, or mouth. Other infection site: central nervous, reproductive tract, bone and joint, and cardiovascular system infections; ICU, intensive care unit; OPD, outpatient department.
DISCUSSION
Large-scale studies have the potential to offer useful information for healthcare providers, consumers, institutions and the government to aid understanding of the overall burden of HAIs. Unlike several previous epidemiological and infectious surveillance studies [Reference Kaech12, Reference Guggenbichler18–Reference Mackintosh and Hoffman23] that focused on the incidence of HAIs and clinical trials [Reference Tsigrelis24–Reference Miller27] addressing the case-fatality rate in past decades, the present study is the first, to our knowledge, to assess the 20-year time trends for three outcomes together by deconstruction of the mortality rate of HAIs by incidence and case-fatality rates. This information provides an overall management viewpoint for the control of HAIs [Reference Kanerva25].
Over the study period the overall mortality rate for HAIs fell by almost 30% but the analysis revealed a strong decreasing trend in the incidence rate accompanied by a weakly rising trend in case-fatalities. A possible explanation of these findings follows. Since 2005, the hospital infection control decision-makers initiated measures using Edward Deming's Plan-Do-Check-Act (PDCA) cycle-combined monitoring for each warning outbreak of HAI incidence [Reference Deming28]. The annual mortality rate in 2006 was 112% of that in 2005. Using the data deconstruction approach, this rate increase was due to a higher incidence rate (12·8% higher), and a small decrease in the case-fatality rate (-0·3%). Thus, to decrease the incidence rate, since 2007 infection control staff introduced a hand hygiene programme that incorporated both an alcohol-based hand rub disinfection site for each ICU bed and an increased number of hand hygiene stands at ward entrances. Alcohol-based hand rubs have been shown to have an important effect on bacterial cross-transmission [Reference Vernaz29, Reference Pittet30]. We set a red warning separation line between the ICU care and work areas and continuing interventions included increasing the comprehensiveness of staff education and the density of hand hygiene stands. Other factors such as reinforcement of the national infection control programme and an audit by the Taiwan joint commission on hospital accreditation may also have contributed to reduce the incidence during this time period. Moreover, the definition of HAI was modified in 2008, during the study period, and the hospital central infection committee changed the inclusion criteria accordingly. The incidence rate of HAIs decreased after 2008 with a lower frequency of surgical site, and bacteraemia, but not for pneumonia and urinary tract infections. This finding was similar to other earlier studies where surveillance and policy change were shown to effectively reduce the incidence of some HAIs [Reference Haley7, Reference Rioux, Grandbastien and Astagneau8]. However, the surveillance effect varies for different sites of infection [Reference Gastmeier6]. Our multivariable regression analysis showed that the department of hospitalization and patient age had significant effects on the incidence rate of HAI. These findings were consistent with other studies [Reference Kaech12, Reference Raymond and Aujard19, Reference Yoshida31].
The intensive care education programme for physicians and junior doctors was launched with the aim of controlling the case-fatality rate but this failed to decline as expected. It is possible that reducing the case-fatality rate becomes more difficult as the severity of illness and the proportion of elderly patients increases [Reference Luzzati10]. Furthermore, several other factors are known to influence this rate including disease severity, antibiotic administration, infection site, gender, and underlying comorbidities [Reference Tsigrelis24]. Our multivariable analysis showed that advanced patient age was also associated with an increased hazard of death and other factors such as ICU admission, admitting department and site of infection reflected the severity and type of patient illness.
These findings underline the need for a multidisciplinary approach to decrease case-fatality rates and considerable care is required in interpreting complex HAI mortality data. We recommend a task force with an integrated periodic monitoring-analysing-intervention feedback system that can be used to execute root cause analysis. The deconstruction of outcomes strategy appears to be an efficient means to differentiate the underlying causes of mortality trends and if utilized over a period of time, can further reveal trends in case-fatality and incidence rates that are embedded within the mortality rate. These trends could also be used for detecting emerging problems.
While this study benefits from the large sample size and the longitudinal time trend deconstruction analysis, there are some notable limitations. First, the database was obtained from the electronic hospital registration and infection control system. Second, our study site was a metropolitan teaching hospital, and thus, the generalization of specific results, such as relative risk, to other levels of healthcare institutions may be limited. These limitations, however, do not negate the value of the analytical approach. Finally, the Poisson distribution assumption for infectious disease incidence may be biased due to erroneous assumption of independence, thus leading to an underestimation of the variance. Therefore, we also performed deviance adjustment and negative binomial distribution to re-evaluate the risk factors in multiple regression analysis, By the deviance adjustment, all of the results remained significant and similar to the Poisson values and likewise by negative binomial assumption with the exception of patient gender (P = 0·0794).
In conclusion, this study demonstrates the evolution of HAIs by time trend deconstruction of the mortality rate of HAIs into incidence and case-fatality rates using data from a tertiary medical centre. The decreasing mortality trend was strongly determined by a decreasing trend in the incidence rate that was offset by a slightly increasing trend in the case-fatality rate. This difference between both time trends may imply a need for different requirements for HAI control interventions and will need to be clarified in future research.
ACKNOWLEDGEMENTS
The authors thank the infection control team, Dr Chien-Hsien Huang, and Dr Wei-Yu Chen for hospital infection quality and data control.
DECLARATION OF INTEREST
None.








