The coronavirus disease 2019 (COVID-19) pandemic has spread at an exponential rate since the first recognition of the novel virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and has placed a disproportionate strain on intensive care resources worldwide. Reference Griffin, Karas, Ivascu and Lief1,Reference Li, Rivers, Tan, Murray, Toner and Lipsitch2
Healthcare facilities have been implicated as centers of transmission, in both tertiary-care hospitals and long-term care facilities. Reference Wang, Hu and Hu3-Reference Schwierzeck, Konig and Kuhn7 However, the frequency of nosocomial transmission in ICUs is less clear, and no large ICU outbreaks have been reported to date. ICUs have been important sites of nosocomial transmission and super-spreading events in previous coronavirus outbreaks, in part due to the increased frequency of use of aerosol-generating procedures (AGPs), particularly endotracheal intubation. Reference Tran, Cimon, Severn, Pessoa-Silva and Conly8,Reference Raboud, Shigayeva and McGeer9
Other noninvasive oxygenation strategies, such as positive pressure noninvasive ventilation (NIV) or high-flow nasal oxygen (HFNO), have been shown to be beneficial in reducing mortality and progression to intubation in hypoxemic respiratory failure. Reference Ferreyro, Angriman and Munshi10 However, from an infection control perspective, these strategies are AGPs with an increased risk of aerosol transmission and environmental contamination via droplet dispersion. The extent of transmission risk through environmental contamination from these procedures remains unclear, and recommendations from different regulatory authorities have varied in their definition of AGP and their relative risk. Reference Harding, Broom and Broom11
Extensive environmental contamination by SARS-CoV-2 in the environments of infected patients has been demonstrated in multiple studies in both healthcare and community settings, Reference Ong, Tan and Chia12-Reference Colaneri, Seminari and Novati20 but no study has focused specifically on the extent of such contamination in an ICU setting nor correlated patient and disease factors with the extent of environmental contamination, including the impact of ventilation modalities. A study found that environmental contamination decreased sharply after day 7 of illness, Reference Chia, Coleman and Tan13 which was hypothesized to be related to the similar decrease in viral load from the upper respiratory tract in the same time frame. Reference Wolfel, Corman and Guggemos21-Reference Young, Ong and Kalimuddin23
In this study, we evaluated the extent of environmental contamination by SARS-CoV-2 in an ICU setting and correlated these findings with patient and disease factors to assess the relative safety of different oxygenation methods with regard to environmental contamination. We hypothesized that despite the increased use of noninvasive ventilatory methods, environmental contamination in the ICU would be lower (1) because most COVID-19 patients typically deteriorate in the second week of illness, during which period viral shedding decreases Reference Zhou, Yu and Du24,Reference Huang, Wang and Li25 and (2) because closed-loop ventilatory circuits contain and limit the spread of contaminating droplets or aerosols. Additionally, we conducted a literature review to assess the extent and frequency of ICU environmental contamination across different healthcare systems worldwide.
Methods
Collection of environmental samples
This study was conducted in 2 dedicated COVID-19 ICUs in the National Centre for Infectious Diseases, the largest outbreak center for COVID-19 in Singapore. These ICUs admitted both patients with confirmed COVID-19 requiring intensive care as well as suspected patients with respiratory symptoms undergoing evaluation to rule out COVID-19. Environmental sampling was carried out at 5 separate time points in the rooms of all patients with active COVID-19 infection, defined by a positive SARS-CoV-2 polymerase chain reaction (PCR) test from any respiratory sample. Patients were housed in single airborne infection isolation rooms (AIIRs) with attached anterooms. Patients who had ceased viral shedding (ie, latest respiratory sample was negative for SARS-CoV-2 PCR) were excluded. In total, 10 sites were sampled from each room (Supplementary Fig. 1 online). In addition, 5 points in the common areas in the ICU were sampled, as well as 5 points in the staff pantry shared between both ICUs (Supplementary Fig. 2 online).
Environmental samples were collected by the same study team member throughout all sampling cycles using EnviroMax Plus premoistened macrofoam sterile swabs (Puritan Medical Products, Guilford, ME). The same surface area was swabbed for each sampling site using a standardized technique. This same environmental sampling protocol has been used in other studies at our center and has achieved consistent detection results. Reference Ong, Tan and Chia12,Reference Chia, Coleman and Tan13 All samples were kept at 4°C and were transported to a biosafety level 3 (BSL-3) laboratory for storage and testing within 3 days of sampling.
Clinical data collection
Clinical data including day of illness, type of oxygenation or ventilatory support, use of AGPs (intubation, extubation, open suctioning, nebulization, or bronchoscopy), and clinical cycle threshold (Ct) value (if available) were collected from the electronic medical record using a standardized case report form. No patient identifiers were recorded, and data were stored on a secured server. Informed consent was waived as clinical data were collected as part of an outbreak investigation under the Infectious Diseases Act, authorized by the Ministry of Health, Singapore.
Cleaning regimen of rooms
Routine twice-daily environmental cleaning in the ICU rooms was performed by housekeeping staff, using 5,000 parts per million (ppm) sodium dichloroisocyanurate (NaDCC) for environmental surfaces and 1,000 ppm NaDCC for the floor. Cleaning of common areas was also performed twice daily with 1,000 ppm NaDCC for the floor and high-touch surfaces. All environmental sampling was conducted in the morning before the scheduled environmental cleaning (ie, the last cleaning time was the afternoon prior to environmental sampling).
Polymerase chain reaction methods
Sample RNA extraction was performed using the QIAamp viral RNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Real-time PCR assays targeting the envelope (E) gene Reference Corman, Landt and Kaiser26 and orf1ab assay adapted from Drosten et al Reference Drosten, Gunther and Preiser27 were used for the detection of SARS-CoV-2 RNA. For the E gene assay, a 20 µL reaction mix was prepared with 12.5 µL of SuperScript III Platinum One-Step qRT-PCR Kit (Thermofisher Scientific, Waltham, MA) buffer, 0.75 mM Mg2SO4, 5 µL of RNA, 400 nM each of the forward primer (E_Sarbeco_F1-ACAGGTACGTTAATAGTTAATAGCGT) and reverse primer (E_Sarbeco_R2-ATATTGCAGCAGTACGCACACA) with 200 nM of probe (E_Sarbeco_P1-(FAM) ACACTAGCCATCCTTACTGCGCTTCG (BHQ1)). Thermal cycling conditions included reverse transcription at 55°C for 10 minutes, an initial denaturation at 95°C for 5 minutes, followed by 45 cycles of 95°C for 15 seconds, 58°C for 1 minute. For the orf1ab assay, a 20 µL reaction mix was prepared with 12.5 µL SuperScript III Platinum One-Step qRT-PCR Kit buffer, 0.5 mM Mg2SO4, 5 µL RNA, 800 nM each of the forward primer (Wu-BNI-F-CTAACATGTTTATCACCCGCG) and reverse primer (Wu-BNI-R-CTCTAGTAGCATGACACCCCTC) with 400 nM of probe (WU-BNI-P-(FAM) TAAGACATGTACGTGCATGGATTGGCTT (BHQ1)). Thermal cycling conditions included reverse transcription at 55°C for 10 minutes, an initial denaturation was conducted at 95°C for 5 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. All samples were run in duplicate and with both assays with positive and negative controls with each sample run. Positive detection was recorded as long as amplification was observed in at least 1 assay.
Virus culture methods
Positive swabs by PCR from the common area and staff pantry were further evaluated for virus viability via cell culture. Monolayers of Vero C1008 cells (ATCC-1586) in T25 flasks were inoculated with 1 mL inoculum (500 µL of the swab sample and 500 µL of Eagle’s MEM) and cultured at 37°C, 5% CO2 with blind passage every 7 days. Also, 140 µL cell culture was used for RNA extraction and real-time PCR twice per week to monitor changes in target SARS-CoV-2 genes as an indication of successful viral replication. In the absence of cytopathic effects and real-time PCR indication of viral replication, blind passages continued for a total of 4 passages before any sample was determined to be negative of viable SARS-CoV-2 virus particles.
Statistical analysis
Extent of ICU contamination was compared with previously published historical data from 30 rooms (27 general ward and 3 ICU) from our center. Reference Chia, Coleman and Tan13 Categorical variables were compared using the Fisher exact test, and continuous variables were compared using the Mann-Whitney U test. Binary logistic regression analysis was used to determine odds ratios (ORs) and 95% confidence intervals (CIs) for variables associated with presence of environmental contamination. P < .05 was considered significant, and all tests were 2-tailed. Analyses were performed using Stata version 13 software (StataCorp, College Station, TX).
Literature review
Other studies evaluating environmental contamination of hospital environments by SARS-CoV-2 were analyzed and compared in relation to our study results. We searched PubMed for manuscripts in English published before July 19, 2020, using varying combinations of the search terms “environmental,” “contamination,” “SARS-CoV-2,” “COVID-19,” and “hospital.” All manuscripts that reported results of environmental sampling in hospital environments were included and results were extracted and compared.
Results
Sample collection and clinical data
In total, 200 samples from 20 patient rooms were collected across 5 sampling time points; 60 samples from the ICU common areas were collected across 3 sampling time points; and 15 samples from the staff pantry were collected across 3 sampling time points. Of the 20 patients whose rooms were sampled, the median age was 51.5 years old (interquartile range [IQR] 39–67.75), and 15 (75%) were men. The median day of illness was day 14 (IQR, 9.25–18.75), and the median clinical Ct value was 31.22 (IQR, 27.31–34.56; n = 18 because 2 patients had only qualitative PCR reported).
Moreover, 7 patients (35%) were intubated and mechanically ventilated, 9 (45%) were receiving HFNO, and 4 (20%) did not receive any supplementary oxygen or ventilatory support. These last 4 patients were in the ICU for closer monitoring for other nonrespiratory complications (eg, myocardial infarction or arrhythmias requiring cardiac monitoring). Also, 3 patients (15%) had AGPs performed within the 24 hours prior to sampling.
Contamination of patient rooms
Overall, 14 rooms had at least 1 site that was contaminated (median number of sites, 2; IQR, 1–2; range 1–5) (Table 1). The most frequently contaminated sites were the bed rail and floor (30%), followed by the air outlet vent (25%) and infusion pumps (20%) (Fig. 1). Presence of environmental contamination was not significantly associated with age, sex, day of illness, ventilatory mode, AGP, or clinical Ct value (Table 2). Contamination was identified in rooms with patients on mechanical ventilation, HFNO, as well as those not requiring any ventilatory support. Viral cell culture was not attempted on patient room samples due to resource limitations.
Table 1. Clinical Data of Patients in Rooms Sampled and Sites of Environmental Contamination
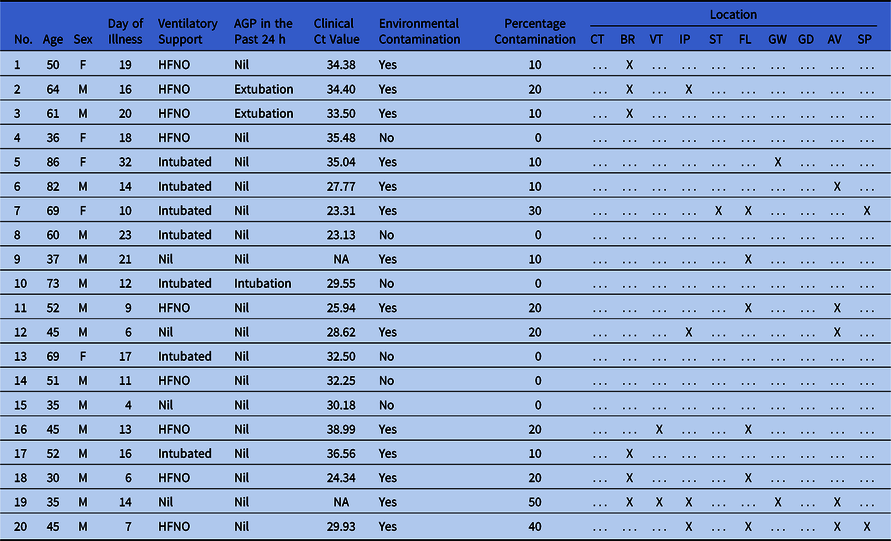
Note. AGP, aerosol-generating procedure; Ct, cycle threshold; CT, cardiac table; BR, bed rail; VT, ventilator; IP, infusion pumps; ST, stethoscope; FL, floor; GW, glass window; GD, glass door; AV, air outlet vent; SP, surgical pendant; F, female; M, male; HFNO, high-flow nasal oxygen; NA, not available; -, no contamination; X, contamination present

Fig. 1. Percentage contamination by sites sampled in patient rooms.
Table 2. Univariate Logistic Regression Analysis of Factors Associated With Presence of Environmental Contamination

Note. CI, confidence interval; IQR, interquartile range; ref, reference; Ct, cycle threshold; AGP, aerosol-generating procedure.
Comparison of ICU and general ward contamination
Results from this study were compared to historical data from 27 general ward rooms and an additional 3 ICU rooms to assess the differences in environmental contamination between both settings. Both studies were conducted done at the same center and by the same study team; thus, the environmental sampling protocol and hospital environmental decontamination protocols were unchanged. Comparing all ICU rooms with all general ward rooms, although the proportion of rooms with any environmental contamination were similar, there appeared to be less contamination in the ICU, with a lower number of sites and percentages of sites contaminated (Table 3). However, due to possible confounding factors, tests were not performed to determine the statistical significance of this difference.

Note. ICU, intensive care unit; IQR, interquartile range; Ct, cycle threshold.
a 30 rooms (3 from ICU, 27 from general ward) were included from historical data in a previously published study for analysis to compare environmental contamination between ICU and general ward rooms.
b Categorical variables are expressed as number (percentage), continuous variables are expressed as median (IQR).
Contamination of common areas and staff pantry
Of the 60 samples collected from the ICU ward common areas, 6 (10%) were positive for SARS-CoV-2: 5 samples from the floor and 1 sample from a desktop computer outside the patient room (Table 4). Of the 15 samples collected from the staff pantry, 2 (13.3%) were positive: 1 sample from the floor and 1 sample from a refrigerator door handle (Table 4). All samples were negative on viral cell culture.
Table 4. Results of Surface Sampling of Intensive Care Unit Common Areas and Staff Pantry

Literature review of environmental sampling studies
In total, 22 studies were identified that conducted environmental sampling of SARS-CoV-2. However, 2 studies were excluded because they were conducted outside of acute healthcare settings, 1 in a hotel quarantine facility and 1 in a community long-term care facility. Reference Jiang, Jiang and Wang18,Reference Nelson, Kassimatis and Estoque28 Of the 20 remaining studies, 9 did not conduct any sampling in the ICU (Table 5). No study specifically focused on environmental contamination in the ICU, and the number of ICU samples ranged from 24 to 218 (median, 35; with 2 studies not stating the precise number of ICU samples). Percentage contamination of all environmental samples from ICU patient rooms ranged from 0 to 44%. Only 2 studies performed viral cultures, and these results were negative for all samples. Because sampling protocol, patient profile, and environmental set-ups differed greatly between studies, further statistical analyses were not performed to assess statistical differences between studies.
Table 5. Comparison of the Extent of Environmental Contamination in Hospital Environmental Sampling Studies
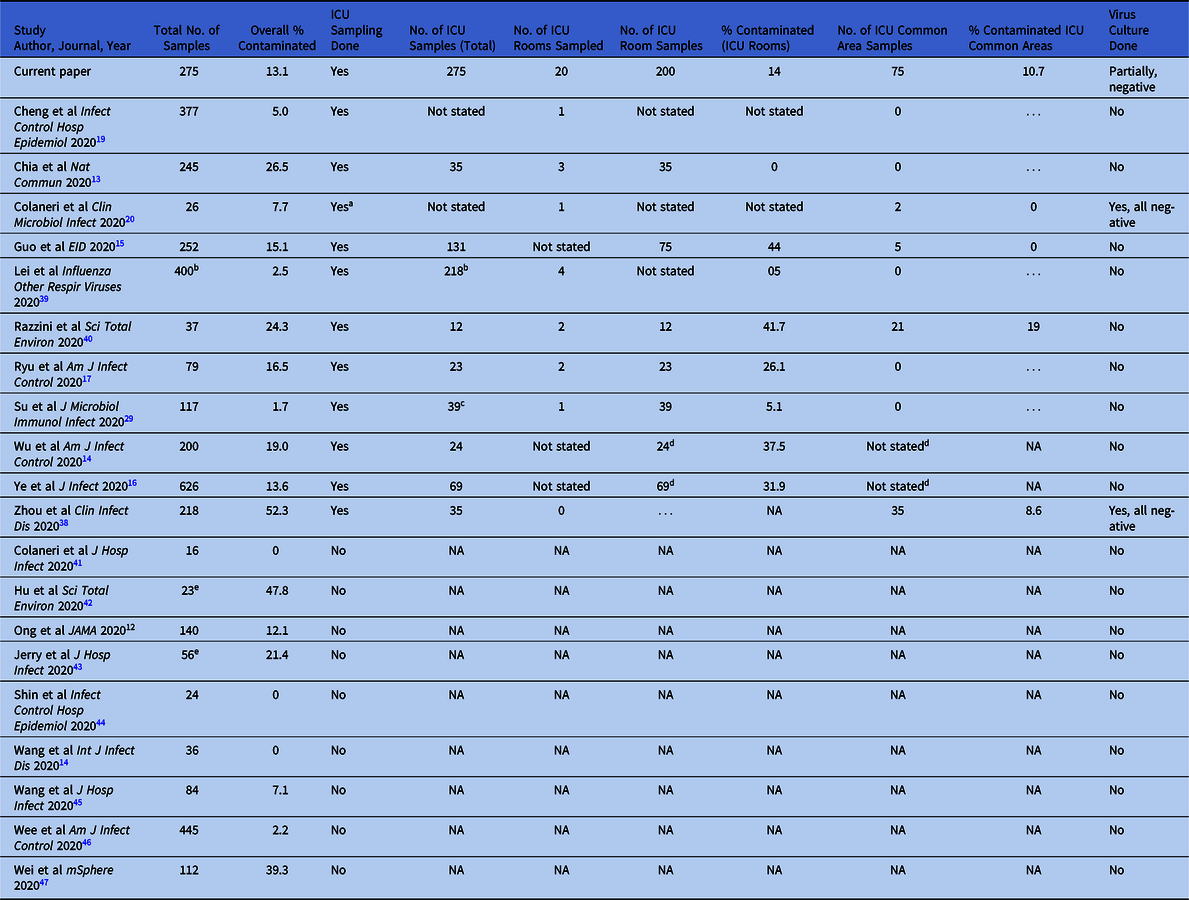
Note. ICU, intensive care unit; NA, not applicable.
a Sampling was done in a “sub-intensive care unit” and emergency unit, and individual numbers were not reported.
b Total number of samples for both air and surface samples; exact number of surface samples not specified.
c One sample, the inside of a closed suctioning tube, was excluded as we did not consider this an environmental sample.
d Not stated in paper whether ICU samples were divided into rooms are common areas. Percentage reported is percentage positivity of all ICU samples.
e Only samples taken before environmental decontamination were included.
Discussion
In this study, we report the presence and extent of environmental contamination by SARS-CoV-2 in a dedicated COVID-19 ICU. The overall contamination rate was low, and there was no difference in environmental contamination between those on mechanical ventilation or HFNO compared to those on room air. We also found limited contamination of the ICU common areas outside patient rooms.
Compared to other environmental sampling studies (Table 4), the degree of environmental contamination in the ICU was lower in our cohort, with 14% of patient room samples testing positive compared to a median of 29% (range, 0–44%) in the other 6 studies from which data were available. However, variation in sampling technique, patient profile, environmental ventilation settings, cleaning methods, and study design limits direct comparisons with other studies. Compared to an earlier study at our center that utilized the same standardized sampling protocol, Reference Chia, Coleman and Tan13 the extent of environmental contamination in the ICU was lower than in the general wards (with overall 26.5% of collected samples testing positive).
The lower extent of environmental contamination seen in the ICUs could be due to several reasons. First, viral shedding has been reported to peak in the first week of illness and to decrease thereafter, Reference Wolfel, Corman and Guggemos21,Reference Wang, Xu and Gao22 which coincides with the time in which most patients develop respiratory complications necessitating ICU admission. Reference Zhou, Yu and Du24,Reference Huang, Wang and Li25 The median day of illness (day 14) during sampling in our cohort is consistent with this. Second, patients in the ICU are confined to their bed and unable to walk around the room, thus reducing the chance of direct or indirect droplet spread. The patient with the greatest contamination in our study (50% of surfaces contaminated) was not requiring ventilatory support and was ambulant. Third, closed ventilatory circuits in mechanically ventilated patients likely limit the extent of aerosol or droplet dispersion from respiratory secretions. Su et al Reference Su, Hung and Lin29 tested environmental samples around 3 patients including 1 ICU patient, and although all environmental samples were negative, swabs taken from inside the ventilation and closed suction tubings were positive, supporting the hypothesis that the closed-loop ventilatory systems prevent environmental contamination.
Increased surface contamination was not associated with mechanical ventilation or HFNO, which suggests that such ventilatory modalities do not enhance SARS-CoV-2 viral dispersion. Reference Li, Fink and Ehrmann30 Although we did not directly measure aerosol or droplet generation, surface contamination may be used as a surrogate in assessing the extent of such generation because aerosols and droplets are deposited on environmental surfaces via gravity. In vitro studies using manikins and smoke dispersion have found that HFNO did not increase dispersion distance compared to simple oxygen or Venturi masks. Reference Hui, Chow and Lo31,Reference Ip, Tang and Hui32 HFNO was also not associated with increased environmental contamination for bacterial pneumonia in a randomized–controlled trial comparing its use with conventional oxygen masks. Reference Leung, Joynt and Gomersall33 Our results add to the data supporting the use of HFNO in hypoxemic respiratory failure in COVID-19 from an infection control perspective.
We did not find AGPs to be associated with increased environmental contamination, though only 3 patients underwent AGPs in the 24-hour window prior to sampling, and this small sample size limits conclusive interpretation. AGPs should still be considered high-risk procedures in terms of infection transmission. Novel engineering solutions, such as protective aerosol barriers or hoods, have been proposed to limit the aerosol and droplet dispersal associated with AGPs. Reference Adir, Segol and Kompaniets34,Reference Hill, Crockett, Circh, Lansville and Stahel35 However, there has been concern regarding breach of PPE and delayed intubation times associated with some of these contraptions, and their routine use cannot be recommended until more data emerge. Reference Begley, Lavery, Nickson and Brewster36
The contamination of surfaces in the common area and staff lounge, while unexpected, is likely to be of low impact in terms of infection transmission risk. The Ct values were high and close to the limit of detection, meaning that the amounts of nucleic acid detected were minute. A lower Ct value has been shown to correlate with successful isolation in viral culture, Reference Wolfel, Corman and Guggemos21,Reference Bullard, Dust and Funk37 with a cutoff of 24 in a study in which clinical samples were assessed. Reference Bullard, Dust and Funk37 Zhou et al Reference Zhou, Otter and Price38 have also demonstrated in in vitro studies that inoculated environmental samples with a Ct value >30 would not be positive on culture. Consistent with this, we were unable to isolate virus from these specimens. Similar to our findings, contamination of common areas, including water dispenser buttons and desktop computers, was also reported by Wu et al. Reference Wu, Wang, Jin, Tian, Liu and Mao14 Small amounts of nucleic acid could have been deposited on surfaces outside patient rooms through cross contamination after contact with the floor, shoes, or other fomites exiting patient rooms. Although the risk of infection from contact with such contaminated surfaces is infinitesimally small, attention should nonetheless be given to rigorous infection control precautions, decontamination protocols, and strict hand hygiene.
This study has several limitations. First, we could not perform viral culture on all samples that tested positive by PCR due to resource limitations, and we tested only a subset of positive samples from the common area and staff pantry, as we considered the downstream implications on infection control policy to be greater if this contamination was viable virus. PCR positivity alone for the samples taken from the patient rooms does not equate to infective virus, and the PCR assays may have detected nonviable viral nucleic acid. However, because we compared environmental contamination across various patient groups, RNA contamination may act as an acceptable outcome measure. Second, the sample size was small with only 20 patient rooms sampled; thus, this study may have been underpowered to detect smaller differences with regard to patient or disease factors affecting the degree of environmental contamination. Third, there were no patients receiving NIV; hence, we could not assess the potential impact of NIV.
In conclusion, environmental contamination was seen in the ICU, both in patient rooms and common areas. Contamination did not differ depending on the mode of ventilatory support, supporting the safe use of HFNO from an infection control perspective. The frequency and extent of contamination in the ICU was lower compared to general ward settings. Although the infectious risk of horizontal transmission from contaminated surfaces is low, attention should be given to the maintenance of strict hygiene, decontamination, and infection control precautions.
Acknowledgments
We thank the DSO environmental detection team and clinical diagnostics team for BSL3 sample processing and analysis. We thank the logistics and repository team for transport of biohazard material, inventory, and safekeeping of received items. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Financial support
This study was funded by the NMRC Seed Funding Program (grant no. TR19NMR119SD), NMRC COVID-19 Research Fund (grant nos. COVID19RF-001, COVID19RF-002), NHG-NCID COVID-19 Center Grant (grant no. COVID19 CG0002), and internal funds from DSO National Laboratories. Oon-Tek Ng is supported by NMRC Clinician Scientist Award (grant no. MOH-000276). Kalisvar Marimuthu is supported by NMRC CS-IRG (grant no. CIRG18Nov-0034). Additional funding support from a private donation from Sumitomo Mitsui Banking Corporation to the National Centre for Infectious Diseases which supplemented funding for this study.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.1278









