Buprenorphine is prescribed as a treatment for opioid dependence (Reference Barnett, Rodgers and BlochBarnett et al, 2001; Reference Lintzeris, Bell and BammerLintzeris et al, 2002). Methadone substitute prescribing is the primary maintenance treatment in Britain and is well established (Ward, 1997). Despite the advantages of methadone maintenance there are associated disadvantages, including potential death following overdose, the inconvenience of daily dosing, the risk of the drug being used by others and stigma (Walsh, 1994).
In 1996 buprenorphine was introduced into Europe as an opioid treatment, and in some countries (e.g. France) is now the main treatment modality. It was licensed for the treatment of opioid dependence in the UK in 2001, and the Royal College of General Practitioners first published guidelines for buprenorphine prescribing within primary care in 2003 (Reference Ford, Morton and LintzerisFord et al, 2003); these were revised in February 2004. It is used widely in England and Wales but less so in Scotland because of buprenorphine misuse in the 1980s (Reference Sakol, Stark and SykesSakol et al, 1989; Reference Lavelle, Hammersley and ForsythLavelle et al, 1991).
Mode of action and administration
Buprenorphine is a partial opioid agonist analgesic that exhibits high affinity for mu receptors and causes blockade of other opioid agonists. Some of its properties are summarised in Box 1. It can be prescribed on alternate days. The tight binding to receptors is thought to be a possible reason for the low level of withdrawal symptoms when it is stopped abruptly. It is most effective when taken in its sublingual tablet form which is licensed for the treatment of drug dependence in the UK. The patch and parenteral forms are used as analgesics.
Box 1. Properties of buprenorphine
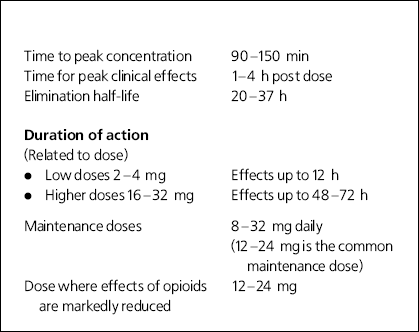
| Time to peak concentration | 90-150 min |
| Time for peak clinical effects | 1-4 h post dose |
| Elimination half-life | 20-37 h |
| Duration of action | |
| (Related to dose) | |
| • Low doses 2-4 mg | Effects up to 12 h |
| • Higher doses 16-32 mg | Effects up to 48-72 h |
| Maintenance doses | 8-32 mg daily |
| (12-24 mg is the common maintenance dose) | |
| Dose where effects of opioids are markedly reduced | 12-24 mg |
Ceiling effect and safety of use
As a partial agonist it has a bell-shaped dose-response curve with a ceiling effect on respiratory depression. This makes it safe with overdose. In one study, doses as high as 32 mg (equivalent to 1000 mg oral methadone) were administered to non-opioid-dependent volunteers with only mild respiratory effects (Reference Walsh, Preston and StitzerWalsh et al, 1994). The maximal effects of sublingual buprenorphine occur over the dose range of 16-32 mg and higher doses do not produce additional effects.
Treatment for opioid dependence
In the treatment of opioid dependence (Reference Mattick, Kimber and BreenMattick et al, 2004) and for opioid detoxification (Reference Gowing, Ali and WhiteGowing et al, 2005), buprenorphine is as effective as methadone if administered at equivalent doses. Some reasons for choosing buprenorphine rather than methadone include patient choice, milder withdrawal and more-rapid transfer to naltrexone to prevent relapse. This could also be a treatment for individuals who have experienced unwanted side-effects or not responded well to methadone.
Barriers to prescribing buprenorphine include possible use within the community and its reinforcing effects that make it a potential drug of misuse. Increased costs compared with methadone and supervised consumption of the sublingual tablet by community pharmacists may also dissuade clinicians from choosing this treatment.
Buprenorphine or methadone?
In the treatment of opioid withdrawal, evidence suggests that buprenorphine may be more effective than methadone and that high drop-out rates occur when methadone is used. Adjunctive behavioural treatments and supportive counselling enhance the retention and abstinence rates. When considering opioid substitution/maintenance, methadone and buprenorphine appear to be equally effective, although methadone is more effective when administered at higher doses (>65 mg). Methadone maintenance seems to be less effective than buprenorphine when administered at lower doses (<20 mg). Overall, buprenorphine maintenance should be used when higher doses of methadone cannot be given. Priority should be given to younger heroin users, smokers and those not wanting methadone or who have failed in trials of methadone treatment (Effective Interventions Unit, 2002).
Adverse effects
Side-effects
The side-effects are similar to those of other opioids and include headaches, nausea, vomiting, sweating, insomnia, constipation, sweating, dizziness, tiredness, erythema (25%), pruritis (22%) and sometimes complaints of a metallic taste (Ghodse et al, 2004).
Drug interactions
Buprenorphine is metabolised by two liver pathways: via glucuronide conjugation (80-90% of metabolism) and N-dealkylation by the CP450 enzyme system (responsible for 20% of metabolism). Benzodiazepine use is prevalent among opioid misusers (between 40 and 90%) and the use of benzodiazepines and/or alcohol with buprenorphine can be fatal (Reference KintzKintz, 2001). Comprehensive and thorough drug assessments and review procedures should be adopted. Alcohol may compound the central depressant effect, and therefore acute alcoholism and delirium tremens are contraindications for buprenorphine use. Antidepressants such as the tricyclic group and monoamine oxidase inhibitors may also compound the central depressant effect of buprenorphine. Antipsychotics can also interact because they are metabolised by similar enzymes (cytochrome P450 3A4 and glucuronyl transferase). Some enzyme-inducing drugs induce the metabolism of buprenorphine and reduce its efficacy These include phenytoin, carbamazepine and phenobarbitone. Psychostimulants (e.g. cocaine, methylene dioxymethamphetamine) are metabolised by different enzymes and hence there have been no reports of significant interactions.
Precipitated withdrawal
This may occur if the individual has commenced buprenorphine too soon after the use of heroin or methadone or in clients who are taking full opioid agonists (e.g. morphine). Those on opioid antagonists (e.g. naltrexone) can experience a delayed withdrawal reaction. Precipitated withdrawal is a result of the high affinity of buprenorphine with displacement of other opioids from opioid receptors, which typically occurs with 1-3 h of the first buprenorphine dose and peaks in 3-6 h. Symptomatic treatment with an alpha-adrenergic agonist such as lofexidine may be used with non-opioid analgesia for muscle aches. If severe, further buprenorphine should not be given until the withdrawal symptoms have subsided.
Starting buprenorphine
It is essential to perform a comprehensive assessment, including history of drug use (drug diaries included), mental health and physical health assessment. Initial liver function tests should be performed and a urine specimen obtained before prescribing buprenorphine. It is usual to commence with a low dose and rapidly titrate to a stabilising dose over the first few days. The first dose is delayed until the individual experiences opioid withdrawal (typically 8 h after the last heroin use or 24-48 h after the last methadone use). This initial dose ranges from 4 to 8 mg and is followed by rapid titration according to clinical response (by up to 4 mg daily). An observation period of 90-120 min allows monitoring for precipitated withdrawal. This is followed by frequent review of the patient with supervised dispensing. Transition from heroin or low-dose methadone (30 mg or less) may occur with few complications, and mild opioid withdrawal symptoms are commonly reported within the first 1-3 days.
Monitoring and supervision
It is recommended that buprenorphine be dispensed daily during induction and for the following 3 months. Consumption should continue to be supervised due to the risks of community diversion. Hepatitis is prevalent among the drug misuser group, and buprenorphine causes an increase in aspartate aminotransferase and alanine aminotransferase. The monitoring of liver function is warranted (Reference Petry, Bickel and PiaseckiPetry et al, 2000). To optimise the benefits of buprenorphine, psychosocial treatments, including relapse prevention, motivational interviewing and support with domestic/social issues, should be included (Reference Kakko, Svanborg and KreekKakko et al, 2003).
Scottish survey
Scottish addiction specialists have been reluctant to prescribe buprenorphine because of past misuse linked with leakage into the community. However, there has been a growing awareness of its increased use as a treatment for opiate dependence. In view of the absence of UK studies indicating its clinical effectiveness and the failure to launch local studies, the Scottish Drug Specialist Committee commissioned a national questionnaire survey to clarify the prescribing practices of buprenorphine among Scottish medical addiction specialists. The survey was conducted in July 2003, and with a 97% response rate (25 respondents) we identified that approximately 50% of clinicians were prescribing buprenorphine. The remaining half were eager to prescribe but felt restrained by the lack of local policies and protocols. Within the prescribing group, there were broad variations in prescribing practices. These included variable supervision times after the initial dose (ranging from no supervision to 180 min), with one-third of clinicians failing to perform urinalysis or oral fluid testing and one-half failing to conduct any liver function tests. Half were providing motivational interviewing and relapse prevention and most (87%) were prescribing without local protocols or procedures.
Discussion
Buprenorphine is proven to be a valid alternative to methadone for the treatment of opioid dependence. The evidence for this is drawn entirely from international research because of the absence of UK studies. The potential risks of diversion into the community with subsequent misuse are cause for concern. This can be prevented by ensuring that a comprehensive initial assessment is completed followed by supervision of administration and monitoring with regular urine and/or oral fluid analysis. Clinical and social outcomes of the treatment of opioid dependence are optimised when the delivery of pharmacological treatments is enhanced with comprehensive psychosocial interventions.
In the Scottish survey, inconsistencies in supervision, monitoring practices and treatment pathways were highlighted. We believe that this is associated with the majority of clinicians prescribing in the absence of local protocols and raises concerns about the repetition of past misuse. We recommend that the development of local and national guidelines is essential for the safe and sensible introduction of buprenorphine.




eLetters
No eLetters have been published for this article.