As a result of increasing cost and limited supplies of fish meal, the aquaculture industry has elevated the levels of lipids in the diet, so as to decrease protein utilisation for energy and increase lipid consumption in the aquaculture industry( Reference Li, Jiang and Liu 1 ). However, prolonged energy imbalance caused by a high-fat diet leads to increased body fat mass and prevalence of the metabolic syndrome in aquatic animals( Reference Holdsworth, Ati and Bour 2 ). Therefore, it is a high attractive issue in present aquatic nutrition research that an efficient lipid-lowering factor could be found to inhibit lipid accumulation, promote lipid as a key energy source to supply and have the protein-sparing effect.
Recent studies have indicated that α-lipoic acid (LA) has potential positive effects on obesity and its related disorders. The research of Kim et al. ( Reference Kim, Park and Namkoong 3 ) showed for first time that α-LA exerted its anti-obesity effect in rodents by reducing food intake through suppressing hypothalamic AMP kinase α (AMPKα) activity. However, other studies provided the evidence to reveal the anti-obesity mechanism of α-LA that it also could increase energy expenditure by activating phosphorylation of AMPKα in muscle( Reference Stark, Ashley and Andrews 4 ). Therefore, there is a dual effect of α-LA on AMPKα in different tissues, but the mechanism of AMPKα activation by LA in different situations and parts of the organism has yet to be elucidated. The pivotal anti-obesity mechanism is to promote the breakdown and obliteration of TAG( Reference Ormseth, Swift and Fazio 5 , Reference Walther and Farese 6 ). Thus, several studies have suggested that α-LA has a positive lipolytic property in animals. Dietary α-LA could increase the release of free fatty acids and glycerin in the plasma of chickens( Reference Hamano 7 ). Lipolysis, as a complicated process, involves several lipases and lipid droplet proteins in various tissues, including adipose TAG lipase (ATGL) and hormone-sensitive lipase (HSL)( Reference Jaworski, Ahmadian and Duncan 8 , Reference Reid, Ables and Otlivanchik 9 ). Further studies indicate that the mechanism of lowering effect on α-LA is to increase ATGL protein content via activating phosphorylation of AMPKα in HepG2 cells( Reference Kuo, Lin and Chen 10 ). However, recent studies demonstrated that increasing lipolysis and free fatty acid release induced by α-LA from adipocyte tissue do not have significant effect on insulin resistance in mammals( Reference Ormseth, Swift and Fazio 5 ). Therefore, α-LA might have the ability to utilise fatty acids that are released from TAG via β-oxidation. Furthermore, the improvement of lipid consumption, like fatty acid β-oxidation, has a protein-sparing effect via decreasing protein utilisation for energy in fish( Reference Li, Jiang and Liu 1 ). However, it is yet to be clarified whether α-LA has protein-sparing effects via regulation of lipid metabolism in fish.
The previous study indicated that α-LA could activate phosphorylation of mammalian target of rapamycin (mTOR) pathway in brain endothelial cells to promote protein synthesis( Reference Xie, Li and Ling 11 ). Besides, α-LA could promote glomerular mesangial cell growth and secretion of matrix proteins via activating the mTOR pathway under high-glucose conditions( Reference Lv, Wu and Zhou 12 ). However, there is no evidence currently suggesting that α-LA promotes protein deposition and synthesis in vivo.
Grass carp (Ctenopharyngodon idellus) is one of the economically important herbivorous freshwater fish farmed in China( Reference Wu, Wang and Angert 13 ) and easily accumulates excessive fat in the abdominal cavity and suffers from fatty liver in aquaculture( Reference Du, Clouet and Zheng 14 ). So grass carp has been regarded as quite a good model for lipid metabolism study in fish.
Above all, in the present study, we aimed to investigate whether α-LA, as an efficient nutrient factor, has a protein-sparing effect via promoting lipid catabolism, increasing the energy supply from lipid sources and sparing protein from energy production to increase protein deposition in grass carp.
Methods
Experimental diets
The formulation and proximate composition of the experimental diets are shown in online Supplementary Table S1. In all, three isonitrogenous (33·0 % crude protein) and isolipidic (5·0 % crude lipid) experimental diets were supplemented with α-LA at different concentrations of 0 (control, without extra α-LA addition), 600 mg/kg (LA1) and 1200 mg/kg (LA2) diet. The dietary level of LA in the present study is based on the results of study by Kütter et al. ( Reference Kütter, Monserrat and Primel 15 ) in Trachinotus marginatus. This study has reported that high reduction in specific growth rate at high doses (1750 and 3000 mg/kg) and diets supplemented with 500 and 1000 mg/kg exhibited the positive effects of LA, such as lower thiobarbituric acid-reactive substances in muscle and higher glutathione S-transferase activity in brain compared with the control group( Reference Kütter, Monserrat and Primel 15 , Reference Kütter, Romano and Ventura-Lima 16 ). α-LA (>99 % purity) was obtained from Suzhou Fushilai Pharmaceutical Co. Ltd. All ingredients were purchased from Hua-qin Husbandry and Technology Co. Ltd. Fish meal, soyabean meal, cottonseed meal and other ingredients were used as dietary protein sources. Soyabean oil (Kerry Oils & Grains Co. Ltd), lard oil (Kangle Market) and linseed oil (Hoval Seasons Bio-Sci Co. Ltd) were added to satisfy the essential fatty acid requirements (1·0 % linoleic acid and 1·0 % α-linolenic acid) of grass carp( Reference Glencross 17 ). The dough was pelleted to proper size (2·5 mm pellet diameter) and the pellets were dried in a cool and well-ventilated room for about 12 h and then kept in a freezer at −20°C until use.
Experimental procedure
The experimental procedures were similar to those described in our previous study( Reference Shi, Jin and Sun 18 ). After fasting for 24 h, 162 fish (initial body weight: 18·99 (sd 1·82) g) were randomly distributed into nine 225-litre tanks (diameter=0·75 m, height=0·7 m) in a recirculation system. Each diet was distributed randomly to triplicate aquariums. The fish were hand-fed the experimental diets three times daily (at 08.30, 12.30 and 16.30 hours, respectively) to apparent satiation for 8 weeks. The feed intake was recorded. Each tank was supplied with a heating rod for maintaining the water temperature at 28°C and the photoperiod was 12 h light–12 h dark. During the feeding experiment, water was circularly filtered and renewed by 1/5 daily to maintain acceptable water quality. The dissolved O2, pH and ammonia content maintained at 15·03 (sd 0·17), 7·32 (sd 0·08) and 0·10 (sd 0·04) mg/l, respectively. Dead fish were weighed and recorded for calculating the mortalities.
Sampling procedure and calculations
The procedures were approved by the Animal Care and Use Committee of Northwest A&F University and performed in accordance with animal welfare and ethics. After 8 weeks of feeding, the fish were fasted for 24 h. All fish were anaesthetised with tricaine methane sulfonate (MS-222; 0·06 g/l; Sigma-Aldrich). Fish were weighed and their body length was measured. In all, two fish per aquarium were kept at −20°C for whole body proximate composition analysis. In all, six fish per aquarium were randomly selected for blood collection from the caudal vein, and sampled blood was allowed to clot at 4°C for 6 h. The serum of six fish was collected after centrifugation of the clot for 10 min (825 g , 4°C), which were pooled as three samples per aquarium, frozen in liquid N2 and stored at −80°C until analysis. The muscle and hepatopancreas that were excised from the six fish per aquarium were stored at −20°C for proximate composition analysis. The remaining fish were killed and dissected. After weighing the total visceral weight, the hepatopancreas, intraperitoneal fat (IPF), kidney, spleen and intestine were stripped and weighed. Afterwards, samples of the hepatopancreas, muscle and IPF from three fish per aquarium were frozen in liquid N2 and then stored at –80°C for gene expression and Western blot analysis, respectively, and hepatopancreas and IPF from the other four fish per aquarium were fixed in paraformaldehyde solution for histology analysis.
The following formulae were used to calculate growth performance and biological parameters:
![]() $$\hskip-10pt{\rm Specific\ growth \ rate }\left \ ( {{\rm %/d}} \right){\rm {\equals}}( {{\rm Ln \ final\ weight }\left \ ( {\rm g} \right ) \ – {\rm \ Ln \ initial \hskip124.5pt{\rm weight (g)} \right) {\rm {\times}100\,/\,d }$$
$$\hskip-10pt{\rm Specific\ growth \ rate }\left \ ( {{\rm %/d}} \right){\rm {\equals}}( {{\rm Ln \ final\ weight }\left \ ( {\rm g} \right ) \ – {\rm \ Ln \ initial \hskip124.5pt{\rm weight (g)} \right) {\rm {\times}100\,/\,d }$$
![]() $${\rm Survival \ rate } \ \left( {\rm \,\%\,} \right){\rm {\equals}final \ number \ of \ fish} \times}{\rm 100\ initial \ number \ of }{\rm \ fish} $$
$${\rm Survival \ rate } \ \left( {\rm \,\%\,} \right){\rm {\equals}final \ number \ of \ fish} \times}{\rm 100\ initial \ number \ of }{\rm \ fish} $$
![]() $$\hskip-10pt\quad{\rm Feed \ conversion \ ratio{\equals} amount \ } {\rm of \ feed \ given \left \ ( {\rm g} \right) \, {\rm weight} {\rm \ gain \ }\left( {\rm g} \right)$$
$$\hskip-10pt\quad{\rm Feed \ conversion \ ratio{\equals} amount \ } {\rm of \ feed \ given \left \ ( {\rm g} \right) \, {\rm weight} {\rm \ gain \ }\left( {\rm g} \right)$$
Cell culture and treatment
The hepatocytes (L8824) of grass carp were bought from the China Centre for Type Culture Collection (CCTCC). The hepatocytes were placed into M199 cell culture medium (Thermo Scientific) with 10 % fetal bovine serum and incubated at 28°C in a 5 % CO2 humidified atmosphere, and the medium was refreshed every 2 or 3 d until cell confluence. For experiment, the confluent hepatocytes were then placed into M199 cell culture medium with 250 μm palmitic acid (PA) (P5585; Sigma-Aldrich Inc.) and cultivated at 28°C in a 5 % CO2 humidified atmosphere for 24 h. Then, the cells were treated with α-LA (T1395; Sigma-Aldrich Inc.) for 24 h. About 20 μm compound C (HY-13418; Medchem Express Inc.) in water, the inhibitor of phosphorylation of AMPKα, was added 30 min before the addition of α-LA.
Proximate composition analysis
The proximate composition of the diets, hepatopancreas, muscle and whole body were determined according to the methods of the Association of Official Analytical Chemists Procedures (1995)( Reference Cunniff 19 ). Briefly, the samples were dried to a constant weight to determine moisture at 105°C. Crude protein was determined by measuring N (N×6·25) of the samples using the Kjeldahl method. Crude lipid was measured by diethyl ether extraction using the Soxhlet method.
TAG measurement
The TAG measurement was performed based on the standard method, as previously described( Reference Zhou, Jian and Hong 20 ). After the treatment, cells in each well were collected for the measurement of glycerol concentration using a Triglyceride Determination Kit (Jiancheng Biotechnology Co.) according to the manufacturer’s protocol. The kit utilises a spectrophotometric method with glycerol-3-phosphate oxidase and phenol aminophenazone. The increase in absorbance at 510 nm is directly proportional to the TAG concentration of the sample.
NEFA and enzyme activity of alanine aminotransferase and aspartate aminotransferase measurement
The NEFA and enzyme activity of ALT and AST from serum, hepatopancreas and muscle were measured by using an assay kit (Jiancheng Biotechnology Co.). The content of NEFA and enzyme activity of ALT and AST were measured based on the user’s manual step by step.
Histological processing and morphological evaluation
The samples of fixed hepatopancreas and IPF were washed in tap water for 12 h, followed by a routine dehydration in a graded series of ethanol (30, 50, 70, 80, 90, 95 and 100 % twice). The samples were then equilibrated in xylene and embedded in paraffin based on the standard histological techniques as described previously( Reference Liu, Ji and Li 21 , Reference Tian, Lei and Ji 22 ). Afterwards, sections were cut at 5 μm with the use of a rotary microtome (RM2235; Leica) and mounted in glass slides, which were then stained with haematoxylin and eosin. Histological samples were observed and photographed by using an upright microscopy (Leica Biosystems). The average adipocyte size per image was quantified using Photoshop (Adobe), as previously described( Reference Osman, Selway and Kępczyńska 23 ). An average value across five non-overlapping images (five/section) was calculated for each group.
Oil Red O staining
Oil red O staining was performed based on the standard method, as previously described( Reference Ramírezzacarías, Castromuñozledo and Kuriharcuch 24 ). The dye was extracted with isopropanol. The optical density (OD) was estimated at an absorbance of 490 nm using a microtitre plate spectrophotometer (Multiskan MK3; Thermo Labsystems). The cellular lipid content was calculated from the OD value.
Real-time quantitative RT-PCR
RNA extraction, complementary DNA synthesis and gene expression measurements of tissues and cell samples in experiments were performed as described previously(
Reference Shi, Jin and Sun
18
,
Reference Shi, Sun and Yang
25
). The primer sequences for β-actin, ATGL, HSL, fork-head box O1 (FOXO1), PPAR
α, lipoprotein lipase (LPL), fatty acid synthase (FAS), diacylglycerol O-acyltransferase (DGAT), PPAR
γ, carnitine palmitoyltransferase-1α (CPT-1α), target of rapamycin (TOR), insulin-like growth factor-1, ribosomal protein S6 kinase (S6K), glutamate dehydrogenase (GLDH), myogenic differentiation antigen (MyoD) and myogenin (MyoG) are listed in online Supplementary Table S2. After the PCR, melting curves were analysed to confirm that single products were obtained in these reactions. A relative quantification method, the comparative C
t method (
![]() $$2^{{{\minus}\Delta \Delta C_{{\rm t}} }} $$
), was used to calculate the gene expression values, as described in the literature(
Reference Livak and Schmittgen
26
,
Reference Pfaffl
27
).
$$2^{{{\minus}\Delta \Delta C_{{\rm t}} }} $$
), was used to calculate the gene expression values, as described in the literature(
Reference Livak and Schmittgen
26
,
Reference Pfaffl
27
).
Western blotting analysis
Tissues were homogenised with glass Tenbroeck tissue grinders (Kimble Chase) on ice and lysed with lysis buffer (Beyotime Technologies Inc.) containing protease and phosphatase inhibitor cocktails (Roche) at 4°C for 1 h and cleared by centrifugation at 13 000 g for 10 min at 4°C. Cultured cells were lysed in lysis buffer containing protease and phosphatase inhibitor cocktails on ice. Crude lysates were centrifuged at 13 000 g for 10 min at 4°C. Total protein concentration from the resultant supernatant was determined by a bicinchoninic acid protein assay kit (Biobox, Biotech. Co. Ltd). Protein samples were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Beyotime Technologies Inc.) by electroblotting. Membranes were incubated overnight at 4°C with primary antibodies. After washing, the secondary antibody was added and incubated for 2 h at room temperature and protein bands were visualised by ECL Plus (ZETA). The membranes were then stripped and reprobed with anti-β-actin antibody. Densitometric quantitation was performed using a Sagecreation imaging system with Sagecreation Quantity One software (Sagecreation Co. Ltd). The following antibodies (Cell Signaling Technology) were used: antibodies against AMPKα (2532), phosphor-AMPKα (Thr172, 2531), protein kinase B (Akt, 9272), phosphor-Akt (Ser473, 9271), TOR (2972), phosphor-TOR (Ser2448, 2971), p70S6K (9202), phosphor-p70S6K (Ser389, 9234), eukaryotic initiation factor 4E binding protein (4E-BP)-1 and phosphor-4E-BP1 (Thr37/46, 9459). All these antibodies were developed using antigenic regions completely conserved in fish, and many had been successfully used in fish and published before( Reference Bian, Jiang and Man 28 ).
Statistical analysis
The values of biological parameters (replicates of sixteen samples per aquarium) and proximate composition of tissues (replicates of six samples per aquarium) are means and standard deviations, n 3. The values of whole body proximate composition analysis (replicates of six samples per group) are means and standard deviations, n 6. And the values of others are means and standard deviations, n 9 (replicates of nine samples per group). Percentage data were arcsine-transformed before analysis. One-way ANOVA was used to compare differences among the experimental groups, followed by Duncan’s post hoc tests. All analyses were conducted using PASW Statistics 18 (SPSS). A significance level of P<0·05 was used for all tests.
Results
Dietary α-lipoic acid inhibits lipid accumulation and promotes protein deposition in grass carp in vivo
No significant differences in the final body weight, specific growth rate, feed conversion ratio, survival rate and other parameters, including VSI, KI, SI and RIL, were observed in all treatment groups (P>0·05) (Table 1). However, the lipid content of the whole body was significantly decreased in those fish fed on the diet with α-LA at the doses of 600 and 1200 mg/kg, compared with the control group (P<0·05) (Table 1). The fish fed on the α-LA diet showed a remarkable decrease in adipocyte size and IPF index (P<0·05) (Fig. 1(J) and (K)). Meanwhile, the TAG content of serum in the treatment at the dose of 1200 mg LA/kg was significantly lower than that of the control group and the dietary α-LA 600 mg/kg group (P<0·05) (Fig. 1(A)), and NEFA content of serum in the control group was remarkably higher than the content in the LA groups (P<0·05) (Fig. 1(B)). In addition, the crude lipid content of the hepatopancreas and muscle showed a significant decrease in fish fed on the α-LA diet (P<0·05) (Fig. 1(E) and 1(G)), with the lipid content of the hepatopancreas of fish in LA groups being observably lower than that in the control group (P<0·05) (Fig. 1(E)). However, NEFA content of the hepatopancreas and muscle was not significant among all groups (P>0·05) (Fig. 1(F) and (H)). The ALT and AST enzyme activity showed a significant decrease in LA groups (P<0·05) (Fig. 1(C) and (D)), although the morphological analysis of the hepatopancreas exhibited no marked change among all groups (Fig. 1(I)).
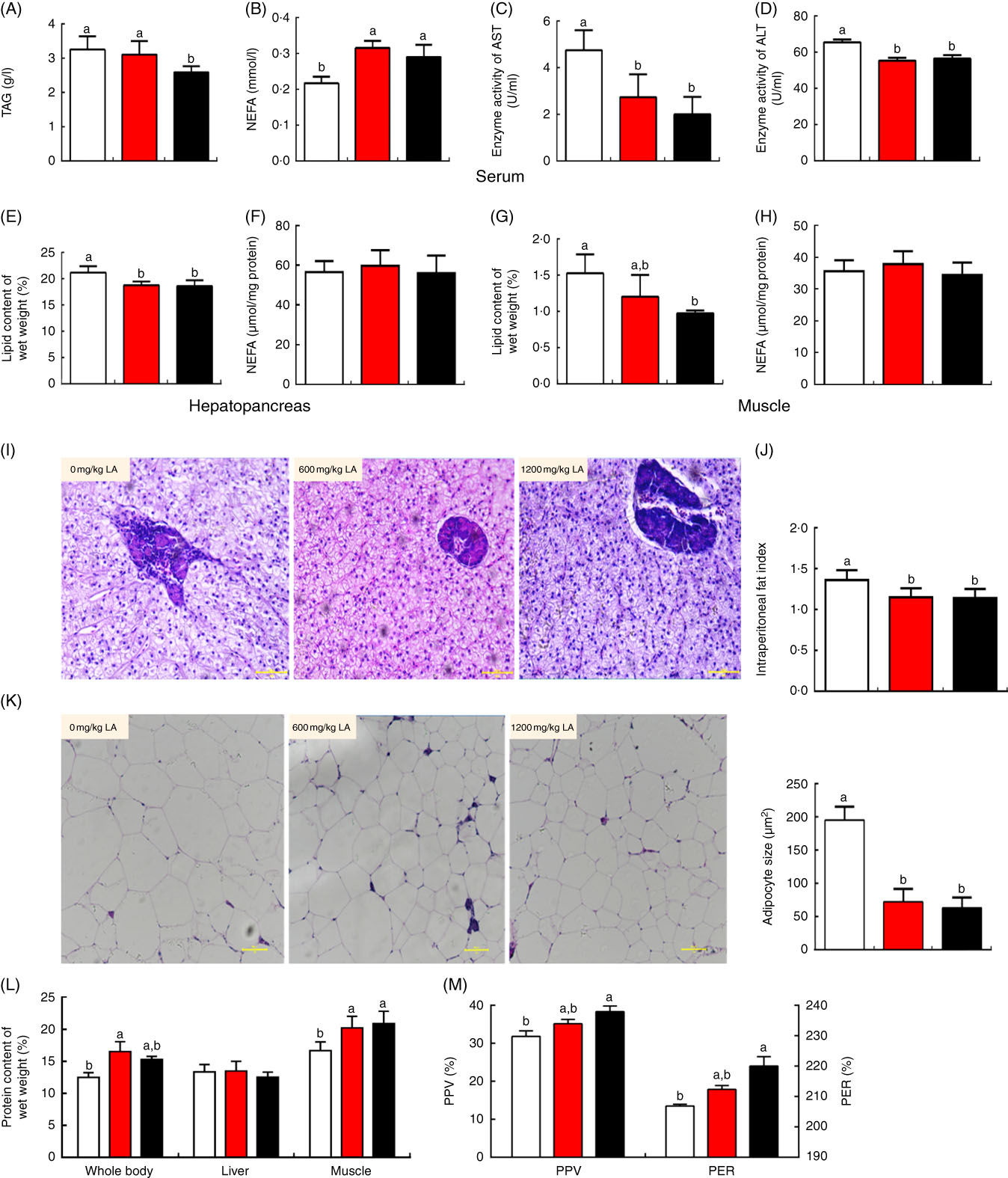
Fig. 1 Dietary α-lipoic acid (LA) inhibits lipid accumulation and promotes protein deposition in grass carp in vivo. (A) TAG content of serum in experimental fish; (B) NEFA content of serum in experimental fish; (C and D) enzyme activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum; (E and F) lipid and NEFA content of the hepatopancreas in experimental fish; (G and H) lipid and NEFA content of muscle in experimental fish; (I) morphology of the hepatopancreas in experimental fish (haematoxylin and eosin staining, original magnification 200×); (J) intraperitoneal fat index; (K) morphology of intraperitoneal fat (haematoxylin and eosin staining, original magnification 200×) and adipocyte size in experimental fish; (L) crude protein content of the whole body, hepatopancreas and muscle in experimental fish; (M) protein efficiency ratio (PER) and protein productive value (PPV) of experimental fish. The values of biological parameters (replicates of sixteen samples per aquarium) and proximate composition of tissues (replicates of six samples per aquarium) are means, with standard deviations represented by vertical bars, n 3. The values of whole body proximate composition analysis (replicates of six samples per group) are means, with standard deviations represented by vertical bars, n 6. Other values are means, with standard deviations represented by vertical bars, n 9 (replicates of nine samples per group). a,b Mean values with unlike letters were significantly different between groups, P<0·05 (one-factor ANOVA, Duncan’s post hoc test). ![]() , 0 mg/kg LA;
, 0 mg/kg LA; ![]() , 600 mg/kg LA;
, 600 mg/kg LA; ![]() , 1200 mg/kg LA.
, 1200 mg/kg LA.
Table 1 Effects of dietary α-lipoic acid (LA) on the biological parameters and whole-body chemical analysis of grass carp (Mean values and standard deviations, n 3 per group)

a,b Mean values with unlike superscript letters were significantly different (P<0·05).
* Calculated as percentage of wet weight.
Interestingly, the crude protein content of the whole body and muscle showed a significant increase in the fish fed on the α-LA diet at doses of 600 and 1200 mg/kg compared with the control group except for which in the hepatopancreas (P<0·05) (Fig. 1(L)). The protein efficiency ratio and protein productive value, which reflect the status of protein deposition in the body, were significantly increased in fish fed on the α-LA diet at the dose of 1200 mg/kg, compared with the control group and fish fed α-LA at the dose of 600 mg/kg (P<0·05) (Fig. 1(M)).
Effect of dietary α-lipoic acid on protein expression and mRNA expression levels of genes involved in lipid metabolism in grass carp in vivo
To expound the mechanism of α-LA inhibits lipid accumulation in grass carp, the protein expression and mRNA expression levels of genes involved in lipid metabolism in the hepatopancreas, muscle and IPF were detected, and the results were shown in Fig. 2 and online Supplementary Fig. S1. Results indicated that the mRNA expression of ATGL was significantly up-regulated in IPF, hepatopancreas and muscle of the fish fed with dietary α-LA at doses of 600 and 1200 mg/kg (P<0·05) (Fig. 2(D)–(F)). Dietary α-LA at doses of 600 and 1200 mg/kg significantly up-regulated the mRNA expression of LPL in the hepatopancreas and muscle (P<0·05) (Fig. 2(E) and (F)). As shown in Fig. 2(D)–(F), dietary α-LA at doses of 600 and 1200 mg/kg could significantly increase the PPAR α mRNA expression level in the IPF and muscle except for the hepatopancreas. However, the mRNA expression of CPT-1α was markedly up-regulated in muscle and hepatopancreas in the treatment at doses of 600 and 1200 mg LA/kg (P<0·05), but not in the IPF (P>0·05) (Fig. 2(D)–(F)). In addition, mRNA expression levels of other genes involved in lipolysis, fatty acid de novo synthesis and re-esterification showed no significant differences among all groups, including HSL, FOXO1, FAS and DGAT1 (P>0·05) (online Supplementary Fig. S1). Similar to mRNA expression results, dietary α-LA significantly increased the ATGL protein expression in muscle, hepatopancreas and IPF. However, α-LA supplementation at doses of 600 and 1200 mg/kg significantly decreased PPARγ protein expression in IPF (P<0·05) (Fig. 2(A)–(C)). Interestingly, the phosphorylation level of AMPKα was significantly increased in the IPF, hepatopancreas and muscle of the fish fed on the α-LA diet at doses of 600 and 1200 mg/kg (P<0·05) (Fig. 2(A)–(C)).
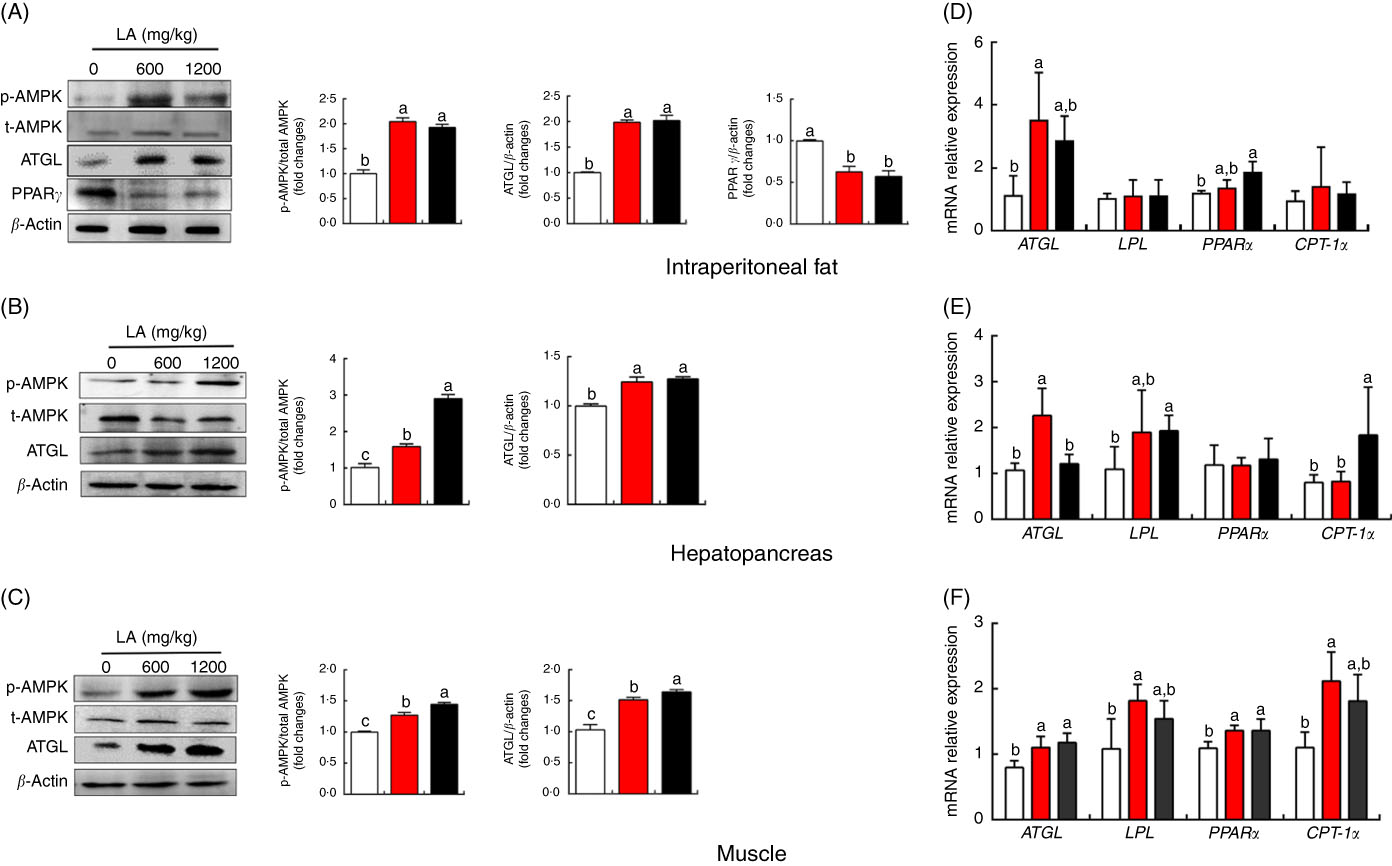
Fig. 2 Effect of dietary α-lipoic acid (LA) on protein expression levels of genes involved in lipid metabolism in intraperitoneal fat (A), hepatopancreas (B) and muscle (C) of grass carp in vivo. Effect of dietary LA on mRNA expression levels of genes involved in lipid metabolism in intraperitoneal fat (D), hepatopancreas (E) and muscle (F) of grass carp in vivo. Values are means, with standard deviations represented by vertical bars, n 9. a,b,c Mean values with unlike letters were significantly different between groups, P<0·05 (one-factor ANOVA, Duncan’s post hoc test). p-AMPK, phosphorylated AMP kinase; t-AMPK, total AMP kinase; ATGL, adipose TAG lipase; LPL, lipoprotein lipase; CPT-1α, carnitine palmitoyltransferase-1α. ![]() , 0 mg/kg LA;
, 0 mg/kg LA; ![]() , 600 mg/kg LA;
, 600 mg/kg LA; ![]() , 1200 mg/kg LA.
, 1200 mg/kg LA.
α-Lipoic acid promotes lipolysis via modulating AMP kinase pathway in grass carp in vivo
To explore the mechanism of lipolysis regulated by α-LA, we examined the effect of α-LA in cell culture. The hepatocytes of grass carp were treated with a series of LA concentrations (0, 50, 250, 500 and 1000 μm) for 24 h. Quantification using oil red O staining was conducted and TAG content was measured, and the results were shown in Fig. 3(A) and (B). Results indicated that α-LA significantly decreased the TAG content in a dose-dependent manner (P<0·05).
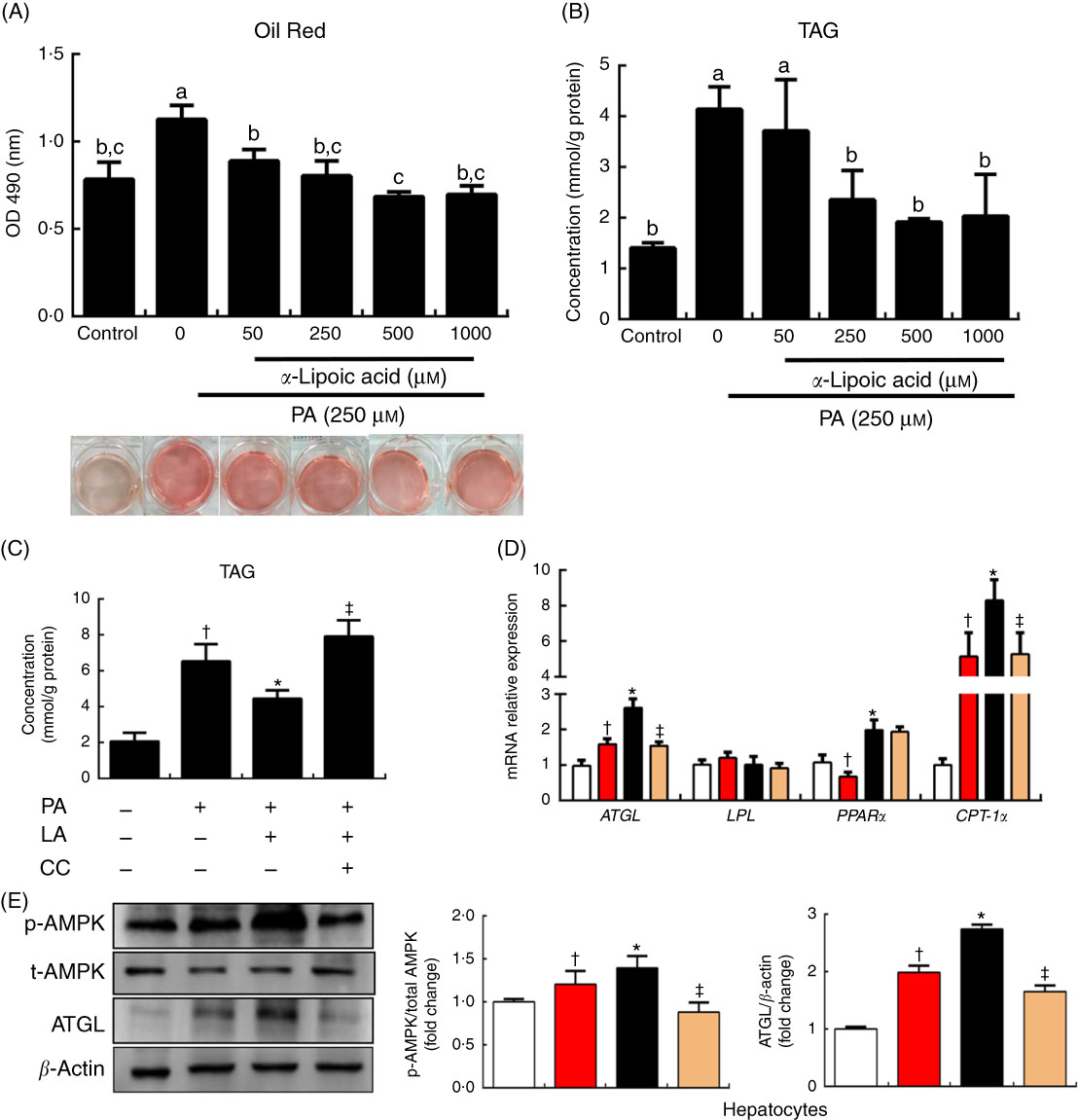
Fig. 3
α-Lipoic acid (LA) modulates lipolysis via the AMP kinase (AMPK)–adipose TAG lipase (ATGL) pathway in grass carp hepatocytes. (A) Oil red O staining of hepatocytes upon 24-h α-LA treatment (0, 50, 250, 500 and 1000 μm) after 24 h 250 μm palmitic acid (PA) treatment. Quantification of Oil red O staining was performed by measuring absorbance at 490 nm. (B) TAG content of the hepatocytes upon 24-h α-LA treatment (0, 50, 250, 500 and 1000 μm) after 24-h 250 μm PA treatment. (C) TAG content of the hepatocytes in medium with 250 μm PA pretreated with compound C (CC; 20 μm) for 30 min before the addition of α-LA (250 μm) for 24 h. (D and E) Protein and mRNA expression levels of genes involved in lipid metabolism in the hepatocytes after treatment. The results were obtained from three independent experiments in triplicate. (A and B) Values are means, with standard deviations represented by vertical bars, n 9. a,b,c Mean values with unlike letters were significantly different between groups, P<0·05 (one-factor ANOVA, Duncan’s post hoc test). (C–E) Values are means, with standard deviations represented by vertical bars, n 9. *P<0·05, relative to control (![]() ) v. PA (
) v. PA (![]() ); †P<0·05, relative to PA v. PA+LA (
); †P<0·05, relative to PA v. PA+LA (![]() ); ‡P<0·05, relative to PA+LA v. PA+LA+CC (
); ‡P<0·05, relative to PA+LA v. PA+LA+CC (![]() ). OD, optical density; LPL, lipoprotein lipase; CPT-1α, carnitine palmitoyltransferase-1α; p-AMPK, phosphorylated AMP kinase; t-AMPK, total AMP kinase.
). OD, optical density; LPL, lipoprotein lipase; CPT-1α, carnitine palmitoyltransferase-1α; p-AMPK, phosphorylated AMP kinase; t-AMPK, total AMP kinase.
To evaluate the ability of α-LA to modify the AMPK pathway involved in the regulation of lipolysis, the PA treatment hepatocytes were treated with compound C for 30 min, before the addition of α-LA (250 μm, 24 h). As shown in Fig. 3, the TAG content was significantly increased following PA treatment, compared with the control group (P<0·05) (Fig. 3(C)). However, α-LA markedly inhibited the increase induced by PA, and compound C dramatically inhibited the ability of α-LA (P<0·05) (Fig. 3(C)). α-LA significantly increased the levels of phosphorylation of AMPK and protein expression of ATGL compared with PA treatment, and compound C remarkably prevented the increase induced by α-LA (P<0·05) (Fig. 3(E)). The mRNA expression levels of ATGL, PPARα and CPT-1α were notably increased after α-LA treatment; however, compound C significantly inhibited the mRNA expression of genes involved in lipolysis and fatty acid β-oxidation (P<0·05) (Fig. 3(D)).
Effect of dietary α-lipoic acid on protein expression and mRNA expression levels of genes involved in protein synthesis in grass carp in vivo
To explore the potential mechanism underlying the ability of α-LA to promote protein synthesis and deposition in grass carp in vivo, the protein and mRNA expression levels of genes involved in protein metabolism of muscle and hepatopancreas were detected, and the results were shown in Fig. 4. Compared with the control group, no significant differences in the phosphorylation levels of mTOR, AKT and S6K were detected in the hepatopancreas among all groups (P>0·05), except for the phosphorylation level of 4E-BP in the fish fed on the α-LA diet (P<0·05) (Fig. 4(A)). Interestingly, LA supplementation at the dose of 1200 mg/kg exhibited the highest phosphorylation levels of mTOR, AKT, S6K and 4E-BP in the muscle of fish (P<0·05) (Fig. 4(B)). Meanwhile, the mRNA expression level of mTOR was markedly up-regulated by α-LA at the dose of 1200 mg/kg compared with the control group and dietary α-LA 600 mg/kg group (Fig. 4(C)). Conversely, mRNA expression of GLDH was obviously down-regulated by α-LA compared with the control group (P<0·05) (Fig. 4(C)). However, the gene expression of MyoD and MyoG was not significantly different in fish fed on the α-LA diet from those of the control group (P>0·05) (Fig. 4(D)).
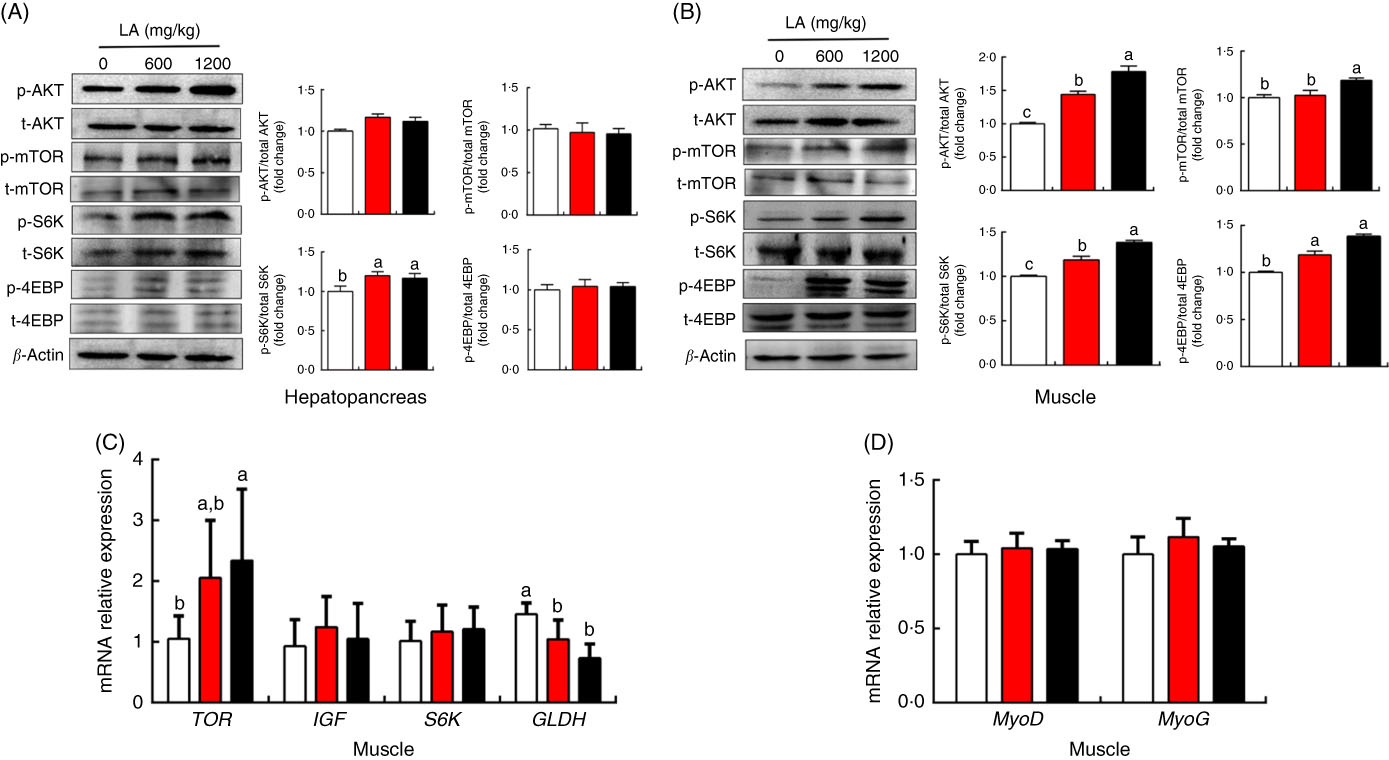
Fig. 4 Effect of dietary α-lipoic acid (LA) on protein expression of genes involved in protein metabolism in the hepatopancreas (A) and muscle (B) of grass carp in vivo. (C and D) Effect of dietary LA on mRNA expression levels of genes involved in protein metabolism in muscle of grass carp in vivo. Values are means, with standard deviations represented by vertical bars, n 9. a,b,c Mean values with unlike letters were significantly different between groups, P<0·05 (one-factor ANOVA, Duncan’s post hoc test). p-AMPK, phosphorylated AMP kinase; t-AMPK, total AMP kinase; p-mTOR, phosphorylated mammalian target of rapamycin; t-mTOR, total mammalian target of rapamycin; p-S6K, phosphorylated ribosomal protein S6 kinase; t-S6K, total ribosomal protein S6 kinase; p-4EBP, phosphorylated eukaryotic initiation factor 4E binding protein; t-4EBP, total eukaryotic initiation factor 4E binding protein; TOR, target of rapamycin; IGF, insulin-like growth factor; S6K, ribosomal protein S6 kinase; GLDH, glutamate dehydrogenase; MyoD, myogenic differentiation antigen; MyoG, myogenin. ![]() , 0 mg/kg LA;
, 0 mg/kg LA; ![]() , 600 mg/kg LA;
, 600 mg/kg LA; ![]() , 1200 mg/kg LA.
, 1200 mg/kg LA.
Discussion
Since protein sources are the most expensive components in aquafeed, from an economic standpoint, protein-sparing by use of non-protein energy sources is gaining more attention from the aquaculture industry; it is believed that this can be achieved by increasing dietary lipid levels. However, increased lipid levels in the diet cause excess lipid accumulation and a higher prevalence of metabolism disorders in aquaculture practice( Reference Wang, Liu and Tian 29 – Reference Boujard, Gelineau and Coves 31 ). Grass carp, a herbivorous freshwater fish, is a good example, which fed high-energy feed easily and exhibit extensive body fat deposits, especially in viscera and liver during the cultivation( Reference Guo, Liang and Fang 32 ). Therefore, it is highly attractive to identify an efficient lipid-lowering factor to inhibit lipid accumulation and promote protein deposition in aquatic nutrition. α-LA is widely accepted that it possesses antioxidant properties in mammals and aquatic organisms(16, Reference Moura, de Andrade and dos Santos 33 ). The present study indicated that α-LA could promote lipolysis without loss of body weight and further promote fatty acid β-oxidation to increase energy supply from lipid catabolism and economise on the protein from energy production to increase protein deposition. In addition, α-LA has positive roles in protein synthesis through activating the mTOR pathway.
Mammal studies and human trials indicate that α-LA has the capability to reduce body weight, decrease food intake and elevate energy expenditure( Reference Kim, Park and Namkoong 34 – Reference Prieto-Hontoria, Pérez-Matute and Fernández-Galilea 36 ). However, in the present study, we revealed a different nutritional pattern in grass carp from mammals. No significant difference in final body weight and the feed conversion ratio was observed among all treatments at doses of 600 and 1200 mg/kg (P>0·05) (Table 1). The similar studies in aquatic organisms is reported that LA supplementation could cause an increase in the growth of Haliotis discus hannai and T. marginatus following a dose of 800 or 1000 mg LA/kg dry food, whereas higher doses (1600 or 3000 mg LA/kg dry food) resulted in a decrease in the growth( Reference Kütter, Monserrat and Primel 15 , Reference Zhang, Chen and Mai 37 ). This is different from previous result in human and other mammals. While the diets in the present study contained 5 % fat which is at ‘normal’ levels for grass carp in order to meet nutritional requirements( Reference Du, Clouet and Zheng 14 ), α-LA supplementation at doses of 600 and 1200 mg/kg significantly decreased the IPF index (Fig. 1(J)) and crude lipid content of the whole body (Table 1), hepatopancreas (Fig. 1(E)) and muscle (Fig. 1(G)) in the fish (P<0·05). Moreover, α-LA remarkably elevated the crude protein content of the whole body and muscle in grass carp (P<0·05) (Fig. 1(L)) although its potential for utilising high-energy diets and for protein-sparing effect is relatively small compared with other aquaculture species, such as salmonids or other carnivorous species( Reference Tian, Lu and Ji 38 , Reference Dabrowski 39 ). This may explain why α-LA prevents lipid accumulation without negative effect on the body weight of grass carp. The PER and PPV founded in the present study suggest a protein-sparing effect of LA at the dose of 1200 mg/kg (Fig. 1(M)) rather than 600 mg/kg. In the study of T. marginatus, the protein concentration in the liver was higher in the groups treated with 500 and 1000 mg/kg compared with the control group and the higher doses group( Reference Kütter, Monserrat and Primel 15 ). The previous study showed that high-dietary lipid levels could decrease protein utilisation for energy and improve lipid consumption( Reference Li, Jiang and Liu 1 ). We deduced that α-LA supplementation at the dose of 1200 mg/kg might promote the consumption of lipids and have a protein-sparing effect in grass carp. Fatty acid β-oxidation is an important source of energy in many animal tissues, including heart, muscle and liver( Reference Tocher 40 ). It was further demonstrated that α-LA supplementation at the dose of 1200 mg/kg observably increased NEFA content and decreased TAG content in serum (P<0·05) (Fig. 1(A) and (B)), while it had no significant effect on the NEFA content of the hepatopancreas and muscle (P>0·05) (Fig. 1(F) and (H)). Therefore, we proposed a hypothesis that the fatty acids transported from blood vessels might be primarily utilised for energy expenditure by β-oxidation in mitochondria rather than be stored in the hepatopancreas and muscle. As shown in Fig. 2(D) and (E), α -LA supplementation at the doses of 600 and 1200 mg/kg significantly increased the mRNA level of LPL in the muscle and hepatopancreas except for IPF. The main biological function of LPL is to catalyse the hydrolysis of TAG in plasma lipoproteins at the luminal surface of capillaries( Reference Mead and Ramji 41 ), so α-LA might promote the transportation of fatty acids from plasma to tissues. The present results further showed that α-LA supplementation at the doses of 600 and 1200 mg/kg could up-regulate the transcription of PPARα and CPT-1α in the muscle (P<0·05) (Fig. 2(F)), the latter protein being the rate-limiting enzyme for fatty acid β-oxidation in mitochondria( Reference Shi, Sun and Yang 25 ). In the previous study, α-LA could increase AMPK and acetyl-CoA carboxylase (ACC) phosphorylation, similar to the present study, leading to an increase in palmitate β-oxidation and decrease in TAG accumulation in C2C12 myotubesReference Chen, Kang and Wang ( 42 ). AMPK phosphorylates ACC, which decreases malonyl-CoA levels in cellsReference Masoodi, Kuda and Rossmeisl ( 43 ); malonyl Co-A is an inhibitor of CPT-1α activity( Reference Borthwick, Jackson and Price 44 ). Compound C was shown to significantly inhibit CPT-1α expression in hepatocytes of grass carp (P<0·05) (Fig. 3(D)). Therefore, α-LA might increase the expression and activity of CPT-1α via activating phosphorylation of AMPKα. Furthermore, α-LA significantly decreased the GLDH mRNA level (P<0·05) (Fig. 4(C)), as the key enzyme of amino acid catabolism, reflecting the status of protein catabolism in vivo ( Reference Smutna and Vorlova 45 ). The present study showed that α-LA might promote NEFA β-oxidation by regulating CPT-1α through activation of AMPKα phosphorylation to increase energy supply from lipid catabolism, as well as to spare protein from energy production to increase protein deposition.
TAG hydrolysation in lipid droplet is a primary source of NEFA( Reference Kim, Park and Namkoong 34 ). NEFA released during lipolysis from adipose tissue is transferred using lipoproteins from blood vessels to other tissues, before being transported into mitochondria, ultimately causing the oxidation and reduction in various cofactors that produce ATP( Reference Holloway, Luiken and Glatz 46 ). ATGL is a major TAG lipase found in many tissues( Reference Reid, Ables and Otlivanchik 9 ). The present result showed that, while the ATGL protein expression level became significantly increased, α-LA dramatically increased the phosphorylation level of AMPKα in the IPF, hepatopancreas and muscle in grass carp (P<0·05) (Fig. 2(A)–(C)). AMPK could integrate hormonal and nutrient signals and transmit these to the hypothalamus. Therefore, AMPK plays an essential role in regulating food intake( Reference Schneeberger and Claret 47 , Reference Targonsky, Dai and Koshkin 48 ). However, phosphorylation of AMPKα activated by α-LA supplementation at the doses of 600 and 1200 mg/kg in the muscle and hepatopancreas had no significant effect on the final body weight and the feed conversion ratio in grass carp (P>0·05) (Table 1). The result of Chen et al. ( Reference Chen, Chen and Chiang 49 ) showed that phosphorylation of AMPKα could increase ATGL protein expression. In the present study, compound C, an inhibitor of AMPKα phosphorylation, markedly inhibited the function of α-LA preventing lipid accumulation via elevating ATGL protein expression in grass carp hepatocytes (P<0·05) (Fig. 3(C)–(E)). The result indicated that α-LA might promote ATGL protein expression via activating phosphorylation of AMPKα. In addition, dietary α-LA at the doses of 600 and 1200 mg/kg has significantly decreased adipocyte size (Fig. 1(K)) and PPARγ protein expression (Fig. 2(A)) in IPF (P<0·05), indicating that α-LA exerts an anti-adipogenic effect in grass carp adipocytes, similar to the results of the study by Cho et al. ( Reference Cho, Moon and Moini 50 ) on 3T3-L1 adipocytes. These results indicated that lipid-lowering function of α-LA in grass carp might promote ATGL protein expression via activating phosphorylation of AMPKα in adipocyte tissue, hepatopancreas and muscle and preventing adipogenesis via mediating PPARγ in adipocytes.
The mTOR pathway plays a key role in protein synthesis and cellular nutrient sensing( Reference Wang and Proud 51 ). The results of the present study showed that α-LA significantly activated the phosphorylation of the AKT/mTOR/S6K/4E-BP pathway in the muscle of the experimental fish (P<0·05) (Fig. 4(B)). In mammals and fish, dietary α-LA could affect the content of free amino acids in the plasma and hippocampal cells( Reference de Freitas, De and Saldanha 52 , Reference Terjesen, Park and Tesser 53 ). The previous studies indicated that, while the level of amino acids changes in myofibrils, mTOR signalling is activated in mammals and fish( Reference Kimball and Jefferson 54 , Reference Lansard, Panserat and Plagnes-Juan 55 ). Therefore, we deduced that α-LA might activate mTOR signalling via altering the amino acid content in fish. However, α-LA has no effect on MyoD and MyoG, both of which play a vital role in muscle cell proliferation and differentiation( Reference Rudnicki, Schnegelsberg and Stead 56 , Reference Wright, Sassoon and Lin 57 ), and it indicated that the promotion of protein synthesis and deposition by α-LA was not through affecting muscle cell proliferation and differentiation. These results indicated α-LA has positive roles in protein synthesis through activating the mTOR pathway.
In conclusion, the present study demonstrated that α-LA could promote lipolysis via activating the AMPKα–ATGL pathway in grass carp without loss of body weight and that it could promote fatty acid β-oxidation by regulating the AMPKα–CPT-1α pathway to increase energy supply from lipid catabolism and spare the protein from energy production to increase protein deposition. Furthermore, α-LA has positive roles in protein synthesis through activating the mTOR pathway. So α-LA supplemented in diets for grass carp at doses between 600 and 1200 mg/kg could have a positive effect on lipid and protein metabolism. These results offer new insights into α-LA nutrition in vertebrates.
Acknowledgements
The authors thank Ankang Fisheries Experimental and Demonstration Station of the Northwest Agriculture and Forestry University for the experimental fish.
This work was financially supported by the National Basic Research Program of China (2014CB138603).
X.-c. S., H. J., Z.-y. D. and L.-q. C. conceived and designed the experiments. X.-c. S. and A. J. performed the experiments and contributed to the analysis of the data. X.-c. S., J. S. and J.-j. T. co-wrote the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451800226X








