Type 2 diabetes mellitus (T2DM) and visceral obesity are closely associated with some long-term consequences such as dyslipidaemia, retinopathy, chronic renal disease and CHD( Reference Holper, Nolte and Bober 1 ). Hyperglycaemia, insulin resistance and intra-parietal accumulation of advanced glycation end products in patients with T2DM would result in macrovascular alterations through the activation of different pathways, particularly NF-κB and mitogen-activated protein kinase( Reference Casella, Bielli and Mauriello 2 ). Recently, a few studies have evaluated the relationship between carotid intima-media thickness (CIMT) and the metabolic syndrome (MetS). Several studies have reported a significant positive association between CIMT and diabetes, blood pressures, lipid profiles and inflammatory cytokines( Reference Yapei, Xiaoyan and Sha 3 , Reference Chua, Kilung and Ong 4 ), whereas other studies have not reported any significant association between the MetS and CIMT( Reference Kawasaki, Cheung and Islam 5 ).
Decreased vitamin D levels were associated with increased CIMT( Reference Hamdy Al-Said, Abd El Ghaffar Mohamed and Salam 6 ), insulin resistance and inflammation( Reference Petchey, Hickman and Duncan 7 ). Furthermore, Braam et al. ( Reference Braam, Hoeks and Brouns 8 ) found that long-term supplementation with vitamins K1 (1 mg) and D (8 µg) had beneficial effects on elastic properties of the carotid artery in postmenopausal women, but it did not influence CIMT. A 270-d course of vitamin K2 supplementation at a dose of 90 μg (menaquinone (MK)-7) in patients with coronary vascular disease reduced the progression of atherosclerosis and CIMT( Reference Kurnatowska, Grzelak and Masajtis-Zagajewska 9 ). Moreover, two recent studies have shown that Ca and vitamin D co-supplementation had beneficial effects on metabolic profiles( Reference Asemi, Karamali and Esmaillzadeh 10 , Reference Tabesh, Azadbakht and Faghihimani 11 ). Vitamin K supplementation for 36 months at a dose of 500 µg/d also decreased progression of insulin resistance in older men( Reference Yoshida, Jacques and Meigs 12 ).
We assume that vitamin D, K and Ca co-supplementation might influence CIMT by improving insulin function as well as biomarkers of inflammation and oxidative stress. Such beneficial effects might be mediated through the beneficial effect on the elastic properties of the arterial vessel wall( Reference Braam, Hoeks and Brouns 8 ), the regulation of cell cycle( Reference Nair-Shalliker, Armstrong and Fenech 13 ) and suppression of parathyroid hormone (PTH)( Reference Zemel 14 ). We are aware of no study that assessed vitamin D, K and Ca co-administration on CIMT and metabolic status in overweight T2DM patients with CHD. The current study was, therefore, performed to evaluate the effects of vitamin D, K and Ca co-administration on CIMT, glycaemic control, lipid concentrations, biomarkers of inflammation and oxidative stress in these patients.
Methods
Participants
This study was a randomised, double-blind, placebo-controlled trial that was registered in the Iranian registry of clinical trials (http://www.irct.ir: IRCT201506185623N45) and was carried out at a cardiology clinic affiliated to Kashan University of Medical Sciences (KUMS), Kashan, Iran, between June and September 2015. Inclusion criteria were as follows: overweight patients (BMI≥25 kg/m2) with T2DM, aged 40–85 years with a CHD condition. According to criteria of the American Diabetes Association( 15 ), patients who have one out of three of the following criteria were diagnosed with T2DM: fasting plasma glucose (FPG)≥126 mg/dl, blood glucose 2-h postprandial ≥200 mg/dl or glycated Hb (HbA1C)≥6·5 %. In addition, patients who had one or more of the following criteria were considered as having CHD: record of myocardial infarction, document of at least 50 % stenosis in one or more coronary vessels upon cardiac catheterisation evaluated by angiography, document of exercise-induced ischaemia by treadmill electrocardiogram or nuclear perfusion stress imaging and a history of coronary re-vascularisation( Reference Welles, Whooley and Karumanchi 16 ). Exclusion criteria were consumption of vitamin D, K and Ca supplements within the last 3 months, occurrence of an acute myocardial infarction within the past 3 months, a cardiac surgery within the past 3 months and major renal or liver failure.
Ethics statements
This study was performed according to the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of KUMS (reference no: IR.Kaums.REC.1394.32). All patients were informed about the aims and protocol of the study, and written informed consent was obtained from all the participants.
Study design
At the onset of the study, patients were first matched one by one according to age, BMI, sex and the dosage and kind of medications used. Next, the matched patients were randomly assigned to the intervention and placebo groups. Subsequently, the participants were randomly allocated into two treatment groups. Group A (intervention; fifteen females and eighteen males: n 33) received 5µg of vitamin D and 90 µg of vitamin K2 to form MK-7 and 500 mg Ca supplements as a tablet and group B (placebo; sixteen females and seventeen males: n 33) received daily placebo tablets for 12 weeks. Owing to lack of information about the appropriate dosage of vitamin D, K and Ca for overweight diabetic patients with CHD, we used the above-mentioned doses of vitamin D plus Ca based on a previous study in postmenopausal women( Reference Schnatz, Jiang and Vila-Wright 17 ) and a dose of vitamin K based on a previous study in older women and men( Reference Yoshida, Jacques and Meigs 12 ). Vitamin D, K and Ca supplements and the placebos (cellulose) were manufactured by Aryan Salamat Pharmaceutical Company and Barij Essence Pharmaceutical Company, respectively. Both vitamin D, K and Ca supplements and placebo tablets had similar packaging, and patients and researchers were not aware of the contents of the package until the end of study. Randomisation and allocation were blinded from researchers and subjects until the main analyses were completed. At the cardiology clinic, a trained nutritionist carried out the randomised allocation sequence and enrolment and assignment of the patients to the groups. At the beginning of the study, patients were requested to maintain their regular diet and levels of physical activity throughout the trial period. Compliance to the consumption of supplements and placebos was examined by checking the containers of the tablets as well as by measuring serum 25-hydroxyvitamin D (25(OH)D) levels. All participants completed three dietary records (2 weekdays and 1 weekend day) at week 2, 5, 8 and 11 of the trial. To obtain nutrient intakes of patients according to 3-d food records, we applied Nutritionist IV software (First Databank) adopted for the Iranian food pattern. In the present study, physical activity was described as metabolic equivalents (MET) in hours per day. To determine the MET for each patient, we multiplied the times (h/d) reported for each physical activity by the related MET coefficient using standard tables( Reference Ainsworth, Haskell and Whitt 18 ).
Assessment of anthropometric measures
In the present study, weight and height (Seca) were quantified at the beginning of the study and after intervention without shoes in minimal clothing in the cardiology clinic by a trained nutritionist. BMI was calculated as weight in kg divided by height in metres squared.
Assessment of outcomes
In the present study, we considered CIMT as the primary outcome and parameters of glucose homoeostasis, lipid profiles, biomarkers of inflammation and oxidative stress as secondary outcomes. Measurement of the CIMT was performed in patients at the 2-cm distance of the common carotid bifurcation, by the same sonographist, at study baseline and after 12 weeks of intervention using a Doppler ultrasonography device (Samsung Medison V20) with linear multifrequencies of 7·5–10-MHz probe. The physician was blinded to any clinical information of the subjects. The examinations were conducted in a quiet room with a controlled temperature of 21±2°C. The measurements were made at the same time of the day to avoid diurnal variations and after a 10–15-min supine rest to stabilise blood pressure levels. Participants were requested to refrain from smoking and consumption of tea, caffeinated drinks or any foods for at least 10–12 h before the examination.
Fasting blood samples (10 ml) were collected at the beginning and at the end of the study at Kashan Reference Laboratory in fasting conditions and centrifuged to separate serum. Next, the samples were stored at −80°C before analysis. Serum 25(OH)D concentrations were quantified using a commercial ELISA kit (IDS) with inter- and intra-assay CV of 4·3–6·5 %, respectively. Serum insulin concentrations were assessed using an available ELISA kit (DiaMetra) with intra- and inter-assay CV of 2·8 and 4·9 %, respectively. The homoeostasis model for assessment of insulin resistance (HOMA-IR), β-cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) were determined according to the suggested formulae( Reference Pisprasert, Ingram and Lopez-Davila 19 ). Enzymatic kits (Pars Azmun) were used to quantify Ca, FPG, serum TAG and VLDL-, total-, LDL- and HDL-cholesterol concentrations. All inter- and intra-assay CV for Ca, FPG and lipid concentrations were <5 %. Serum high-sensitivity C-reactive protein (hs-CRP) concentrations were evaluated using a commercial ELISA kit (LDN) with intra- and inter-assay CV of 2·7 and 4·8 %, respectively. The plasma nitric oxide (NO) concentrations were assessed using the Griess method( Reference Tatsch, Bochi and Pereira Rda 20 ). Plasma total antioxidant capacity (TAC) concentrations were determined by the ferric reducing antioxidant power method developed by Benzie & Strain( Reference Benzie and Strain 21 ), total GSH levels were determined using the method of Beutler & Gelbart( Reference Beutler and Gelbart 22 ) and malondialdehyde (MDA) concentrations were determined by the thiobarbituric acid reactive substances spectrophotometric test( Reference Janero 23 ). All inter- and intra-assay CV for NO, TAC, GSH and MDA concentrations were <5 %. Measurements of vitamin D, Ca, FPG, insulin, lipid concentrations, biomarkers of inflammation and oxidative stress were conducted in a blinded manner, in duplicate, in pairs (pre/post-intervention) at the same time, in the same analytical run and in random order to reduce systematic error and inter-assay variability.
Statistical methods
In the present study, we used a randomised clinical trial sample size calculation formula where type-one (α) and type-two errors (β) were 0·05 and 0·20 (power=80 %), respectively. According to the previous trial( Reference Braam, Hoeks and Brouns 8 ), we used 0·06 mm as sd and 0·05 mm as the change in mean (d) of CIMT as a main variable; based on the formula, we needed twenty-six patients in each group. After considering seven dropouts in each group, the final sample size was thirty-three patients in each group.
To determine the normal distribution of the variables, we applied the Kolmogrov–Smirnov test. The intention-to-treat (ITT) analysis of the primary study end point was applied for all of the randomly allocated participants. To detect differences in the general characteristics and daily dietary macronutrient and micronutrient intakes between the two groups, independent samples Student’s t test was used. To identify within-group differences (pre- and post-intervention), we used paired samples t tests. To determine the effects of vitamin D, K and Ca co-administration on CIMT, glycaemic control, lipid profiles, biomarkers of inflammation and oxidative stress, we used one-way repeated-measures ANOVA. All the findings and P-values were based on Bonferroni’s correction for pairwise comparisons. To evaluate for several confounders, we adjusted all analyses for baseline values of biochemical variables, age and baseline BMI to avoid potential bias. A P-value lower than 0·05 was considered significant. All statistical analyses were performed using Statistical Package for Social Science version 18 (SPSS Inc.).
Results
In the present study, three participants each from the vitamin D, K and Ca supplements group and the placebo group discontinued from the study because of personal reasons (Fig.1). However, all sixty-six participants were included in the final analysis using ITT principle. Overall, the compliance rate was high, such that higher than 90 % of tablets were consumed throughout the study in both groups.

Fig. 1 Summary of patient flow diagram.
Mean duration of DM, consumption of antidiabetic and antilipidaemic drugs, and hypertension rate of the study participants were not statistically different between the two groups (data not shown). There were no significant differences between the two groups in terms mean age, height, baseline weight, baseline BMI and mean changes in weight and BMI during the trial (Table 1).
Table 1 General characteristics of the study participants (Mean values and standard deviations)
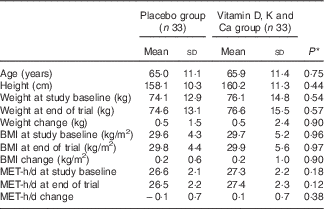
MET, metabolic equivalents.
* Obtained from independent t test.
On the basis of the 3-d dietary records obtained throughout the intervention, we observed no significant change in dietary macronutrient and micronutrient intakes, including energy, carbohydrate, protein, fat, SFA, PUFA, MUFA, cholesterol, total dietary fibre, Ca, Mg, Mn, Zn and vitamins D and K between the two groups (Table 2).
Table 2 Dietary intakes of study participants throughout the study (Mean values and standard deviations)

TDF, total dietary fibre.
* Obtained from independent t test.
After 12 weeks of intervention, vitamin D, K and Ca co-supplementation resulted in a significant reduction in maximum levels of left CIMT (−0·04 (sd 0·22) v. +0·04 (sd 0·09) mm, P=0·02); however, it did not influence mean levels of left and right CIMT and maximum levels of right CIMT compared with the placebo group (Table 3). In addition, changes in serum vitamin D (+6·5 (sd 7·8) v. +0·4 (sd 2·2) ng/ml, P<0·001), Ca (+0·6 (sd 0·3) v. +0·1 (sd 0·1) mg/dl, P<0·001) and insulin concentrations (−0·9 (sd 3·1) v. +2·6 (sd 7·2) µIU/ml, P=0·01), HOMA-IR (−0·4 (sd 1·2) v. +0·7 (sd 2·3), P=0·01), HOMA-B (−2·1 (sd 9·0) v. +8·9 (sd 23·7), P=0·01) and the QUICKI (+0·007 (sd 0·01) v. −0·006 (sd 0·02), P=0·01) in supplemented patients were significantly different from those in patients in the placebo group. Supplementation resulted in significant changes in serum HDL-cholesterol (+2·7 (sd 7·0) v. −2·5 (sd 5·7) mg/dl, P=0·002), hs-CRP (−1320·1 (sd 3758·3) v. +464·0 (sd 3053·3) ng/ml, P=0·03) and plasma MDA concentrations (−0·4 (sd 0·5) v. −1·0 (sd 1·1) µmol/l, P=0·007) compared with the placebo group. We did not observe any significant differences in changes in other lipid concentrations and biomarkers of oxidative stress when comparing the two groups.
Table 3 Carotid intima-media thickness (CIMT), metabolic profiles, biomarkers of inflammation and oxidative stress at baseline and 12 weeks after the intervention in patients with type 2 diabetes mellitus, overweight and CHD (Mean values and standard deviations)

FPG, fasting plasma glucose; HOMA-IR, homoeostasis model for assessment of estimated insulin resistance; HOMA-B, homoeostasis model for assessment of estimated β cell function; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; MDA, malondialdehyde.
* P values represent paired-samples t test.
† P values represent the time×group interaction (computed by analysis of the one-way repeated-measures ANOVA).
There was a significant difference in the baseline levels of biochemical variables, Ca, insulin, total cholesterol, HDL-cholesterol, NO, TAC and MDA between the two groups; however, additional adjustments for these variables did not influence the results except for findings on HDL-cholesterol (P=0·07) and MDA (P=0·36) (Table 4). Similarly, further adjustments for age and baseline BMI did not influence our findings.
Table 4 Adjusted changes in metabolic variables in patients with type 2 diabetes mellitus, overweight and CHDFootnote * (Mean values with their standard errors)

CIMT, carotid intima-media thickness; FPG, fasting plasma glucose; HOMA-IR, homoeostasis model for assessment of estimated insulin resistance; HOMA-B, homoeostasis model for assessment of estimated β cell function; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; MDA, malondialdehyde.
* Values are adjusted for baseline values, age and BMI at baseline.
† Obtained from ANCOVA.
Discussion
In the present study, which to the best of our knowledge is the first of its kind, we demonstrated that vitamin D, K and Ca co-supplementation for 12 weeks among these patients had beneficial effects on maximum levels of left CIMT, glycaemic control, HDL-cholesterol, hs-CRP and MDA levels; however, it did not have any effect on other lipid profiles and biomarkers of oxidative stress.
We found that vitamin D, K and Ca co-supplementation resulted in a significant reduction in maximum levels of left CIMT; however, it did not influence mean levels of left and right CIMT and maximum levels of right CIMT compared with the placebo. Previous studies have shown that there is a difference between the left and right CIMT. It was speculated that haemodynamics status, age, sex, metabolic profiles especially lipid concentrations, blood glucose levels and other risk factors would result in different effects on the left and right CIMT( Reference Luo, Yang and Cao 24 ). In a study by Luo et al. ( Reference Luo, Yang and Cao 24 ), left CIMT correlated better with blood biochemical indices such as total cholesterol, LDL-cholesterol and blood glucose levels. In the present study, decreased biomarkers of inflammation and lipid peroxidation in the intervention group may have resulted in decreased maximum levels of left CIMT. Hileman et al. ( Reference Hileman, Turner and Funderburg 25 ) demonstrated that decreased markers of oxidative stress, inflammation and monocyte activation resulted in improved CIMT. Furthermore, the effect of vitamin D, K and Ca co-supplementation on maximum levels of left CIMT could be a chance finding. We did not observe any significant effect of vitamin D, K and Ca co-supplementation on mean levels of left and right CIMT. This might be explained by the normal levels of CIMT in the study population (85 % in the placebo group and 82 % in the intervention group). A few studies have evaluated the association between vitamin D levels and CIMT. In a study by Choi et al. ( Reference Choi, Lo and Mulligan 26 ), it was observed that there is a negative relation between vitamin D levels and CIMT in HIV-infected persons. In addition, vitamin D and K supplementation among postmenopausal women for 3 years did not influence CIMT, but affected elastic properties of the arterial vessel wall( Reference Braam, Hoeks and Brouns 8 ). However, there was no direct association between serum 25(OH)D and CIMT in HIV-infected youth( Reference Eckard, Tangpricha and Seydafkan 27 ). Vitamin K intake may affect CIMT through the effect on the post-translational processing of matrix Gla-protein (MGP)( Reference Braam, Hoeks and Brouns 8 ), which is synthesised in a number of non-hepatic tissues, notably cartilage and the arterial vessel wall and is regarded as a major inhibitor of soft tissue calcification( Reference Price, Faus and Williamson 28 ). In addition, vitamin D has a role in the regulation of MGP gene expression, which may support the control of CIMT. In a study by Fraser & Price( Reference Fraser and Price 29 ), it was indicated that the MGP promotor contains a vitamin D response element that is responsible for a 2–3-fold enhancement of MGP expression after vitamin D binding.
The present study demonstrated that combined vitamin D, K and Ca supplementation in overweight patients with T2DM and CHD for 12 weeks was associated with a significant reduction in serum insulin, HOMA-IR and HOMA-B and a significant increase in QUICKI and serum HDL-cholesterol levels, but it did not affect FPG and other lipid profiles. Supporting our findings, Tabesh et al. ( Reference Tabesh, Azadbakht and Faghihimani 11 ) demonstrated that an 8-week supplementation with joint 1000 mg/d Ca and 1250µg/week vitamin D supplementation among patients with T2DM improved glycaemic control and lipid profiles. We have previously shown that 1000 mg Ca/d and a 1250µg vitamin D3 twice during the study (at study baseline and on day 21 of the intervention) among patients with gestational diabetes mellitus resulted in improved parameters of glucose homoeostasis as well as LDL-cholesterol and HDL-cholesterol levels( Reference Asemi, Karamali and Esmaillzadeh 10 ). Furthermore, vitamin K supplementation for 36 months at a dosage of 500 µg/d decreased HOMA-IR among older men, but it did not affect older women( Reference Yoshida, Jacques and Meigs 12 ). Vitamin D and Ca supplementation may improve metabolic profiles through their effects on up-regulation of the insulin receptor genes( Reference Maestro, Molero and Bajo 30 ) and the regulation of insulin secretion from the pancreatic β-cells( Reference Sergeev and Rhoten 31 ). Furthermore, previous studies have proposed that osteocalcin and vitamin K administration may improve insulin sensitivity through the enhancement of β-cell proliferation, adiponectin expression( Reference Lee, Sowa and Hinoi 32 ) and suppression of inflammation( Reference Reddi, Henderson and Meghji 33 ).
Our study showed that compared with placebo, vitamin D, K and Ca co-administration for 12 weeks decreased serum hs-CRP and plasma MDA concentrations, whereas it did not influence NO and other biomarkers of oxidative stress in overweight diabetic patients with CHD. Our previous study among patients with polycystic ovary syndrome has indicated that taking Ca plus vitamin D supplements for 8 weeks significantly decreased hs-CRP, MDA and increased TAC and GSH, whereas it did not affect NO levels( Reference Foroozanfard, Jamilian and Bahmani 34 ). In addition, joint vitamin D and Ca supplementation among patients with congestive heart failure for 14 weeks improved biomarkers of oxidative stress( Reference Zia, Komolafe and Moten 35 ). However, taking 2 g/d Ca plus 20µg vitamin D for 6 months had no significant effects on hs-CRP levels among patients with colorectal adenoma( Reference Hopkins, Owen and Ahearn 36 ). Less production of PTH after taking Ca and vitamin D supplements would result in decreased production of inflammatory cytokines( Reference Brandi 37 ), which in turn might explain the effects of Ca and vitamin D on hs-CRP. A decrease in reactive oxygen species and pro-inflammatory cytokines by vitamin D supplements might explain its favourable effects on oxidative stress( Reference Jain and Micinski 38 ).
To interpret the findings of the present study, some limitations need to be considered. Owing to limited funding, we did not assess the effects of vitamin D, K and Ca co-supplementation on vitamin K levels, osteocalcin or MGP and other inflammatory cytokines. We agreed that compliance and dispensability are related to elasticity, and that is the outcome mostly affected by vitamin K; however, due to funding limitations, we did not evaluate vascular compliance and dispensability. In addition, the treatment effects observed in our study result from an indistinguishable component of the supplements. Therefore, further studies are needed with single supplements used in the present study in order to evaluate the beneficial effects on CIMT and metabolic status. In the present study, the sample size was also small. Furthermore, our study was relatively of short duration of intervention. Long-term interventions with bigger sample size might result in greater changes in the clinical end points. It must also be kept in mind that the beneficial effect seen in the present study by vitamin D, K and Ca co-supplementation might be explained by the fact that the mean serum 25(OH)D concentrations were low in both groups at baseline. Vitamin D, K and Ca supplementation has been hypothesised to act jointly rather than independently. Thus, a relatively low dose of cholecalciferol (10 µg) and vitamin K (180 µg) that was effective in this population might not be in others with better status. As the study was carried out during summer (June through September), we did not expect to see a difference in serum vitamin D concentrations between the two groups. Furthermore, the study participants were elderly subjects who normally have limited physical activity levels and sun exposure than others.
Overall, vitamin D, K and Ca co-supplementation for 12 weeks among diabetic patients with stable CHD had beneficial effects on maximum levels of left CIMT, glycaemic control, HDL-cholesterol, hs-CRP and MDA levels; however, it did not affect maximum levels of right CIMT, mean levels of left and right CIMT, other lipid profiles and biomarkers of oxidative stress. Furthermore, the effect of vitamin D, K and Ca co-supplementation on maximum levels of left CIMT could be a chance finding.
Acknowledgements
The present study was funded by a grant from the Vice-chancellor for Research, KUMS, Iran.
The study was supported by a grant from KUMS.
Z. A. and A. E. contributed to the conception, design, statistical analysis and drafting of the manuscript. F. R., F. B., Z. R., H. R. T., M. R., S. P., M. D. M., S. T. and A. A. M. contributed to the data collection process and manuscript drafting. A. E. supervised the study. All the authors approved the final version for submission.
The authors declare that there are no conflicts of interest.








