Introduction
Corrective surgeries for complex CHD have significantly improved patient survival. However, the long-term course after these complex surgeries is frequently complicated by atrial and ventricular arrhythmias, haemodynamic deterioration, and heart failure. Reference Khairy, Aboulhosn and Gurvitz1– Reference Moore, Anderson and Nisbet5 In 1964, Rastelli et al. described the first surgery involving an extracardiac valved pericardial conduit from the venous ventricle to the pulmonary artery and an intracardiac baffle that tunnelled the systemic ventricle to the aorta via a ventricular septal defect. Reference Rastelli, Ongley, Davis and Kirklin6 Initially, this technique was used to treat transposition of the great arteries in the presence of a ventricular septal defect with severe pulmonary stenosis. However, with time, several modifications of the technique and conduit materials have been described with an expansion of indications. Reference Dearani, Danielson and Puga7 Currently, this procedure is also used for correction of truncus arteriosus, pulmonary atresia with ventricular septal defect, double outlet right ventricle, and other complex CHDs that have a ventricular septal defect, overriding of great arteries, and outflow obstruction. Reference Backer and Mavroudis8
Arrhythmias have emerged as significant late complications in adult patients after surgical correction of CHDs. Reference Khairy, Aboulhosn and Gurvitz1– Reference Moore, Anderson and Nisbet5 The pattern of late arrhythmia following corrective surgery is dependent on multiple factors including the inherent gross and ultrastructural changes associated with a specific disease, sites of surgical incisions, and haemodynamic consequences of the correction. Hence, the burden and pattern of arrhythmias are heterogeneous among procedures. Although the Rastelli procedure has been used for a large number of patients since 1969, there is a paucity of data on arrhythmic outcomes in adults after this surgery. We, therefore, sought to describe the long-term prevalence of arrhythmias, their mechanisms, and contributing factors after Rastelli surgery.
Methods
Study population and data collection
A retrospective cohort study was conducted on all adults (age >18 years) who underwent Rastelli surgery and have been followed up by the adult CHD clinic and electrophysiology services at Toronto General Hospital and University Health Network. The term “Rastelli surgery” was used for the originally described surgery or any of its modifications – a common factor being a venous ventricle to pulmonary artery conduit.
After obtaining approval from the institutional research review board, patient demographic, clinical, and imaging details including echocardiography and cardiac magnetic resonance imaging were retrospectively reviewed from hospital electronic medical records. As this was a retrospective study, we relied on archived echocardiography data. When technically feasible and the ventricular geometry was conducive, we applied standard definitions from published guidelines to assess chamber dilatation, ventricular dysfunction, and other structural abnormalities. Reference Lang, Badano and Mor-Avi9 For the morphological left ventricle, an ejection fraction of less than 50% was considered indicative of ventricular dysfunction. In cases where ejection fraction measurement was not feasible due to complex ventricular geometry, ventricular dysfunction was assessed by an experienced echocardiographer through a combination of visual assessment (“eye-balling”) and fractional area change. Valvular regurgitation of at least moderate severity was classified as haemodynamically significant. When available, cardiac magnetic resonance reports were utilised for the assessment of ventricular function, following standard definitions from published guidelines for ventricular dysfunction as assessed by cardiac magnetic resonance. Reference Kawel-Boehm, Hetzel and Ambale-Venkatesh10
Any arrhythmia documentation was noted from electrocardiography, electrocardiography monitoring with Holter, and device logs from cardiac implantable electronic devices. For patients who underwent implantation of cardiac implantable electronic devices or radiofrequency catheter ablation, procedure-related data were obtained by reviewing the procedure notes. The mechanism of tachycardia was also obtained from procedural notes, and the intervention was considered successful if the tachycardia was not inducible at the end of the procedure.
Statistical analysis
Continuous variable distributions are expressed as means ± standard deviation or median with interquartile range. Comparisons between groups were performed using the student’s t-test or Mann–Whitney U test. Categorical variables are summarised as frequency and percentage. Comparisons between groups were performed with Chi-square test or Fisher’s exact tests. In cases with missing values for a variable, the case was excluded from the analysis for that variable. Kaplan–Meier analysis was used to estimate time-to-event. A two-sided P-value of less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (Ver. 21.0, IBM, Chicago, IL, USA).
Results
Patient characteristics
A total of 55 patients (36.4% female) were included in the study. The patient characteristics are summarised in Table 1. The median age at Rastelli surgery was 4.0 (2.0–7.0) years. Most patients underwent Rastelli surgery for d-TGA with ventricular septal defect and pulmonary stenosis/pulmonary atresia (78.2%). The median follow-up time was 24.2 (20.6–31.0) years after surgery.
Table 1. Patient characteristics

Note: Data are number (%), mean ± standard deviation or median (25th percentile – 75th percentile); TGA = transposition of great arteries, DORV = double-outlet right ventricle, ARB = angiotensin receptor blocker, MRA = mineralocorticoid antagonist.
Structural and haemodynamic abnormalities as assessed by echocardiography and/or cardiac magnetic resonance are summarised in Supplementary Table S1. Forty-two (76.4%) patients had significant haemodynamic abnormalities in the form of valvular regurgitation, residual shunt, outflow obstruction, or ventricular dysfunction.
Tachyarrhythmias and catheter ablation
Symptomatic sustained tachyarrhythmias were seen in 21 (38.4 %) patients (Table 2). Fifteen patients had only atrial arrhythmias, 5 had only ventricular arrhythmias, and 1 patient had both. An additional four patients had device-detected nonsustained arrhythmias. The median age at arrhythmia onset was 29.0 (20.0–42.0) years. Arrhythmia-free survival after surgery is shown in Figure 1. Older age at surgery was significantly associated with symptomatic arrhythmias (median 4.0 [1.8–4.2] years in those without arrhythmias vs. 6.0 (3.5–12.0) years in those with arrhythmias, p = 0.022) (Supplementary Table S2). No other factor was significantly different between the group with arrhythmias and the group without arrhythmias (Supplementary Table S2).

Figure 1. Arrhythmia-free survival after surgery.
Table 2. Arrhythmia characteristics
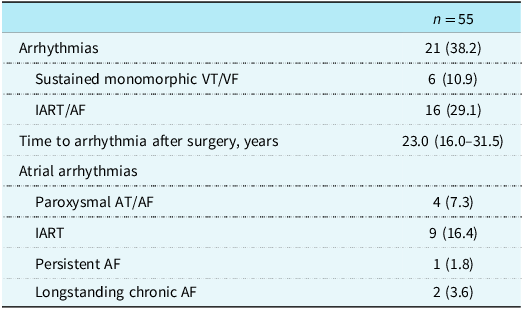
Note: Data are number (%) or median (25th percentile - 75th percentile). AT: atrial tachycardia; AF: atrial fibrillation; IART: intraatrial reentrant tachycardia; VT: ventricular tachycardia; VF: ventricular fibrillation.
Paroxysmal or persistent intra-atrial reentrant tachycardia was the presenting arrhythmia in 13 (81.3%) of the 16 patients with atrial arrhythmias. Atrial fibrillation was the first arrhythmia in the rest. Five (9.1 %) patients had sustained monomorphic ventricular tachycardia, and one had sudden cardiac arrest. All six patients with ventricular arrhythmias had dTGA with ventricular septal defect and pulmonary stenosis/pulmonary atresia as the underlying CHD. Nine patients (42.9%) among the 21 with arrhythmias underwent catheter ablation because they were either resistant to antiarrhythmic agents or did not tolerate them. One patient had sudden cardiac death.
Nine patients underwent a total of 16 catheter ablation procedures. Stable tachycardia was inducible in 13 of the 16 procedures. The rest had substrate modification aided by pace mapping due to unstable or noninducible tachycardia. Acute success was seen in 12 of 13 (92.3%) procedures with inducible arrhythmias. There were no procedure-related complications. Six patients with atrial arrhythmias underwent catheter ablation, and all of them had intra-atrial reentrant tachycardia. In three patients, the intra-atrial reentrant tachycardia was dependent on the cavotricuspid isthmus. All three had successful ablation without recurrence. Two patients had a right lateral intra-atrial reentrant tachycardia related to the surgical incisional scar (Supplementary Figure S1). Both these patients had successful ablation of their arrhythmias but later developed cavotricuspid isthmus-dependent flutter, which was ablated during a second procedure. One patient, who had an atrial switch along with Rastelli surgery, had two non-cavotricuspid isthmus dependent intra-atrial reentrant tachycardia involving both venous and systemic atria.
Three patients underwent ventricular tachycardia ablation. One had macro-reentrant ventricular tachycardia around the conduit with the isthmus between the conduit and tricuspid annulus (Supplementary Figure S2A). Another patient had a focal ventricular tachycardia (likely micro-reentrant) on the posterior aspect of the RV-PA conduit (Supplementary Figure S2B). One patient underwent substrate modification by cryoablation at the ventricle-conduit junction during conduit revision surgery.
Cardiac implantable electronic devices
Sixteen (29.1%) patients had cardiac implantable electronic devices implantation. Six patients had pacemaker implantation for post-op AV block. Another six patients underwent pacemakers late after surgery, mostly for sinus node dysfunction. Only one patient developed AV block twelve years after surgery. Six of the patients with AV block had dTGA with ventricular septal defect and pulmonary stenosis/pulmonary atresia as the underlying heart disease, and one patient had ccTGA with ventricular septal defect and pulmonary stenosis. Overall, 7 (58.3 %) patients with initial pacemakers required up-gradation – five to ICD, two to CRT-P, and one to CRT-D.
Other outcomes
During follow-up, 10 (18.2 %) patients developed heart failure. Four (7.3%) patients died – three due to heart failure and one due to infective endocarditis. Congestive heart failure was significantly associated with arrhythmias (5.9 % in those without arrhythmias vs. 38.1% with arrhythmias, p = 0.004). All four deaths were preceded by arrhythmias, a median of 3 years before death. Thirty-two (58.2%) patients did not have any events (arrhythmia, heart failure, and death) during the follow-up. Median survival after surgery without any events (heart failure, arrhythmia, or death) was 31.6 (28.1–35.1) years.
Discussion
This study explores arrhythmia outcomes on late follow-up after Rastelli surgery. The salient findings are (1) clinically significant arrhythmias occurred in about two-fifths of the patients, with a prevalence that increased in the second decade after surgery, (2) older age at surgery was associated with a higher incidence of arrhythmias, (3) atrial arrhythmias were predominantly in the right atrium – with most intra-atrial reentrant tachycardias involving the cavotricuspid isthmus or right atrial incisional scar, (4) ventricular arrhythmias were mostly related to the substrate at the base of the RV-PA conduit, and (5) atrioventricular block, when it occurred, was mostly perioperative with late AV blocks being rare. The main strengths of the study include a fairly large number of patients with this uncommon surgery and a long follow-up duration.
Tachyarrhythmias were seen in about 38% of our patients and most occurred >15 years after surgery. The proportion was higher than previously reported in patients after Rastelli surgery. Reference Kreutzer, De Vive and Oppido11,Reference Alsoufi, Awan and Al-Omrani12 Several reasons could have contributed. First, previous studies reporting low incidence (0–7%) were in children who were followed up immediately after surgery as compared to the long-term follow-up in our cohort. Second, this study focuses on the prevalence of arrhythmias among adults with Rastelli surgery. Patients who died during childhood were not represented in this study. As such, an overall survival rate cannot be inferred from this study due to immortal time bias. Nevertheless, a clear pattern for arrhythmias emerged consisting of a quiescent period of about 15 years from surgery, after which arrhythmia prevalence starts increasing. This is consistent with studies in patients with other forms of complex CHD that indicate that tachycardia circuits evolve with time and the incidence of arrhythmias increases with advancing age. Reference Khairy, Aboulhosn and Gurvitz1,Reference Gelatt, Hamilton and McCrindle13– Reference Gewillig, Wyse, de Leval and Deanfield16
The only factor significantly associated with arrhythmias in this study was older age at surgery. In contrast to previous studies, the presence of significant haemodynamic abnormalities – ventricular dysfunction, shunt, valve regurgitation, or outflow obstruction – was not associated with arrhythmias. Not surprisingly, tachyarrhythmias were significantly associated with CHF and deaths. Tachyarrhythmias can be a marker of haemodynamic deterioration of the conduit, systemic ventricular dysfunction, and subsequent heart failure or death. Reference Dearani, Danielson and Puga7 They also contribute directly to heart failure decompensation.
Arrhythmias after surgery for complex CHD can be highly symptomatic due to compromised haemodynamic reserve. Antiarrhythmic drugs may be poorly tolerated owing to drug-induced bradycardia, systemic toxicity, and risk of pro-arrhythmia due to structural heart disease. Reference Gewillig, Wyse, de Leval and Deanfield16,Reference Driscoll, Offord, Feldt, Schaff, Puga and Danielson17 About half of the patients with arrhythmias in this study were either resistant to or did not tolerate antiarrhythmics in this study, thereby prompting catheter ablation. The prevalence of sustained atrial arrhythmias (29.1%) and ventricular arrhythmias (10.9%) after Rastelli surgery was comparable to the prevalence of arrhythmias after surgical repair for tetralogy of Fallot. Reference Khairy, Aboulhosn and Gurvitz1
Several areas of surgical scars and the potential for arrhythmias exist in patients after Rastelli surgery (Figure 2). However, despite the presence of multiple scars, it is known that cavotricuspid isthmus-dependent flutters are the most common atrial arrhythmias in adults after CHD surgery when a cavotricuspid isthmus is present. Reference Lesh, Kalman, Saxon and Dorostkar18 Cavotricuspid isthmus-dependent flutter was the most common atrial arrhythmia in this series. All non-cavotricuspid isthmus-dependent intra-atrial reentrant tachycardia circuits involved a right atrial incisional scar (Supplementary Figure S1). While Rastelli surgery in our series was primarily performed for dTGA, three patients underwent both Rastelli and atrial switch surgery for ccTGA. It is important to note that the atrial arrhythmia substrate in these three ccTGA patients is fundamentally different from that of the dTGA/Rastelli group, aligning more closely with the TGA/atrial switch patients rather than the dTGA/Rastelli group. Interestingly, in two patients who had non-cavotricuspid isthmus-dependent intra-atrial reentrant tachycardia during the first catheter ablation, there was recurrent arrhythmia due to cavotricuspid isthmus-dependent flutter requiring further catheter ablations. This observation suggests that there may be a role for empiric ablation of the cavotricuspid isthmus in patients with Rastelli surgery who undergo catheter ablation for intra-atrial reentrant tachycardia. All intra-atrial reentrant tachycardias in our series were from the right atrium. Whether prophylactic cryoablation of potential isthmus sites during the surgery, as performed in other forms of complex CHD, effectively decreases the incidence of these intra-atrial reentrant tachycardias remains speculative. This cannot be advocated for index surgery as it is nowadays done in the first months of life. However, prophylactic cavotricuspid isthmus ablation during reoperation can be considered.

Figure 2. Potential arrhythmia substrates after Rastelli surgery. The picture represents the most common form of Rastelli operation done for TGA-VSD-PS. (PFO = patent foramen ovale, RV = right ventricle, PA = pulmonary artery, VSD = ventricular septal defect).
The ventricle-conduit junction is a potential site for ventricular arrhythmias. The literature is limited to a few case reports of catheter ablation for ventricular tachycardia in post-Rastelli patients. Reference Koa-Wing, Linton and Kojodjojo19– Reference Obeyesekere, Mechulan, White, Bergin, Khairy and Gula22 Two of the ablated ventricular tachycardias had an isthmus at the base of the conduit with a circuit around the conduit Reference Koa-Wing, Linton and Kojodjojo19,Reference Te, Chung, Lin, Prabhu, Lee and Chen20 . In one report, the ventricular tachycardia isthmus was localised to the suture line of the ventricular septal defect. Reference Te, Chung, Lin, Prabhu, Lee and Chen20 Another case reported using pace mapping to localise the site of origin to the RVOT below the closed stump of the native RVOT. Reference Obeyesekere, Mechulan, White, Bergin, Khairy and Gula22 In the cases from our series and the reports noted above, an inexcitable scar was present in the region between the RV-PA conduit, tricuspid annulus, and the closed stump of the native pulmonary artery (Supplementary Figure S2). This closed stump is observed only when the Rastelli operation is performed for d-transposition of great arteries with ventricular septal defect and pulmonary stenosis (transposition of great arteries-ventricular septal defect-PS) and is not typically seen when the procedure is done for other conditions. An approximate analogy can be drawn between the substrate in Rastelli and the anatomical isthmuses described in patients after surgery for tetralogy of Fallot. Reference Zeppenfeld, Schalij and Bartelings23 Isthmus 1 (between right ventriculotomy scar and tricuspid annulus) and 2 (between right ventriculotomy scar and pulmonary annulus) roughly correspond to the scars described above.
All but one of the AV blocks in the series was perioperative (first surgery or revision). Only one patient developed a late AV block a decade after surgery. Later pacing indications were mostly for sinus node dysfunction or drug-induced bradycardia. Unlike perioperative AV nodal injury leading to early postoperative AV block, late-onset sinus node dysfunction can be explained by progressive sinoatrial nodal electrophysiological remodelling from the progression of early injury during surgery, interruption of sinoatrial nodal blood supply from the atrial incision, and atrial stretching due to haemodynamic stress.
Limitations
This is a single-centre study with a limited sample size. As we had the follow-up data of only adult patients, our study cannot ascertain the prevalence of arrhythmias in children and adults combined given that the total population who underwent surgery (i.e., the denominator) is unknown. Only a subset of patients with arrhythmias underwent catheter ablation that allowed arrhythmia mechanisms to be identified, thereby precluding the generalisation of mechanisms and sites of origin to all arrhythmias in this population.
Conclusions
In this single-centre study, the prevalence of tachyarrhythmias following the Rastelli procedure was substantial and increased in the second decade after the surgery. Atrial arrhythmias were more common than ventricular arrhythmias and predominantly consisted of intra-atrial reentrant tachycardia circuits involving the cavotricuspid isthmus or atrial incisional scar. Ventricular arrhythmias were related to the substrate at the base of the conduit. Catheter ablation was safely and effectively performed in a subset of patients, with some requiring multiple procedures. Atrioventricular block predominantly occurred in the perioperative setting with late AV blocks being rare.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124026623.
Acknowledgements
The authors thank Ms. Mia Marie Overgaard for her contribution to the graphics.
Financial support
None.
Competing interests
None.







