Introduction
Monogenea, a class of the Platyhelminthes, are typically host-specific monoxenous ectoparasites of fish, infecting especially their skin and gills. This can lead to mass mortalities when secondary infections (e.g. bacteria, virus and fungi) are triggerd, e.g. under aquaculture conditions (Zhang et al. Reference Zhang, Li, Chi, Ling and Wang2015; Kotob et al. Reference Kotob, Menanteau-Ledouble, Kumar, Abdelzaher and El-Matbouli2016). Cephalopods and, more rarely, amphibians, reptiles and cetaceans can be parasitized, one species infects the hippopotamus.
Only few species became endoparasitic in fishes, utilizing e.g. the urogenital and gastro-intestinal system. Besides showing a simple life cycle, a posterior attachment organ called (opist)haptor, armed with clamps, anchors and/or hooks, is unique and differentiates the hermaphroditic Monogenea from other flatworms (Mehlhorn, Reference Mehlhorn2001). Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) shed light on the morphology of the often misinterpreted anchor apparatus of Pseudempleurosoma Yamaguti, 1965, presenting the first laser confocal images of a ‘Diplectanotrema group’ member. Within this group (Diplectanotrema Johnston and Tiegs, Reference Johnston and Tiegs1922; Neodiplectanotrema Gerasev, Gayevskaya & Kovaleva, 1987; Paradiplectanotrema Gerasev, Gayevskaya & Kovaleva, 1987; and Pseudodiplectanotrema Gerasev, Gayevskaya & Kovaleva, 1987), the species are adapted to attach to the inner epithel cells of the oesophagus, stomach and intestine of a wide range of host species, which is unusual for the commonly ectoparasitic and highly host specific Monogenea. Pseudempleurosoma haywardi, Theisen et al., 2017, from the oesophagus of croakers (Sciaenidae) was only recently described as the first endoparasitic Monogenea from Indonesia (Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017).
The genus Paradiplectanotrema was established by Gerasev et al. in Reference Gerasev, Gaevskaya and Kovaleva1987, describing the four species P. antigoni, P. chlorophthalmi, P. cytti and P. lepidopi, all from N, NW and SW Africa (Atlantic Ocean). They also transferred Diplectanotrema trachuri Kovaleva, Reference Kovaleva1970 into Paradiplectanotrema as the new type species P. trachuri (Kovaleva, Reference Kovaleva1970) Gerasev, Gayevskaya & Kovaleva, 1987. According to the key to the genera by Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017), Paradiplectanotrema differs from the above mentioned genera by having a combination of (i) an accessory piece on the male copulatory organ (MCO) and two ventral anchors with (ii) one ventral bar each (one pair of ventral anchors and one pair of ventral bars).
The monogenean species described herein represents a new member of Paradiplectanotrema, the first record of this genus from Indonesia, and the second endoparasitic Monogenea species from the eastern Indian Ocean. We document Synodontidae as a new fish host family for Paradiplectanotrema. A morphometrical comparison is made among the valid species within this genus, also via detailed three-dimensional confocal microscopy, and first DNA sequence data are presented, securing the phylogenetic position of these marine endoparasites. For the first time, in situ images of endoparasitic monogeneans attached to tissue of the oesophageal folds inside their hosts are presented.
Materials and methods
Sample collection and processing
The synodontid grinners Saurida tumbil (Bloch, 1795) (n = 24) were sampled from Kedonganan fish market, South Bali coast, Indonesia (8°45′25.60″S, 115°10′05.94″E) in November 2016 (rainy season). The fishes were transferred on ice to the Marine and Fisheries Faculty Laboratory, Udayana University (UNUD), Kampus Bukit, Jimbaran, Bali, Indonesia.
Morphometrical data for each fish were taken [total and standard lengths (TL and SL) to the nearest 0.1 cm, total and gutted weight (TW and GW) to the nearest 0.1 g]. The body cavity was opened, the stomach was cut at the level of the pharynx right behind the gill rakers and transferred to Petri dishes containing NaCl solution (0.9%), and studied for parasites under a Zeiss Stemi DV4 binocular microscope. Endoparasitic monogenean parasites were isolated from the pharyngeal/oesophageal folds, washed in saline solution and pre-identified under a Novel XSZ-107BN microscope. Most worms were fixed using AFA (Alcohol: Formalin: Acetic acid) or 4% neutral buffered formalin and then transferred and stored in 70% EtOH for further morphological analyses (light microscopy). Some were either compressed and directly transferred to 95% ethanol for confocal laser scanning microscopy or to 99.8% EtOH for DNA analysis. Type specimens are deposited in the Berlin Natural History Museum, Germany [‘Museum für Naturkunde’, catalogue ‘Entozoa’, collection ‘Vermes’, numbers E.7439 (holotype) and E.7440a–E.7440 h (paratypes)] and the Zoological Museum Bogor, Indonesia (MZBTr240–MZBTr245, paratypes). The ecological parameters for fish infections with Paradiplectanotrema klimpeli sp. nov. (prevalence, intensity and abundance) were calculated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Light microscopy, drawing and morphological investigation
Following standard parasitological methods, specimens were cleared and mounted with glycerin after fixation. Some compressed samples from 70% ethanol were prepared as whole mounts, stained with acetic carmine and studied for sclerotized organs. Measurements are given as the range followed by mean in parentheses (in micrometers), according to the genus description by Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) and Kovaleva (Reference Kovaleva1970), respectively. A camera lucida drawing tube was used to illustrate the new species while photographs were taken with a digital camera Olympus UC30 attached to an Olympus BX53 DIC light microscope or a Zeiss SZX10 binocular magnifier, supported with Cellsens Dimension software (Olympus Soft Imaging Solutions GmbH) for measurements.
Confocal microscopy
Confocal laser scanning microscopy was performed following the procedure described in Galli et al. (Reference Galli, Strona, Villa, Benzoni, Fabrizio, Dogila and Kritsky2006) and Marchiori et al. (Reference Marchiori, Pariselle, Pereira, Agnèse, Durand and Vanhove2015) with specimens fixed in 95% ethanol, using a Leica TCS SP2 confocal microscope (laser with HeNe at 543 nm wavelength, minimum setting: 200 z section) equipped with an inverted Leica DMIRE2 microscope and a PL APO 363 oil immersion objective (×63 magnification, numerical aperture 5 1.4) at the Live Cell Imaging Center, Department of Biology, University of Rostock.
DNA analysis
For molecular analysis, total genomic DNA was extracted from individual adult worms fixed in 99.8% ethanol using QIAamp mini DNA kit (Qiagen) according to the manufacturer's instructions with some modification. In order to increase the DNA yield, the obtained DNA was completely evaporated using Concentrator plus/Vacufuge® (Eppendorf), dehydrated DNA was then re-suspended with 50 µL of elution buffer and stored in a − 20°C freezer. Amplification of D1–D3 fragments of the large subunit region (LSU) was achieved using the following primers: C1 (forward; 5′-ACCCGCTGAATTTAAGCAT-3′) and D2 (reverse; 5′-TGGTCCGTGTTTCAAGAC-3′) (Mendlová et al. Reference Mendlová, Pariselle, Vyskočilová and Šimková2010). The reaction was performed using illustra™ puReTaq Ready-To-Go PCR beads (0.2 ml tubes, 96 reactions), containing 5 pmol of each primer, 5 µL of DNA suspension and nuclease-free water to a total volume of 30 µL. Obtained PCR products (1 µL) were viewed on a 0.8% agarose gel stained with ethidium bromide. Contiguous sequences were aligned and assembled using BioEdit v.7.0.9 (Hall, Reference Hall1999). The generated sequences [GenBank accession numbers (GBAN) MG763101] were aligned with their closest matches in GenBank [15 ingroup and Actinocleidus recurvatus (GBAN AJ969951) as an outgroup taxa]. Phylogenetic analysis were perfomed in MEGA version 7 (Kumar et al. Reference Kumar, Stecher and Tamura2016) based on the best scoring model (Bayesian Information Criterion), maximum likelihood was used for the best fitting tree based on the Tamura–Nei model, rates among sites were gamma distributed with invariant sites (G + I), the number of discrete gamma categories was 5, and using complete deletion of gaps as gaps missing data treatment. The robustness of the inferred phylogeny was assessed using a bootstrap procedure with 1000 replications (Wu et al. Reference Wu, An, Xing and Ming2005).
Nomenclatural acts
The description of P. klimpeli sp. nov. (Life Science species Identifier number (ZooBank LSID): urn:lsid:zoobank.org:act:18989757-3EE3-462E-A389-491A132A6672) complies with the requirements of the International Commission on Zoological Nomenclature (ICZN). The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix ‘http://zoobank.org/’. The LSID for this publication is: urn:lsid:zoobank.org:pub:F44C225F-795C-462F-BD33-D1E4BBED9067. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central and ResearchGate. DNA sequences are available in GenBank under the GBAN MG763101.
Ethics
No experiments were performed on live vertebrates. Freshly caught dead fish was used and therefore no ethics statement is required. Samples were taken in cooperation with Udayana University, Denpasar, Bali under a valid Memorandum of Understanding (MoU). The fishes were obtained from an official fish market (see the ‘Material and Methods’ section), national laws regulate captures and fishermen/salesmen are licensed, sampled fish species are common in Indonesia, not protected, and the number of sampled 24 fish were all available individuals.
Results
Taxonomy and description
Family Ancyrocephalidae Bychowsky & Nagibina, 1968
Paradiplectanotrema Gerasev, Gayevskaya & Kovaleva, 1987
History of type species: Diplectanotrema trachuri Kovaleva, 1970 became P. trachuri (Kovaleva, Reference Kovaleva1970) Gerasev, Gayevskaya & Kovaleva, 1987
Paradiplectanotrema klimpeli sp. nov.
urn:lsid:zoobank.org:act:18989757-3EE3-462E-A389-491A132A6672
Type-host: Common/Greater Lizardfish/Grinner, Saurida tumbil (Synodontidae)
Type-locality: Kedonganan fish market, South Bali coast, Indonesia (8°45′25.60″S, 115°10′05.94″E)
Habitat: oesophageal/pharyngeal folds
Type-material: holotype E.7439; paratypes E.7440a-E.7440h (paratypes) and additional paratypes MZBTr240-MZBTr245
Deposition of specimens: Berlin Natural History Museum, Germany [‘Museum für Naturkunde’, catalog ‘Entozoa’, collection ‘Vermes’, numbers E.7439 (holotype) and E.7440a–E.7440 h (paratypes)], the Zoological Museum Bogor, Indonesia (MZBTr240–MZBTr245, additional paratypes)
Infection: four of 24 fishes harboured 47 specimens, prevalence 17%, mean intensity 12, intensity 1–21, mean abundance 2
Etymology: the specific name is to honor Dr Sven Klimpel for his work on marine fish parasites
Description (all μm) (Figs 1–3): based on 17 specimens from the type host Saurida tumbil

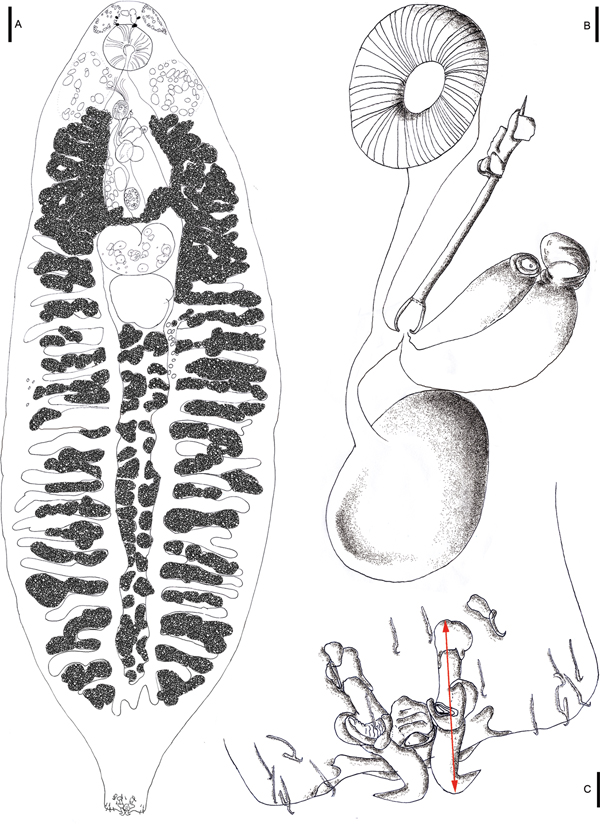
Fig. 1. Drawings of Paradiplectanotrema klimpeli sp. nov. Holotype from the type fish host Saurida tumbil (A), with detailed view of reproductive system with genital atrium/disc (mga), male copulatory organ (MCO) with accessory piece, and prostatic reservoir (pr) and gland at base; and egg in uterus (B) and opisthaptor with anchors (one dorsal and one ventral pair), bars (one between dorsal anchors and one attached to each ventral anchor = three) and seven pairs of hooklets (C); scale bars A: 100 µm; B & C: 10 µm.

Fig. 2. Light microscopy illustrations of Paradiplectanotrema klimpeli sp. nov. Worms in situ, attached to its hosts oesophageal epithel folds (A, binocular), detailed individual in situ (B), gladiator breast-plate shaped dorsal bar of four different individuals (C, DIC).

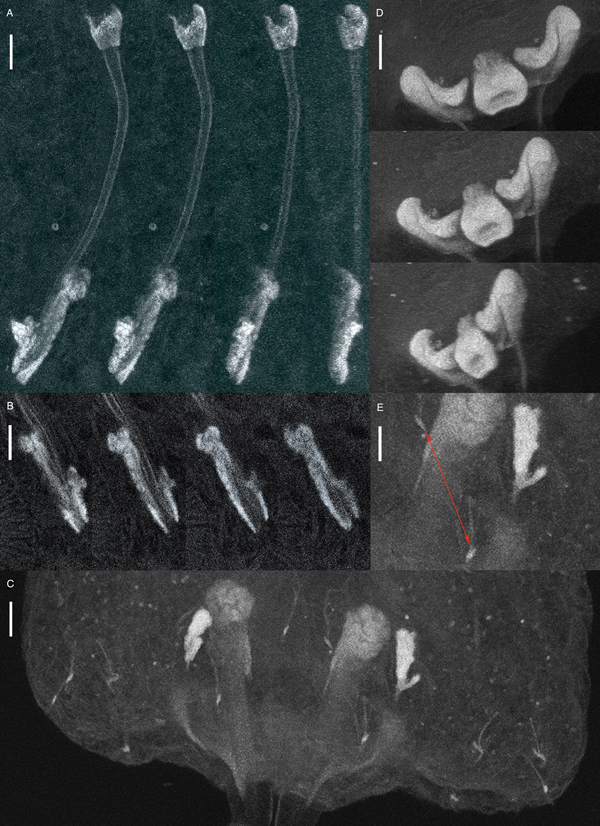
Fig. 3. Confocal laser scanning illustrations of Paradiplectanotrema klimpeli sp. nov. Male copulatory organ (MCO) in different angels (A) and levels (B), haptor (C) with dorsal anchors’ saucer-type excrescences and dorsal bar stained, from different angels (D), and detailed view of two central hooks (red arrow), base of a dorsal anchor and a ventral anchor with its associated, irregular ventral bar (E); scale bars: A–D: 10 µm; E: 15 µm.
Body slender, unspined, 2050–2880 (2390) long; maximum width 600–980 (800) (posterior to testis). Pair of small head glands anterior to oesophagus lateral to eye spots; second, larger pair lateral at level of intestinal bifurcation. Two pairs of eye-spots. Oral aperture ventral; pharynx globular, 106–164 × 112–167 (140 × 142.5) (length × width) (Fig. 1). Intestinal caeca overlaid posterior to testis, coexisting with vitelline follicles, with lateral diverticula lacking haematin pigment. Haptor, 92–131 × 100–147 (116.5 × 125.5) (length × width), not distinctly set off from body, but laterally slightly lobed; with two pairs of dissimilar hamuli (~anchors) and 14 marginal hooks (~hooklets) (Figs 1–3). Dorsal hamuli, from the tip of the ventral root to the height of curve of the blade (with the inner dorsal root being shorter than the outer ventral root in this genus), 55–62 (58) in length, connected by a single gladiator breast-plate shaped dorsal bar with a 1:1 length:width ratio (Figs 1–3), 15–19 (17) from end to end (=width), with a distance of 14–19 (16.5) from anterior to posterior border (=length) (Figs 1 and 3). Ventral hamuli 9–11 (10) in length, each one with one wide (>5) (connected) bar of 9–13 (11) length (Figs 1 and 3). Marginal hooks 11–15 (13) long (Figs 1 and 3). MCO sclerotized, tubular, 67–96 (79.5) long, with seminal vesicle and prostatic reservoir at base; accessory piece sclerotized, irregular in shape, 25–32 (27.5) long (Figs 1 and 3). Testis post-ovarian, shaped like a rounded cumulus cloud, widest anterior, median or posterior, 120–237 × 110–225 (183 × 180) (length × width) (Fig. 1). Ovary turned back on itself, giving an appearance of being kidney shaped, wider than long, 122–159 × 176–239 (143.5 × 207) (length × width) (Fig. 1). Uterus hose shaped, ends as muscular aperture of 33–63 × 40–59 (51.5 × 49) (length × width), in a smooth genital atrium, almost round in shape (Fig. 1). Vagina simple, opens at the level of muscular genital atrium. Vitelline follicles arranged longitudinally in lateral fields along body (Figs 1 and 2). Vitelline ducts unite in a slender line directly anterior to ovary. Eggs oviform, 50–104 × 46–66 (84 × 52) (length × width) (Fig. 1).
Remarks
Based on Santos et al. (Reference Santos, Mourão and Cárdenas2001) and Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017), the new species belongs into Paradiplectanotrema due to the presence of an accessory piece on the MCO and one bar associated with each ventral anchor as well as one bar connecting the two dorsal anchors (one pair of dorsal anchors, one pair of ventral anchors, one pair of ventral bars, one dorsal bar).
The specimens represent a new species, as seen in Table 1. While the sizes of the marginal hooklets, MCO and eggs are similar in all species of this genus (Table 1), other morphological measurements are distinct. Paradiplectanotrema klimpeli sp. nov. differs from all congeners by having a longer body with a mean of 2390 (2050–2880). So far, only few specimens of P. trachuri have been reported being longer than 2 mm; however, the mean length of P. trachuri [1680 (1030–2220)] is smaller (see Table 1). The body width of P. klimpeli sp. nov. [800 (600–980)] is wider than the congeners’ (mean of 450–500) (Table 1). The widest part of the body lies half way between testis and opisthaptor (while in Pseudempleurosoma it lies at the level of the testis). With a length of 55–62 (58), the dorsal anchors are the longest in the genus, even though the size of the dorsal anchor of P. antigoni is almost as large (up to 60 with a mean of 57.5). Thus, the dorsal anchor length alone is characteristic to distinguish P. klimpeli sp. nov. from its congeners except P. antigoni.
Table 1. Morphological measurments of the now six species of Paradiplectanotrema

Main differences are in bold.
a Only mean/one value is given in Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) (thus, no statistical tests could be performed); note that details of former measurements are only available for the species P. lepidopi and P. trachuri (both show similar values and were sampled in the same region), although all species were (re-)described in one publication (see text) with n > 1 for each species except for P. citty (see also discussion).
b Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) provided different values for this character within one species (partially shown in italics), resulting from mounting (squeezing) techniques and/or omitting juvenile specimens from the measurements.
c Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) identified Cyttus sp. as host, however, out of the three valid Cyttus spp., only C. traversi is known from the African West coast (Atlantic).
A unique character of the new species is the shape of the dorsal bar being gladiator breast-plate shaped, with a 1:1 length:width ratio, while all so far decribed dorsal bars of the congeners have different shapes and ratios (Gerasev et al. Reference Gerasev, Gaevskaya and Kovaleva1987). Thus, the two structures of the dorsal anchor complex, namely, the dorsal hooks and the dorsal bar of P. klimpeli sp. nov., are unique. The ventral anchor is the smallest in this new species. With sizes of 9–11 (10), it differs from its congeners with means between 12.5 and 15. The bar of the ventral hamuli has smaller sizes (mean 11) compared with the congeners (means between 12.5 and 16) (Table 1).
The amount of eggs in P. klimpeli sp. nov. is noteworthy, with up to four eggs per specimen, similar to other species within the genus (up to three eggs), but in comparison with P. haywardi, that had nil to a maximum of one egg in utero. Other differences from the congeners is the host species, with the Synodontidae representing a new host family for the genus, and the geographic range in Indonesia, South-East Asia. The sampled grinners had sizes of 14.5–34.2 cm SL, with TW of 32.6–447.2 g. The infected four-type host fish specimens were comparably large, with 26.6/239.5, 29.9/285.1, 29.6/304.4 and 34.2/447.2 [SL (cm)/TW (g)].
DNA
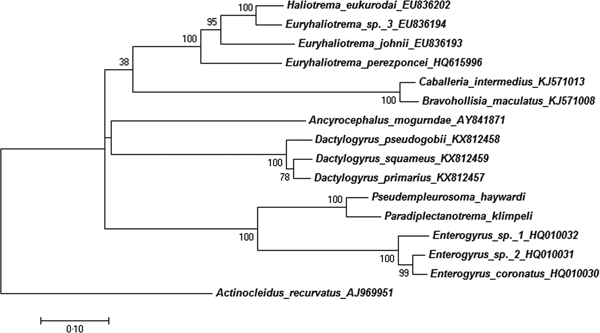
The partial LSU rDNA sequences were 838 bp long (with primers). A NCBI GenBank blast showed closest similarity to the only so far available sequence of a member of the Diplectanotrema group, namely P. haywardi (GBAN MF115714–MF115717), followed by freshwater endoparasitic monogeneans, namely Enterogyrus ‘sp. 1 AS-2010’, Enterogyrus ‘sp. 2 AS-2010’ [both ex Sarotherodon galilaeus (L.)], and Enterogyrus coronatus Pariselle, Lambert & Euzet, 1991 ex Coptodon zillii (Gervais, 1848) (GBAN: HQ010032, HQ010031, HQ010030). The obtained sequence of P. klimpeli sp. nov. formed a well-supported sister clade with P. haywardi (bootstrap value of 100%) (Fig. 4). The new sequence is available under the GBAN MG763101.

Fig. 4. Maximum-likelihood tree inferred from the analysis of LSU rDNA. The generated sequences were aligned with their closest matches in GenBank (15 ingroup and Actinocleidus recurvatus as outgroup) and formed a sister clade with Pseudempleurosoma haywardi (bootstrap value 100%), and with a branch of freshwater endoparasitic Monogenea from Cichlidae. Phylogenetic analysis based on the Tamura–Nei model with complete deletion used as gaps missing data treatment. The robustness was assessed using a bootstrap procedure with 1000 replications (Wu et al. Reference Wu, An, Xing and Ming2005; Kumar et al. Reference Kumar, Stecher and Tamura2016).
Discussion
Morphological characterization
Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) described several species of the Diplectanotrema group and named them after their respective teleost host, as seen in the description of the five Paradiplectanotrema spp. The morphological measurements of these species, all documented from the same region (African Atlantic coast), appear similar (Table 1). However, the descriptions were based on few specimens (n = 2 P. antigoni, n = 7 P. chlorophthalmi and n = 1 P. cytti), making detailed comparisons rather difficult. In addition, Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) provided a broader range of intraspecific measurements for the same character, possibly resulting from mounting (squeezing) techniques and/or omitting juveniles. Future DNA analyses of the West African species of Paradiplectanotrema are needed to confirm their validity and so far recorded host specificity (see Table 1). On the other hand, P. klimpeli sp. nov. can be clearly distinguished by the body and dorsal anchor length, the shape of the dorsal bar being gladiator breast-plate shaped (in upside-down view, see Fig. 2), and further parameters as summarized in Table 1. A number of misinterpretations of the morphology of the anchor apparatus of the Diplectanotrema group has been published over the years (compare Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017). Confocal images can clearly illustrate these important structures for diagnoses, and we herewith provide the first confocal images for a Paradiplectanotrema species (Fig. 2). Confocal microscopy enabled us to position structures, providing angle of views for illustrating and comparison as well as accurate measurements, for example the saucer-type excrescences, the exact length of the curved MCO, the irregular shape of its accessory piece, the connection of the hooks and bars or the dorsal bar, showing its length, width and shape accurately (Fig. 3). This methodology is found to be suitable to detect minor, often difficult to describe differences in the haptoral structures of these small monogeneans (also see Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017).
The (sub-)generic (re-)descriptions of Diplectanotrema by Johnston and Tiegs (Reference Johnston and Tiegs1922) and Price (Reference Price1937) are based on the type species Diplectanotrema balistes (MacCallum, 1915) Johnston and Tiegs, Reference Johnston and Tiegs1922. According to Gerasev (pers. comm. 2017), all (re-)descriptions seem to have misinterpreted the anchor apparatus (discussion on Pseudempleurosoma in Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017), with D. priacanthi Lebedev, 1968 still awaiting re-examination in the future. The first adequate diagnosis for Pseudempleurosoma as well as Paradiplectanotrema were provided by Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987). However, confocal illustrations of specimens belonging to all so far described genera within the Diplectanotrema group, describing the sclerotized structures of the anchor apparatus, are needed to distinguish the so far five valid genera (incuding Diplectranotrema, see Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017), especially their Table 1) and clarify the synonymies within this group of monogeneans.
Zoogeography and host range
The five so far described Paradiplectanotrema species have been reported exclusively from the Atlantic Ocean, off the coasts of West Africa [Guinea-Bissau, Western Sahara, Strait of Gibraltar (NW) and Angola (SW, also W Mediterranean)], along with their to date reported hosts (a caproid fish for P. antigoni, two chlorophthalmid species for P. chlorophthalmi, a zeid fish for P. cytti, a trichiurid fish for P. lepidopi and three carangid hosts for P. trachuri) (Table 1). This is the second description of an endoparasitic marine monogenean in South East Asia, the Indian Ocean respectively, suggesting a wider distribution also for other endoparasitic monogeneans of the Diplectanotrema group.
Here we establish a new host and family record for Paradiplectanotrema. It was already pointed out by Santos et al. (Reference Santos, Mourão and Cárdenas2001) and Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) that, although monogeneans are known for being host specific, site selecting and well adapted, the closely related Pseudempleurosoma (see below the ‘Phylogeny and Evolution’ section) has a variety of different host families and internal sites (from the beginning to the end of the gastro-intestinal system), and was also randomly collected from the gills. Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) summarized 14 different host species from seven families reported for the seven Pseudempleurosoma spp. worldwide (circum-equatorial). It is possible that Paradiplectanotrema might also have a lower host specificity (see Table 1 and Table 1 in Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) and a worldwide equatorial occurrence as well (Atlantic and Pacific, see also below the ‘Phylogeny and Evolution’ section). The oesophagus and pharynx are widely neglected host sites in the research of endoparasitic monogeneans, and the worms are hidden in the oesophageal folds (Fig. 2), covered by mucus. Consequently, further samples are needed to answer the real host and site specificity within this genus.
Concerning the host ecology, the so far known host species of Paradiplectanotrema spp. are, just like in Pseudemplerosoma spp., associated with muddy bottoms, infecting bottom-dwelling and sometimes schooling fish. However, while all hosts of Pseudempleurosoma spp. are associated with coastal waters and reefs, the host species of Paradiplectanotrema are deep-water fishes (see Table 2 here and Table 3 in Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017)).
Table 2. Paradiplectanotrema spp. fish host species ecology and economic value

Note that all host species are – in contrast to hosts of the members of coastal, reef-associated Pseudempleurosoma – deep-water fishes, sometimes schooling, and – similar to Pseudempleurosoma – associated with muddy bottoms; some fish host species have commercial value, N.e.: not evaluated, bold font marks similarities.
a Data from Froese and Pauli (Reference Froese and Pauly2017).
b 1 = P. antigoni, 2 = P. chlorphthalmi, 3 = P. cytti, 4 = P. lepidopi, 5 = P. klimpeli sp. nov., 6 = P. trachuri.
c Gerasev et al. (Reference Gerasev, Gaevskaya and Kovaleva1987) identified Cyttus sp. as host; however, out of the three valid Cyttus spp., only C. traversi is known from the African West coast (Atlantic) (Froese and Pauli, Reference Froese and Pauly2017).
Infection pattern
We provide infection rates with SLs of the host fishes, also for the sciaenid P. haywardi hosts (Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017, including unpublished data). Within the sample of grinners [14.5–34.2 SL (in cm), 32.6–447.2 TW (in g)], the infected four type host fish specimens were comparable in size. With four of 24 fishes harbouring 47 specimens, the prevalence was 17%, with a mean intensity of 12 and mean abundance of 2. According to own observations, the infections of Indonesian croakers with P. haywardi showed higher prevalences of 63% (22 of 35 fishes infected with 65 worms for Nibea soldado) and 46% (16 of 35 fishes infected with 32 worms for Otolithes ruber), but lower mean intensities of 3 (N. soldado) and 2 (O. ruber), intensities of 1–7 (N. soldado) and 1–6 (O. ruber), mean abundances of 2 (N. soldado) and 1 (O. ruber). Analysed soldier croakers had an SL of 20.0 (17.5–22.5) and a TW of 155.0 (99.5–211.4), the infected ones were of 17.5–21.8 SL and 99.5–203.3 TW, and the largest individual was negative. Investigated tiger-toothed croakers had an SL of 15.2 (13.7–17.1) and TW of 54.8 (41.6–78.1), the infected ones were of 13.7–16.7 SL and 41.6–71.0 TW, with the largest investigated individual being also negative (Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017, including unpublished data). Only two J. amblycephalus (SL 22.6/24.6, TW 258.2/202.2) were screend for P. haywardi (see Theisen et al. Reference Theisen, Palm, Al-Jufaili and Kleinertz2017), and both were found to be infected (with three and five worms, respectively). Therefore, we can summarize that Indonesian fishes infected with endoparasitic monogeneans of the Diplectanotrema group are bottom-dwelling, are genarally schooling, and of a middle size range, showing a TL/TG of 13.7–24.6/41.6–258.3 for croakers and 26.6–34.2/239.5–447.2 for grinners. It is interesting to note that the eggs in this monogenean group are few (maximum four eggs per specimen) and might infect the host of origin. However, bottom-dwelling schooling fish often ingest and disgorge bottom material such as mud and sand while searching for food, which might be a possible way for transmitting the eggs and parasite infection to another host.
Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) sampled P. haywardi in May and July 2011 (dry sesason) off the South coast of Central Java, and again during sampling of P. klimpeli sp. nov. in November 2016 (rainy season) off South Bali. The re-covering of the species within only two examined potential host organisms, almost 1000 km remote off the type locality, and 5 years later, during the rainy season, demonstrate the real abundance of P. haywardi in coastal Indonesian sciaenids. We conclude that, firstly, Indonesia is a marine reef fish biodiversity hotspot, secondly, Pseudempleurosoma spp. show low host specifity, and thirdly, fish parasitology research is still limited in Indonesian waters and the infection site, the oesophagus and pharynx, has been neglected in Monogenea research surveys. Furthermore, the tiny transparent worms are regularly covered by mucus in the pharyngeal folds of their host and are difficult to detect (compare Fig. 2A: magnified, transilluminated and without mucus). Taking deep-sea fishes into account, which also show a high biodiversity in Indonesia, we suggest that many more host records and probably species of these endoparasitizing genera might be present in the region.
Phylogeny and evolution
Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) provided the first DNA sequences for a marine endoparasitic monogenean, namely P. haywardi, a member of the Diplectanotrema group, and ensured its position within the Dactylogyridae. Thus far, it was assumed that evolution towards an endoparasitic lifestyle must have happened multiple times within the Monogenea, for freshwater and marine species separately. This might still be possible, because lacking DNA sequence data of Montchadskyella, marine endoparasitic Monogenea of armorheads (Histiopterinae) in Southern Australia, are not affiliated to the Diplectanotrema group, and might have been evolved separately. However, Theisen et al. (Reference Theisen, Palm, Al-Jufaili and Kleinertz2017) demonstrated the close relationship of Pseudempleurosoma and Enterogyrus, the latter is a freshwater endoparasitic monogenean from African Cichlidae fishes.
With the present study, we provide the first DNA sequences for the genus Paradiplectanotrema. The 28S region is found to be suitable for differentiation of diplectanotrem species and genera, however, the detected differences between the two diplectanorems of 2% in query cover and 8% in identity (according to NCBI GenBank Nucleotid Blast) can be primarily attributed to the two analysed species belonging to different genera, with similar generic differences occuring for example between respective species in the genus Dactylogyrus (or Enterogyrus, see Fig. 4). The relationship of two different genera within the Diplectanotrema group, was only based on morphology and ecology, but could be also now confirmed genetically (Fig. 4). The necessity of analysing further diplectanotrem species and genera will (i) help to understand the distribution patterns and evolution of a monogenean endoparasitic lifestyle through the world's oceans, and (ii) help to confirm validity and synonymy within the diplectanotrem taxa of less described species.
Conclusion
A new species of endoparasitic monogeneans in the marine lizardfish S. tumbil from Indonesian waters was described as P. klimpeli sp. nov., based on unique morphological characteristics, zoogeography, host distribution and DNA markers. Indonesian fish infections with endoparasitic monogeneans of the Diplectanotrema group can be abundant, their hosts known so far show medium sizes (13.7–34.2 cm SL, 41.6–447.2 g TW). All hosts of Paradiplectanotrema and Pseudempelurosoma are related to muddy bottoms, show schooling behaviour, and some are of commercial value. While the genus Pseudempleurosoma is associated with coastal reefs, Paradiplectanotrema occurs strictly in deep waters.
The phylogeny of marine endoparasitic Paradiplectanotrema was postulated for the first time, securing the position of these endoparasites and supporting an African freshwater origin. The Diplectanotrema group is ideally suited to analyse the spreading of a respective lifestyle and the worldwide colonization out of African waters into the world's oceans.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/pao.2018.8
Acknowledgements
We are thankful to Endang Wulandari Suryaningtyas, Pande Gde Sasmita Julyantoro and I Ketut Wija Negara from Udayana Universitas, for their help with issuing authorities, sampling, transport, laboratory work and providing work space. This is publication no. 11 under the Memorandum of Understanding between the Faculty of Veterinary Medicine, Udayana University, Bali, and the Faculty of Agricultural and Environmental Sciences, Aquaculture and Sea-Ranching, University of Rostock, Germany, in order to promote fish parasite and biodiversity research in Indonesia. Finally, we thank the collegues from the University of Rostock for providing the chance for confocal microscopy (PD Dr Sergei A. Kuznetsov from the Live Cell Imaging Center (LMZ), Department of Biology), and our colleague from our institute for providing and translating Russian literature, namely Dr Ekaterina Pikalov.
Financial Support
Open access article processing charge was paid by the open access publication fund of Rostock University, funded by the Deutsche Forschungsgemeinschaft (German Research Foundation).
Conflict of Interest
None.