In the Multiethnic Cohort (MEC), very high diabetes incidence rates (per 1000 person-years) were observed for Native Hawaiians (17·5 in men and 15·9 in women) and Japanese Americans (16·2 in men and 12·7 in women) as compared with Caucasians (7·1 in men and 4·9 in women)( Reference Maskarinec, Erber and Grandinetti 1 ). These ethnic differences in risk can be partially explained by BMI, physical activity and dietary factors( Reference Maskarinec, Erber and Grandinetti 1 , Reference Steinbrecher, Morimoto and Heak 2 ), in particular meat and dietary fibre( Reference Steinbrecher, Erber and Grandinetti 3 , Reference Hopping, Erber and Grandinetti 4 ). Reports from other cohorts have also suggested a protective effect of drinking coffee( Reference van Dam 5 ), a widely consumed beverage that contains not only caffeine, but also minerals, phenolic compounds and niacin( Reference van Dam 5 , Reference Natella and Scaccini 6 ). Despite some concerns, primarily in the general population, about possible harmful effects of coffee, the totality of the evidence indicates inverse relationships with diabetes type 2 incidence and mortality( Reference van Dam 5 , Reference Floegel, Pischon and Bergmann 7 – Reference Freedman, Park and Abnet 9 ). The most recent meta-analysis of eighteen cohort studies suggested a statistically significant 7 % lower diabetes risk with each additional cup of coffee consumed per day( Reference Huxley, Lee and Barzi 10 ). In that report, only 20 % of the cohort participants were from non-white populations( Reference van Dam 5 , Reference Natella and Scaccini 6 , Reference Huxley, Lee and Barzi 10 ), indicating a lack of research in different ethnic groups, such as Japanese Americans and Native Hawaiians, who have higher incidences of diabetes. To address to the limited data related to ethnicity, we conducted the present analysis among participants in the Hawaii component of the MEC, comprised of men and women of Caucasian, Japanese American and Native Hawaiian background, in order to examine the association of coffee consumption with diabetes risk in each group.
Methods
Study population
The MEC study of diet and cancer was established between 1993 and 1996, and recruited men and women aged 45–75 years from five major ethnic groups (Caucasians, Japanese Americans and Native Hawaiians, primarily in Hawaii; and African Americans and Latinos, primarily in Los Angeles)( Reference Kolonel, Henderson and Hankin 11 ). The current analysis is limited to the Hawaii component, for which diabetes incidence data were available( Reference Maskarinec, Erber and Grandinetti 1 ). The Hawaii study population consisted of 103 898 participants, largely recruited using driver's licence files as the sampling frame. To enter the cohort, individuals completed a 26-page, self-administered mailed survey that included a quantitative FFQ (QFFQ) covering habitual diet during the past year, as well as demographic information, medical conditions, anthropometric measures and lifestyle factors( Reference Stram, Hankin and Wilkens 12 ). Japanese Americans had higher (46 % for men and 51 % for women) and Native Hawaiians had lower (28 % for men and 35 % for women) response rates than Caucasians (39 % for men and 47 % for women). Nevertheless, participants in the MEC were fairly representative of the general population, as evidenced by a comparison of the cohort distributions across educational levels and marital status with corresponding census data( Reference Kolonel, Henderson and Hankin 11 ). After exclusion of 10 028 self-reported prevalent cases, 8797 participants of other ethnicities and 9933 individuals with missing information for diabetes status, follow-up, dietary intake or other pertinent covariates, 36 120 men and 39 020 women were included in the current analysis.
Ethical approval
The MEC was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Committee on Human Studies at the University of Hawaii and by the Institutional Review Board of Kaiser Permanente. Written informed consent was obtained from all participants at cohort entry.
Case ascertainment
Annual linkages with state death certificate files provided information on vital status. As described previously, incident diabetes cases were identified through three sources( Reference Maskarinec, Erber and Grandinetti 1 ). After excluding all prevalent cases, i.e. participants who reported a diagnosis of diabetes at cohort entry, new cases were found through a follow-up questionnaire sent to MEC members during 1999–2003 (88 % response rate), a medication questionnaire administered at the time of blood collection in 2001–2007 (38 % response rate) and a 2007 linkage of all MEC members known to be alive with the two major health plans in Hawaii, which are estimated to cover >90 % of the population( 13 ). To reduce the number of false positives, the health plans identified cases based on an algorithm that used multiple sources indicating diabetes-related services, including hospital stays, laboratory results, repeated outpatient visits and pharmacy records. Individuals who never reported diabetes and were not identified as diabetics by a health plan were categorized as non-cases, although it is possible that a diabetes diagnosis was missed for a small percentage of these individuals. However, the 8582 incident cases included in the present analysis were all confirmed by a health plan; 812 self-reported cases not identified by one of the two health plans were excluded( Reference Maskarinec, Erber and Grandinetti 1 ).
Dietary assessment
The QFFQ has been described in detail elsewhere( Reference Stram, Hankin and Wilkens 12 ). In brief, the questionnaire included nine frequency categories ranging from never/hardly ever to two or more times daily for each food item or group, with three options for portion sizes. For beverages, usual portion sizes such as cup/mug, bottle, can or glass were used, and the frequency categories ranged from never/hardly ever to four or more times daily. Intake of nutrients was determined using a customized food composition database that includes ethnic-specific foods. This database was developed from the US Department of Agriculture nutrient database, as well as other available nutrient databases, and with additional analyses performed in Hawaii( Reference Kolonel, Henderson and Hankin 11 ). A calibration study using 24 h recalls showed acceptable values; the average correlations for nutrient densities in men and women, respectively, were 0·66 and 0·61 for Japanese Americans and 0·66 and 0·74 for whites( Reference Stram, Hankin and Wilkens 12 ). Values for Native Hawaiians and for specific foods, such as coffee, were not reported. For the present analysis, three beverage groups were examined: regular caffeinated coffee, decaffeinated coffee and espresso/cappuccino. For each item, intake was calculated as g/d and converted into cups (1 cup = 240 g) per day. Total coffee intake was computed as the sum of the three beverage items; regular caffeinated coffee and decaffeinated coffee were examined separately. We created five categories (almost never, <1, 1, 2 and ≥3 cups/d) for total and regular coffee. Because the number of individuals in the lowest category was very small for total coffee intake, we created an additional category that combined 0 and <1 cups/d. Due to the limited number of consumers, decaffeinated coffee was divided into only four categories (almost never, <1, 1 and ≥2 cups/d).
Statistical methods
Statistical analyses were performed using the SAS statistical software package version 9·3. Cox proportional hazards regression models (PROC PHREG) were applied to analyse the effect of total, regular caffeinated and decaffeinated coffee on diabetes risk. Hazard ratios (HR) and 95 % confidence intervals were calculated using follow-up time as the underlying time metric. To ensure that the estimation procedure was based on comparisons of participants of the same age, we controlled for age at cohort entry through stratification. The three coffee intake variables were analysed in categories of consumption frequency (cups/d) among men and women separately. Due to previously reported associations with diabetes risk, ethnicity (Japanese American and Native Hawaiian v. Caucasian), BMI (23·0–24·9, 25·0–29·9 and ≥30·0 kg/m2 v. <23·0 kg/m2), physical activity (log transformed), education (13–15 and ≥16 years v. ≤12 years), smoking status (never, past, current), history of hypertension, energy intake (log transformed), alcohol intake (<1 drink/month, ≥1 drink/month to <1 drink/d, ≥1 drink/d), sugared soda intake (0, <2 sodas/week, ≥2 sodas/week), dietary fibre and processed meat intake (log transformed, density per 4184 kJ) were included as potential confounders. Mg intake was added to the overall models to explore whether this mineral is responsible for the apparent protective effect of coffee( Reference Hopping, Erber and Grandinetti 4 ). Ordinal variables representing each coffee consumption category were included to test for linear trends.
Results
Among the 36 120 men and 39 020 women included in the present analysis, Caucasians and Native Hawaiians were somewhat older and had a higher BMI than Japanese Americans (Table 1). Most participants reported some coffee intake; only 15·4 % of men and 18·9 % of women never consumed coffee, with higher percentages among Native Hawaiians. In men and women, respectively 8·6 % and 11·3 % consumed decaffeinated coffee only, whereas 51·3 % and 48·1 % drank regular coffee only. Men reported significantly higher levels of smoking and hypertension than women and had higher intakes of all foods and nutrients except fibre (Table 1). Consumers of decaffeinated coffee reported a lower BMI, more physical activity, less current smoking, more hypertension, lower intakes of energy, alcohol and red meat, and a higher consumption of dietary fibre and sugared sodas. However, the differences were very small (data not shown).
Table 1 Baseline characteristics of participants in the Hawaii component of the Multiethnic Cohort Study, 1993–2007Footnote *
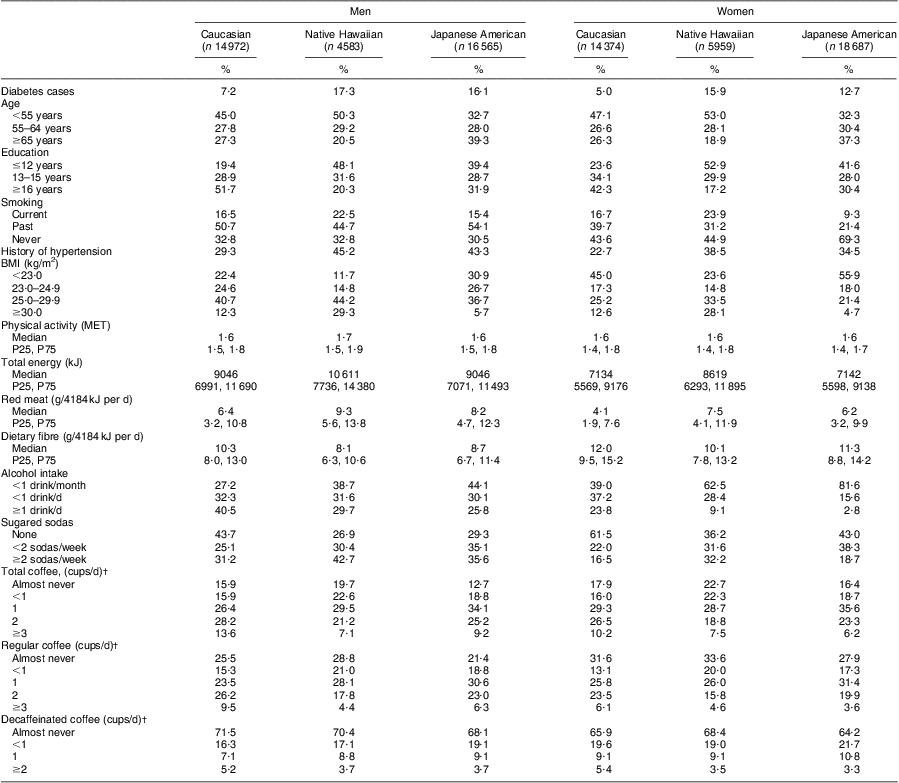
P25, 25th percentile; P75, 75th percentile; MET, metabolic equivalents.
* Data are presented as percentages or as medians with 25th and 75th percentiles.
† 1 cup is the equivalent of 240 g.
The association of diabetes with coffee consumption differed significantly by sex (P interaction < 0·0001). Total coffee intake was not significantly associated with diabetes incidence among men (HR = 0·95; 95 % CI 0·84, 1·08; P trend = 0·30) when the reference group was restricted to participants in the ‘almost never’ intake category (Table 2). When 0 to <1 cup/d was used as the reference, the highest intake group showed a weak inverse association (HR = 0·89; 95 % CI 0·80, 0·99; P trend = 0·09), which was suggestive for Caucasians (HR = 0·84; 95 % CI 0·69, 1·03; P trend = 0·05) only. Examining regular coffee, we found that drinking ≥3 cups/d was related to a 14 % lower diabetes risk (HR = 0·86; 95 % CI 0·75, 0·98; P trend = 0·09), again predominantly for Caucasians (HR = 0·79; 95 % CI 0·62, 1·00; P trend = 0·07). In contrast, no association was seen for decaffeinated coffee intake (HR = 1·07; 95 % CI 0·93, 1·23; P trend = 0·71) in any ethnic group. Despite small differences in the stratified analyses, none of the interaction tests with ethnicity were significant, e.g. P interaction = 0·20 for total and P interaction = 0·48 for regular coffee.
Table 2 Diabetes risk associated with coffee intake in the Hawaii component of the Multiethnic Cohort, men, 1993–2007Footnote *
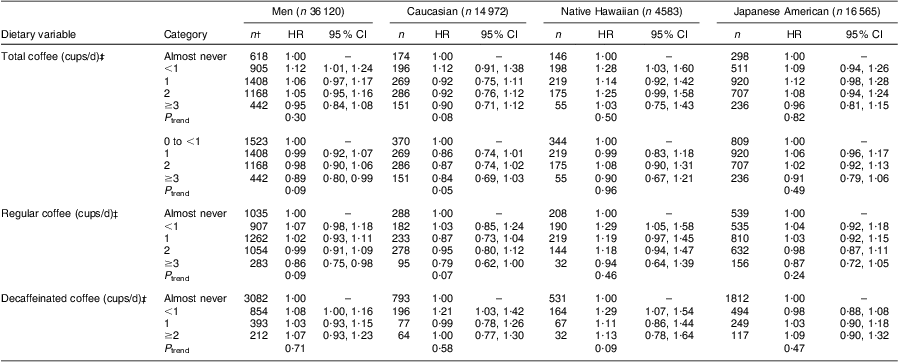
HR, hazard ratio.
* Hazard ratios and 95 % confidence intervals obtained from Cox regression models stratified by age and adjusted for ethnicity (Japanese American, Native Hawaiian v. Caucasian), BMI (23·0–24·9, 25·0–29·9 and ≥30·0 kg/m2 v. <23·0 kg/m2), physical activity (logarithm of continuous), education (13–15 and ≥16 years v. ≤12 years), history of hypertension, energy (logarithm of continuous), alcohol intake (<1 drink/month, <1 drink/d, ≥1 drink/d), smoking status (current, past, never), sugared sodas (0, <2 sodas/week, ≥2 sodas/week), dietary fibre per 4184 kJ (logarithm of continuous) and processed meat per 4184 kJ (logarithm of continuous).
† n represents diabetes cases.
‡ 1 cup is the equivalent of 240 g.
In women (Table 3), total coffee intake was strongly inversely related with diabetes risk (≥3 cups/d v. 0 to <1 cups/d: HR = 0·66; 95 % CI 0·58, 0·77; P trend < 0·0001). This protective association was detected in all ethnic groups (24 % lower risk for Caucasians, 34 % for Native Hawaiians and 37 % for Japanese Americans). A similar inverse association with diabetes risk was found for regular coffee intake (HR = 0·65; 95 % CI 0·54, 0·78; P trend < 0·0001), with risk reductions of 22 % in Caucasians, 40 % in Native Hawaiians and 38 % in Japanese Americans. Decaffeinated coffee was weakly but not significantly associated with diabetes risk in women (HR = 0·85; 95 % CI 0·72, 1·01; P trend = 0·73). The interaction terms between ethnicity and coffee intake were not statistically significant in any category, e.g. P interaction = 0·88 for total and 0·86 for regular coffee.
Table 3 Diabetes risk associated with coffee intake in the Hawaii component of the Multiethnic Cohort, women, 1993–2007Footnote *
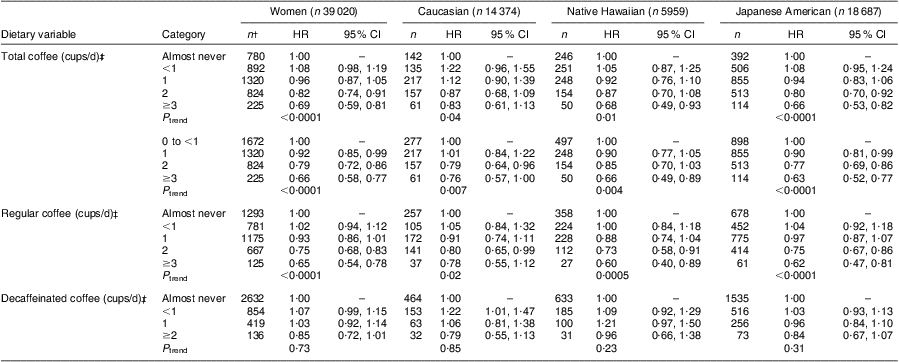
HR, hazard ratio.
* Hazard ratios and 95 % confidence intervals obtained from Cox regression models stratified by age and adjusted for ethnicity (Japanese American, Native Hawaiian v. Caucasian), BMI (23·0–24·9, 25·0–29·9 and ≥30·0 kg/m2 v. <23·0 kg/m2), physical activity (logarithm of continuous), education (13–15 and ≥16 years v. ≤12 years), history of hypertension, energy (logarithm of continuous), alcohol intake (<1 drink/month, <1 drink/d, ≥1 drink/d), smoking status (current, past, never), sugared sodas (0, <2 sodas/week, ≥2 sodas/week), dietary fibre per 4184 kJ (logarithm of continuous) and processed meat per 4184 kJ (logarithm of continuous).
† n represents diabetes cases.
‡ 1 cup is the equivalent of 240 g.
Unadjusted models also showed the stronger associations for women and for regular coffee, indicating that the influence of confounders was similar by sex and by type of coffee. For men, the unadjusted HR for regular and decaffeinated coffee were 0·86 (95 % CI 0·75, 0·98; P trend = 0·03) and 1·09 (95 % CI 0·95, 1·25; P trend = 0·86). The respective values for women were 0·61 (95 % CI 0·50, 0·73; P trend < 0·0001) and 0·79 (95 % CI 0·66, 0·93; P trend = 0·17). To explore whether Mg was responsible for the protective effect of coffee, dietary Mg intake was included as a covariate, but no significant improvement in model fit was detected (data not shown).
Discussion
In this multiethnic population, the highest category of regular coffee intake was related to a 35 % lower diabetes risk in women but only a borderline 14 % reduction of risk in men. The benefit of coffee consumption was detected in women of all three ethnic groups. Despite some variation in effect size, the interaction term with ethnicity was not significant. No association between decaffeinated coffee consumption and diabetes risk was seen in men, but a weak protective effect that did not reach significance was suggested in women.
Our findings agree with two meta-analyses that found inverse associations between coffee consumption and diabetes incidence( Reference Huxley, Lee and Barzi 10 , Reference van Dam and Hu 14 ). The first one included nine cohorts with 193 473 participants in America and Europe and reported that habitual coffee consumption of 4–6 and 6–7 cups/d lowered risk by 28 % and 35 %, respectively( Reference van Dam and Hu 14 ). The second meta-analysis summarized eighteen cohort studies including 457 922 individuals with more than 21 000 cases of new-onset diabetes; each additional cup of coffee per day contributed to a 5–10 % lower risk( Reference Huxley, Lee and Barzi 10 ). That analysis also reported a reduced diabetes risk for those who drank 3–4 cups of decaffeinated coffee daily (HR = 0·64; 95 % CI 0·54, 0·77)( Reference Huxley, Lee and Barzi 10 ). Three cohort studies published since the meta-analyses are also in agreement. The European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study reported a 23 % lower incidence for caffeinated and a 30 % lower risk for decaffeinated coffee intakes of ≥4 cups/d( Reference Floegel, Pischon and Bergmann 7 ). The Black Women's Health study( Reference Boggs, Rosenberg and Ruiz-Narvaez 15 ) and a Japanese cohort study( Reference Oba, Nagata and Nakamura 16 ) reported lower risk for regular coffee but no association for decaffeinated coffee. Thus, while most studies report inverse associations between diabetes risk and regular coffee consumption, associations with decaffeinated coffee remain inconsistent. The small number of consumers and the low consumption of decaffeinated coffee in the MEC as compared with other studies( Reference Happonen, Voutilainen and Salonen 8 ) may be responsible for this difference. Decaffeinated coffee drinkers reported healthier lifestyles in terms of physical activity, weight and diet, but adjustment for these factors did not appear to attenuate the association between decaffeinated coffee intake and diabetes risk. The results for decaffeinated coffee may be due to bias introduced by reverse causation and by residual confounding. Since caffeine is regarded as an ‘unhealthy’ substance by some, individuals diagnosed with hypertension or heart disease may have switched to decaffeinated coffee after early signs of illness were noted( Reference Kubo Shlonsky, Klatsky and Armstrong 17 ).
Adjusted and unadjusted models within the MEC suggested that the protective effect of coffee intake is stronger for women than for men. Two other studies( Reference Kato, Noda and Inoue 18 , Reference Bidel, Silventoinen and Hu 19 ) showed similar results. A Japanese cohort study suggested an 18 % lower risk in men and a 60 % lower risk in women who drank ≥5 cups/d( Reference Kato, Noda and Inoue 18 ). A study in Finland reported a 29 % lower risk in men and a 53 % lower risk in women( Reference Bidel, Silventoinen and Hu 19 ). However, two studies observed that men received more benefits than women( Reference Paynter, Yeh and Voutilainen 20 , Reference Salazar-Martinez, Willett and Ascherio 21 ), while the protective effects were similar across sex in another two studies( Reference Oba, Nagata and Nakamura 16 , Reference Iso, Date and Wakai 22 ). We cannot exclude that residual confounding by unmeasured risk factors was stronger in men than in women, but this explanation appears unlikely given the robust findings in unadjusted and adjusted models. Hormonal mechanisms for a sex-differential aetiology have been proposed( Reference Tuomilehto, Hu and Bidel 23 ), but appear unlikely given the relative consistency of effect sizes in meta-analyses( Reference van Dam 5 , Reference Natella and Scaccini 6 ). Another possibility is that reporting of coffee consumption was less accurate in men than in women and led to attenuated risk estimates. The original calibration study provides some evidence for lower correlations among Caucasian men than women but not for Japanese Americans( Reference Stram, Hankin and Wilkens 12 ). Finally, our results could be a chance finding.
Only a few of the cohort studies have provided information by ethnicity. Four studies in Asia have been conducted( Reference Oba, Nagata and Nakamura 16 , Reference Kato, Noda and Inoue 18 , Reference Iso, Date and Wakai 22 , Reference Odegaard, Pereira and Koh 24 ), but no studies specifically analysed Japanese Americans. Two studies in Native Americans( Reference Zhang, Lee and Cowan 25 , Reference Saremi, Tulloch-Reid and Knowler 26 ), one on African-American women( Reference Boggs, Rosenberg and Ruiz-Narvaez 15 ) and one in Puerto Ricans( Reference Fuhrman, Teter and Barba 27 ) agreed that coffee is associated with a lower risk of type 2 diabetes. This is consistent with the MEC, in particular with the stronger association for women.
Coffee may be protective against diabetes through a number of active ingredients, including caffeine, chlorogenic acid and Mg( Reference Natella and Scaccini 6 ). These and other components of coffee may positively affect glucose metabolism and insulin sensitivity leading to a reduced risk of diabetes( Reference Huxley, Lee and Barzi 10 , Reference van Dam and Hu 14 ), or act through thermogenic, antioxidant, anti-inflammatory or chelating effects( Reference Natella and Scaccini 6 ). Coffee contains relatively high concentrations of Mg (approximately 6·8 mg/240 ml cup), which may provide a protective effect through its positive effect on glucose metabolism and insulin sensitivity( Reference Barbagallo, Dominguez and Galioto 28 ), as observed in our previous report( Reference Hopping, Erber and Grandinetti 4 ). Given that the MEC population consumed >300 mg Mg/d( Reference Hopping, Erber and Grandinetti 4 ), however, the small proportion of Mg from coffee probably does not explain its protective effect.
Potential weaknesses of the current prospective analysis are the small number of participants who consumed decaffeinated and espresso-type coffee frequently. Accurate assessment of coffee intake, e.g. considering aspects of coffee preparation such as cup size and brew strength, challenges the comparison with other reports. Although US cup sizes are larger (240–250 ml) than those typical of European countries (∼125–150 ml), Europeans tend to drink stronger brews, which may compensate for the difference( Reference van Dam and Hu 14 ). As in other studies, the ‘regular coffee’ item does not provide information about the preparation method( Reference Floegel, Pischon and Bergmann 7 , Reference Boggs, Rosenberg and Ruiz-Narvaez 15 , Reference Oba, Nagata and Nakamura 16 , Reference Zhang, Lee and Cowan 25 ), but most published studies reflect filtered coffee intake due to the widespread use of this method( Reference Floegel, Pischon and Bergmann 7 , Reference van Dam and Hu 14 ). Unfiltered coffee, which is rarely consumed in the USA, appears to increase LDL-cholesterol concentrations due to substances that would be removed by the filtering process( Reference van Dam 5 ).
Strengths of the present study include a 14-year follow-up, a large number of participants, a high incidence of diabetes, a multiethnic group representative of the population in Hawaii, and the ability to account for many possible confounders. The validity of case identification was high because all incident cases were confirmed by a health plan( Reference Maskarinec, Erber and Grandinetti 1 ). The participants from the three ethnic groups completed the same questionnaire, which provides consistency in the data, and represent a wide range of anthropometric and lifestyle risk factors. Information bias was reduced through the verification of diabetes cases through health plans.
The current analysis adds to the growing understanding of dietary factors that are associated with the risk of diabetes despite the overwhelming importance of body weight and adiposity( Reference Steinbrecher, Morimoto and Heak 2 ). After controlling for the previously described effects of dietary fibre( Reference Hopping, Erber and Grandinetti 4 ), meat intake( Reference Steinbrecher, Erber and Grandinetti 3 ) and physical activity( Reference Steinbrecher, Erber and Grandinetti 29 ), higher consumption of regular coffee was inversely associated with diabetes risk in women from three ethnic groups and to a lower degree in men, while no significant association was seen for decaffeinated coffee in either group, possibly due to the small number of participants who consumed substantial amounts of decaffeinated coffee.
Acknowledgements
Sources of funding: This work was supported by the National Institutes of Health (grant numbers R37CA54281 to L.N.K. and R21DK073816 to G.M.). Conflicts of interest: None of the authors has any conflicts of interest to declare. Authors’ contributions: L.N.K. established the cohort. G.M. and L.N.K. designed the study, obtained the funding and planned the analyses. G.M. and A.S. oversaw the data analysis. A.S., Y.M. and T.D. conducted the statistical programming. T.D. drafted the paper and prepared the tables. T.D. and Y.M. conducted the literature review and prepared the Discussion section of the text. All authors provided feedback on the initial draft of the manuscript and approved the final version.





