Introduction
Osteoarthritis (OA) is a common reason for consultation in primary care with 4% of adults aged 45 years and over consulting each year for OA (Jordan et al., Reference Jordan, Jöud, Bergknut, Croft, Edwards, Peat, Petersson, Turkiewicz, Wilkie and Englund2013).
Although traditionally viewed as a degenerative disease of joints, OA can be considered to have different phenotypes of disease with distinct clinical characteristics or causal factors (Bijlsma et al., Reference Bijlsma, Berenbaum and Lafeber2011). Obesity is a risk factor for OA (Cooper et al., Reference Cooper, Inskip, Croft, Campbell, Smith, McLaren and Coggon1998) but this may be more than a purely mechanical effect (Sellam and Berenbaum, Reference Sellam and Berenbaum2013). Diabetes has been associated with different musculoskeletal conditions and has also been identified as a risk factor for OA, independently of body mass index (BMI) (Louati et al., Reference Louati, Vidal, Berenbaum and Sellam2015). Conversely an increased risk of type 2 diabetes in people with OA has also been identified (Rahman et al., Reference Rahman, Cibere, Anis, Goldsmith and Kopec2014). This OA phenotype has been described as an expression of the metabolic syndrome (Velasquez and Katz, Reference Velasquez and Katz2010). Insulin resistance is thought to be associated with the development of OA (Hamada et al., Reference Hamada, Maynard, Schott, Drinkwater, Ketz, Kates, Jonason, Hilton, Zuscik and Mooney2015).
Statin treatment for primary or secondary prevention of vascular disease has been found to be associated with a reduction in some manifestations of clinical OA (Kadam et al., Reference Kadam, Blagojevic and Belcher2013). Although a causal mechanism for this association has not been established, it is plausible that the relationship is due to OA forming a part of the metabolic syndrome.
Metformin as a treatment for type 2 diabetes has previously been investigated to determine whether it is associated with a reduction in the risk of cardiovascular events and all-cause mortality, but with conflicting findings (Boussageon et al., Reference Boussageon, Supper, Bejan-Angoulvant, Kellou, Cucherat, Boissel, Kassai, Moreau, Gueyffier and Cornu2012).
There is limited knowledge about the effects of metformin on risk of OA. It has been hypothesised that metformin is associated with bone health through promotion of differentiation of osteoblasts and their regulation and protection from hyperglycaemia (Yan and Li, Reference Yan and Li2013), and metformin is considered to have beneficial effects on insulin resistance (Wiernsperger and Bailey, Reference Wiernsperger and Bailey1999).
We hypothesised that patients with type 2 diabetes treated with metformin may show a reduced risk of OA compared with people with type 2 diabetes not so treated. To investigate this, we conducted a longitudinal analysis using routinely recorded electronic health record data.
Methods
Study design and setting
This study used a cohort design using the Consultations in Primary Care Archive (CiPCA) database, an anonymised database of routinely recorded information from 13 general practices in North Staffordshire, UK (Porcheret et al., Reference Porcheret, Hughes, Evans, Jordan, Whitehurst, Ogden and Croft2004; Jordan et al., Reference Jordan, Clarke, Symmons, Fleming, Porcheret, Kadam and Croft2007). Practices undergo regular assessment, feedback and training on the quality of their morbidity recording (Porcheret et al., Reference Porcheret, Hughes, Evans, Jordan, Whitehurst, Ogden and Croft2004). Prevalence of consultation for musculoskeletal conditions has been shown to be similar to national and international databases (Jordan et al., Reference Jordan, Jöud, Bergknut, Croft, Edwards, Peat, Petersson, Turkiewicz, Wilkie and Englund2013). Practices contributing to CiPCA use the Read code system for recording morbidity as is most common in UK primary care.
Study population
To be eligible, patients had to be aged 40 or over and have had either a recorded diabetes diagnosis or diabetes treatment between January 2002 and December 2003 (the “baseline period”). Read codes for diabetes are available from our website www.keele.ac.uk/mrr. Each patient’s index date was defined as the first occurrence of a diagnosis of diabetes or prescription of a diabetic drug. Patients with a prior record of OA in the past two years were excluded, as were patients with a record of type 1 diabetes (identified either through Read code or through linked consultation text). All eligible participants had a minimum of one year prior registration at their practice.
Exposure
Prescription information was available for all participants for their time in the study and at least 12 months before cohort entry. Those patients prescribed a drug for diabetes (BNF Chapter 6.1) would typically have multiple repeat prescriptions, and may switch between metformin and non-metformin treatments. A non-metformin prescription was defined as any other diabetic drug, or a diet and lifestyle advice (no drug) treatment only. Two approaches were conducted to investigate association of exposure to metformin with OA.
The first analysis compared risk of future OA diagnosis based on baseline treatment (metformin prescription or not in the baseline period 2002–2003).
The second analysis incorporated change in pharmacological treatment of diabetes (metformin versus non-metformin) over time. Patients not prescribed metformin in the baseline period but later prescribed metformin were deemed to be exposed to metformin from the date of the first such prescription. If a patient prescribed metformin was then not recorded as having a metformin prescription for six months at any subsequent point during follow-up, metformin exposure was deemed to have ended 28 days after the last recorded prescription If a prescription was recorded within six months of a prior prescription, metformin exposure was deemed to have continued uninterrupted. This is consistent with previous work within our Research Institute and with guidelines that GPs should prescribe a maximum of a 28 day supply of medication per prescription.
Outcome
The primary outcome of interest was the first occurence of an OA diagnosis during the follow-up period, defined by Read code no. 5 “Osteoarthritis and allied disorders” and all child codes. Follow-up continued to the end of 2011, the end of patient registration at their practice, end of practice records in CiPCA, or the first record of OA.
Covariates
Covariates considered to be potential confounders of the relationship of metformin with OA diagnosis included age at index date, gender, GP practice, neighbourhood deprivation, and comorbidity. Comorbidity was defined as number of different prescription drugs (based on British National Formulary codes) prescribed during the baseline period and categorised into four groups; 0–5, 6–9, 10–13, and 14+, based on quartiles. This measure has been shown to be an efficient measure of comorbidity for healthcare use (Perkins et al., Reference Perkins, Kroenke, Untzer, Katon, Williams and Hope2004).
Measurement of neighbourhood deprivation was based on the Index of Multiple Deprivation (IMD) 2007, a small area-level measure of deprivation across England (Department for Communities and Local governments, 2007). This variable was categorised based on quintiles, the first category representing the most deprived in the population, and the fifth category representing the least deprived.
Statistical analyses
Cox proportional hazards regression models were fitted with Gamma frailty term. This is essentially a random effects model to address variability in outcomes across patients (ie, different underlying frailty) related to unobserved covariates (Hougaard, Reference Hougaard1995). The shared frailty term in this case assumes that the frailty is common to patients within the same practice.
The proportional hazards assumptions were checked for both models fitted, and sensitivity analyses were conducted to test the robustness of the results to the distributional assumptions placed on the random effect. In place of a Gamma distribution, the commonly used Gaussian frailty term was added to the model (Yashin, Reference Yashin, Iachine, Begun and Vaupel2001).
All analyses were completed using R version 3.2.2 through R studio version 0.99.473 for Windows.
Results
A total of 54 006 patients aged 40 and above were registered at the 13 CiPCA practices in 2002. There were 4164 patients with a record of diabetes in 2002 or 2003. Of these 133 were excluded due to having a record of type 1 diabetes; 98 due to having no consultation information recorded during follow-up; 712 due to having a diagnosis of OA before their start date in the study; and a further four were removed due to having a diagnosis of OA on their index date. The remaining 3217 patients were eligible to be included in the analysis.
Baseline exposure analysis
Initially patients were split into treatment groups based on prescriptions received during the baseline period. There were 1838 (57.13%) patients prescribed metformin, and 1379 (42.87%) not prescribed metformin; 13.92% of those in the non-metformin group were on lifestyle and diet changes only, whilst the remaining 86.08% received a prescription for another anti-diabetic drug.
Those prescribed metformin at baseline tended to be younger [mean age 64.08 (SD: 11.33) years versus 68.64 (SD: 11.90)] but were similar in terms of gender, deprivation and median number of other prescription drugs during baseline (Table 1).
Table 1 Baseline characteristics for participants during the baseline period (2002–2003)
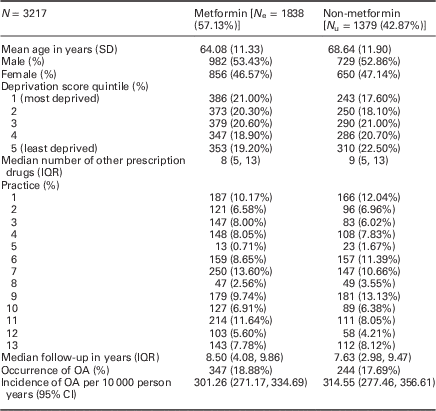
IQR=interquartile range; OA=osteoarthritis.
Median follow-up was 8.50 (IQR: 4.08, 9.86) years for those prescribed metformin, and 7.63 (IQR: 2.98, 9.47) for those not prescribed metformin.
A total of 347 (18.88%) of those prescribed metformin had a diagnosis of OA during follow-up [incidence: 301.26; 95% CI: (271.17, 334.69) per 10 000 person years); 244 (17.69%) of those not prescribed metformin at baseline had a diagnosis of OA (314.55/10 000; 95% CI: 277.46, 356.61)].
There was no association of baseline prescription of metformin with OA [unadjusted HR: 0.97 (95% CI: 0.87, 1.10), adjusted HR: 1.02 (95% CI: 0.91, 1.15)] (Table 2).
Table 2 Hazard ratios, 95% confidence intervals (CIs), and significance for the fitted Gamma frailty term model for the baseline exposure and time-varying analyses
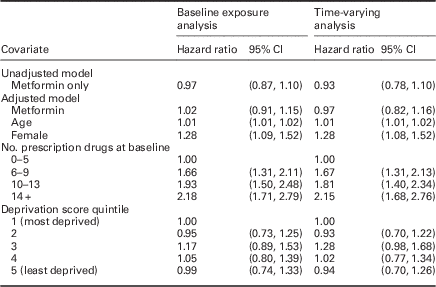
Age [HR: 1.01 per year, (95% CI: 1.01, 1.02)], female gender [HR: 1.28, (95% CI: 1.09, 1.52)], and more prescription drugs 14+ versus 0–5 [HR: 2.18, (95% CI: 1.71, 2.79)] were associated with OA diagnosis during follow-up, whereas deprivation was not associated with OA diagnosis. The gamma frailty term was significant, indicating significant heterogeneity between GP practices.
One practice had only 36 registered patients in the analysis and appeared to violate the proportional hazards assumption. Its removal from the analysis did not change the findings.
Changing the gamma frailty term to a Gaussian did not substantially change the hazard ratios.
Time-varying analysis
In all, 2289 (71.11%) patients had a metformin prescription at some point during follow-up; 196 (8.56%) of these patients received a diagnosis of OA whilst they were on a metformin prescription.
A total of 2885 (89.62%) patients had a period of follow-up when they were not prescribed metformin, of which 395 (13.69%) were diagnosed with OA whilst they were on a non-metformin prescription.
Prescription of metformin (allowing exposure to vary over time) was not associated with a new OA diagnosis [unadjusted HR 0.93 (95% CI: 0.78, 1.10), adjusted HR 0.98 (95% CI: 0.82, 1.16)]. There was a similar relationship with OA for gender, age, deprivation score, and number of recorded prescriptions as in the analysis conducted on the baseline data (Table 2).
The addition of the random effect was again significant.
The proportional hazards assumption was checked and satisfied for this model. Using a Gaussian rather than Gamma frailty term did not substantially change the estimated HRs.
Discussion
In this cohort of diabetes patients with up to 10 years of follow-up, no significant association was found between prescription of metformin and diagnosis of OA.
Consistent with previous literature we identified an increase in risk with increasing age and an increased risk for females. We also identified a dose response relationship with number of other prescription drugs as a marker of comorbidity and risk of OA.
Diabetes has been shown to have an independent association with OA (5). This is the first population-based cohort study to examine whether metformin, a common treatment for diabetes, can have a protective effect against OA in those with diabetes. A major strength of this project is the large sample size from a primary care population database that has been found to give similar results for prevalence of musculoskeletal conditions compared with national databases (Jordan et al., Reference Jordan, Jöud, Bergknut, Croft, Edwards, Peat, Petersson, Turkiewicz, Wilkie and Englund2013). The findings are therefore likely to be generalisable to the United Kingdom as a whole.
There is variability between clinicians in diagnosing and recording OA, with some preferring a non-specific ‘joint pain’ term (Jordan et al., Reference Jordan, Tan, Edwards, Chen, Englund, Hubertsson, Jöud, Porcheret, Turkiewicz and Peat2016). We have used only the OA diagnostic term in this study and so may have under-ascertained cases of OA but are likely to have included patients with more severe joint pain (Jordan et al., Reference Jordan, Clarke, Symmons, Fleming, Porcheret, Kadam and Croft2007).
A total of 27.2% patients had no recorded type associated with a diabetes diagnosis but were assumed to have type 2. This may be have led to a degree of misclassification but there is no reason to believe this should have biased the results. We did not assess OA in specific joints. As the metabolic phenotype of OA has been suggested to predominantly affect the hand, knee, and generalised OA (Bijlsma et al., Reference Bijlsma, Berenbaum and Lafeber2011), further work to examine the effect of metformin on site-specific OA would be appropriate. Patients may have had a previous diagnosis of OA more than two years before the index date, and so in some cases we may have identified new consulting episodes of OA rather than first ever diagnosis.
Although the estimated association between metformin and OA was adjusted for potential confounders such as age and gender, we lacked information for this analysis about other pertinent covariates such as BMI. As the prescription of metformin in clinical practice is linked to increased BMI, the lack of adjustment for BMI may hide any association of metformin with reduced risk of OA.
Similarly any effect of metformin may plausibly be linked to dosage and duration which we have not investigated here.
In conclusion, this study has not identified evidence of an association of metformin with OA but further research should assess the effects of dosage and duration on treatment, incorporate BMI, and ascertain associations with site-specific OA.
Ethics approval
Ethical approval for CiPCA was granted by the North Staffordshire Research Ethics Committee.
Acknowledgements
The Keele GP Research Partnership, the Informatics team at the Arthritis Research UK Primary Care Centre, and the National Institute for Health Research (NIHR).
Financial Support
CiPCA database is funded by the North Staffordshire Primary Care Research Consortium and Keele University Research Institute for Primary Care and Health Sciences. Lauren Barnett is funded by a National Institute for Health Research (NIHR) Research Methods Fellowship. This project presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of Interest
The authors have declared no conflicts of interest.





