1. Introduction
Depression (a depressive disorder, DD) is the most common mental disorder. All around the world, 300 million people suffer from DD Reference WHO[1]. Moreover, by 2020, depression will have become the second health and economic problem, second only to the ischemic heart disease Reference Reddy[2]. Additionally, about 1/3 patients does not respond to the conventional therapy, and untreated or inappropriately treated depression may lead to suicide attempts Reference Iwata, Ota and Duman[3]. Previous studies showed that the DD is regarded as multi-casual disease and its pathology remains unclear [Reference Gałecki, Maes, Florkowski, Lewiński, Gałecka and Bieńkiewicz4–Reference Maes, Galecki, Verkerk and Rief7]. Additionally, depression is associated with numerous somatic disease − it increases the risk of developing the atherosclerotic heart disease, type 2 diabetes mellitus, cancer − and increases mortality rates [Reference Clarke and Currie8–Reference Preiss, Brennan and Clarke10]. Furthermore, recent studies showed, that DD occurrence is also strongly associated with obesity [Reference Hryhorczuk, Sharma and Fulton11–Reference Lin, Huang, Tai, Lin, Kao and Tsai14]. Interestingly, obese patients, similarly to depressed patients, were characterized by an imbalance of tryptophan catabolites (TRYCATs) pathway Reference Chaves Filho, Lima, Vasconcelos, de Lucena, Maes and Macedo[15]. Our earlier study of single nucleotide polymorphisms (SNPs) of genes encoding tryptophan hydroxylase suggests that the tryptophan metabolism may play the key role in the pathophysiology of DD Reference Wigner, Czarny, Synowiec, Bijak, Białek and Talarowska[16]. Another study showed that a decreased level of tryptophan or an elevated concentration of harmful TRYCATs, i.e. kynurenine, quinolinic acid, 3-hydroxyanthranilic acid, 3-hydroxytryptophanmay cause the DD symptoms to surface Reference Maes, Leonard, Myint, Kubera and Verkerk[6]. Although tryptophan is converted into toxic kynurenine, it also is a precursor of serotonin (5-HT) and melatonin − a main neurotransmitter that regulates the mood Reference Hamon and Blier[17]. The first step of TRYCATs pathway, which is degradation of tryptophan to N-formylkynurenine, is catalysed by tryptophan 2,3-dioxygenase (TDO) or indoleamine-pyrrole-2,3-dioxygenase (IDO). There are rate-limiting enzymes of this pathway. TDO is expressed in liver and converts tryptophan only, whereas IDO is expressed in placenta, lungs, brain, blood − and, aside from tryptophan, it also metabolizes melatonin and serotonin [Reference Hayaishi18, Reference Watanabe, Fujiwara, Yoshida and Hayaishi19]. Furthermore, the activity of IDO may be regulated by cytokines, whereas TDO does not depend on this regulation. Pro-inflammatory cytokines − interferon-γ (INFγ), interferon-α (INFα), and tumour necrosis factor-α (TNFα) − may act as potent activators of IDO. On the other hand, the anti-inflammatory cytokines may be inhibitors of the enzyme [Reference MacKenzie, Worku and Daubener20–Reference Wichers and Maes22]. Clinical trials showed that the activity of IDO may be assessed by the examination of kynurenine/tryptophan ratio or by expression level of IDO [Reference Maes5, Reference Maes, Leonard, Myint, Kubera and Verkerk6]. The previous studies suggest that an increased activity of IDO and TDO may be an associated with occurrence of the depressive-like behaviours [Reference Henry, Huang, Wynne, Hanke, Himler and Bailey23, Reference Moreau, André, O'Connor, Dumich, Woods and Kelley24, Reference Maes5, Reference Maes, Leonard, Myint, Kubera and Verkerk6]. Moreover, an elevated kynurenine/tryptophan ratio may cause a development of anhedonia, which is the primary symptom of depression Reference Gabbay, Ely, Babb and Liebes[25]. Animal studies confirmed that depression may be associated with an increased IDO expression/activity and levels of kynurenine, 5-hydroxykynurenine, quinolinic acid in brain areas, i.e. hippocampus, hypothalamus and amygdala [Reference Connor, Starr, O'Sullivan and Harkin26–Reference Launay, Callebert, Surget, Belzung and Barone29]. Moreover, the IDO activation and serum level of the harmful TRYCATs were associated with the onset and severity of the disease symptoms [Reference Maes, Verkerk, Bonaccorso, Ombelet, Bosmans and Scharpé30–Reference Savitz, Drevets, Wurfel, Ford, Bellgowan and Victor33]. Interestingly, the study showed that the female patients with the DD were characterized by a higher serum concentration of IDO, TNFα, INFγ but by a lower level of serotonin when compared to healthy volunteers. Moreover, the levels of IDO and TNFα were decreased in patients after anti-depressant therapy [Reference Zoga, Oulis, Chatzipanagiotou, Masdrakis, Pliatsika and Boufidou34, Reference Robinson, Shirey and Carlin35]. In the same study, Zoga et al. Reference Zoga, Oulis, Chatzipanagiotou, Masdrakis, Pliatsika and Boufidou[34] found a strong positive correlation between the concentration of IDO and INFγ. Moreover, transcription of IDO1, a gene encoding one of the protein isoform, is strongly controlled by cytokines. The gene promoter contains multiple sequence elements that induction of responsiveness to type I (INFα and −β) and type II (INFγ) interferons [Reference Mellor and Munn36, Reference Taylor and Feng37]. Induction of the expression by the latter one is mediated by a signal transducer and activator of transcription 1 (STAT1) and INF-regulatory factor 1 Reference Chon, Hassanain and Gupta[38]. Moreover, IDO metabolizes the serotonin degradation into N-formyl-5-hydroxykynurenine; thus its over-expression results in a deficiency of the neurotransmitter Reference Pertz and Back[39]. Accordingly, the increased activity of the IDO and an impaired central serotonin system may lead to development of depression in patients with inflammatory disease [Reference Cryan and Leonard40, Reference Lee, Jeong, Kwak and Park41]. The same study proved that glial cells secrete interleukin-6 (IL-6), interleukin-1 beta (IL-1β), TNF-α, and INF-γin response to injury and infection. Furthermore, it was found that DD could be potentially attributed to a hippocampal depletion of tryptophan, degradation of serotonin and increased level of kynurenine derived from serotonin degradation Reference Maes, Leonard, Myint, Kubera and Verkerk[6].
The next important step of TRYCATs pathway includes a conversion of kynurenine into kynurenic acid (enzyme − kynurenine formamidase) or, alternatively, into 3-hydroxykynurenine (3-HK), enzyme − kynurenine aminotransferase, up to date, its four isoforms have been described: KAT1/glutamine transaminase K (GTK)/cysteine conjugate beta-lyase (CCBL) 1, KAT2/aminoadipate aminotransferase (AADAT), KAT3/CCBL2 and KAT4/glutamicoxaloacetic transaminase (GOT) 2/mitochondrial aspartate aminotransferase (ASAT)or anthranilic acid (enzyme − kynurenine hydroxylase) Reference de Souza, Fontes, da Silva, Coutinho, Leib and Agnez-Lima[42]. Subsequently, 3-HK can be metabolized by kynureninase to form 3-hydroxyanthranilic acid (3-HAA), which can be further metabolized to form the quinolinic acid (QUIA) Reference Stone and Darlington[43]. Although QUIA is neurotoxic, it cannot penetrate the blood-brain barrier. On the other hand, kynurenine is not neuro-active; however it may cross the blood-brain barrier and it generates free-radical-producing 3-HK or 3-HAA or is converted to the glutamatergically-active QUIA Reference Stone and Darlington[43]. The harmful actions of TRYCATs may be related to an oxidative damage, inflammation, mitochondrial dysfunction, cytotoxicity, excitotoxicity, neurotoxicity and lowered neuroplasticity in central nervous system, e.g. 3-hydroxykynurenine may initiate neuronal apoptosis [Reference Guillemin, Kerr, Smythe, Smith, Kapoor and Armati44, Reference Maes, Mihaylova, RuyterDe, Kubera and Bosmans45, Reference Maes, Leonard, Myint, Kubera and Verkerk6, Reference Maes, Galecki, Verkerk and Rief7]. In contrast, some TRYCATs induce begin effects, e.g. kynurenic acid manifests antioxidant and neuroprotective properties which based on block N-methyl-d-aspartate (NMDA) receptors [Reference Guillemin, Kerr, Smythe, Smith, Kapoor and Armati44, Reference Maes, Mihaylova, RuyterDe, Kubera and Bosmans45, Reference Maes5]. This acid is converted from kynurenine by kynurenineaminotransferase (KAT1 and KAT2/AADAT) Stone and Darlingtone, 2002. Therefore, the kynurenine/kynurenic acid (KYN/KA) ratio indicates a KAT activity (the ratio increase in inverse proportion to the KAT activity) and was found to be higher in depressed patients than controls Reference Maes, Galecki, Verkerk and Rief[7]. Accordingly, an elevated expression of kynurenine aminotransferase in skeletal muscles can protect from depression Reference Schlittler, Goiny, Agudelo, Venckunas, Brazaitis and Skurvydas[46]. Interestingly, the same study showed that physical exercise may be used as an alternative treatment of DD and that endurance exercise led to an increased expression of KAT in muscles, while the patients who were practising some sports/taking the training were characterized by an increased level of kynurenic acid in plasma. Another piece of evidence supporting involvement of TRYCATs pathway in depression development is the fact that abnormal activity of IDO may lead to impairment of serotonin metabolism and a decrease of melatonin level, and, in consequence, may trigger sleep disorders, all of these symptoms often present in the course of the disease Reference Zoga, Oulis, Chatzipanagiotou, Masdrakis, Pliatsika and Boufidou[34]. Moreover, patients with depressive suicidal attempts suffer more frequently from severe insomnia than patents without the attempts Reference Heitzman[47].
The exact pathogenesis of the DD is unclear, genetic, environmental, and behavioral factors as well as interaction between them may be involved. Although the DD is recognized as a multifactorial disease, genetic factors may play the crucial role in its development, as was confirmed by segregation analyses, genetic epidemiological data and gene mapping studies. Several gene loci/chromosomal regions for DD have been mapped by genome-wide linkage analysis, including 12q23.3–q24.11 and 13q31.1–q31.3, 15q25.2 McGuffin et al., 2005, 3p21.1 Sullivian et al., 2013,19q12., 11p14.2, 8q22.2, 8q12.1, 8q23.3, 3p26.1, 2p25.1, 11p14.3, 6p22.3, 1q32.1, 3q26.1 (Shyn et al. 2011). These findings show a heterogeneous and complex genetic nature of the DD. Moreover, according to the study report, the genomic regions significantly associated with the DD were localized on chromosome 1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14,15, 16, 17, 18, 22 Wray et al., 2017. Therefore, in this paper, we examine the link between single nucleotide polymorphisms (SNPs) of enzymes involved in TRYCATs pathway: c.*456G > A of KAT1 (rs10988134, is located on 9q34.11), c.975-7T > C of AADAT (rs1480544, is located on 4q33), c.-1849C > A (rs3824259) and c.-1493G > C (rs10089084)of IDO1 (is located on 8p11.21) and incidence of depression. Moreover, we detected differences between male or female groups in examined polymorphisms frequency. Interesting results were also obtained for the analysis an impact the single-nucleotide polymorphisms of genes encoding TRYCATs enzymes on effectiveness of antidepressant therapy.
2. Materials and methods
2.1 Subjects
281 patients with depression (age 49.53 ± 10.18, n male = 148, n female = 133) hospitalized at the Department of Adult Psychiatry of the Medical University of Lodz (Poland) and 236 healthy volunteers (age 53.19 ± 12.61, n male = 121, n female = 115) participated in the study. The Table 1. shows a detailed characteristic of depressed patients. All subjects in the control and depressed groups were native Poles from central Poland (not related), randomly selected without replacement sampling. All the participants had to meet the inclusion criteria outlined in ICD-10 [48]. The axes I and II disorders, other than the DD, severe and chronic somatic diseases, injuries of the central nervous system, inflammatory or autoimmune disorders and unwillingness to give informed consent were exclusion criteria. Moreover, familial prevalence of mental disorders other than recurrent depressive disorders was also a factor of exclusion from the examined groups. Standardized Composite International Diagnostic Interview (CIDI) served as the guide to conducting a medical history for all cases before the study began. Evaluation and classification of depression severity were based on the Hamilton Depression Rating Scale (HDRS) Reference Hamilton[49]. The intensity of DD symptoms was measured in the study according to the grades proposed by Demyttenaere and De Fruyt Reference Demyttenaere and De Fruyt[50] before and after antidepressant therapy with a selective serotonin reuptake inhibitor (SSRI). The same psychiatrist examined each patient before the start of the study and after 8 weeks of pharmacotherapy. Participation in this study was voluntary. Its subjects were informed about the details of the study and assured of their voluntary participation in the experiment. Moreover, patients were guaranteed that their personal data would be kept secret before making a decision to participate in the study. During hospitalization, all the participants were treated according to antidepressant treatment standards. According to the protocol approved by the Bioethics Committee of the Medical University of Lodz (no. RNN/70/14/KE), all the subjects consented to participation in the study.
Table 1 Detailed characteristic of patients taking part in the study.

2.2 Selection of single nucleotide polymorphism
The public domain of the National Center for Biotechnology Information the Single Nucleotide Polymorphisms database (NCBI dbSNP) at http://www.ncbi.nlm.nih.gov/snp was used to identify potentially functional polymorphisms in genes encoding IDO and KAT. The four polymorphisms were chosen with a minor allele frequency (MAF) higher than 0.05 in the European population, respectively (submitter population ID: HapMap-CEU for both; http://www.ncbi.nlm.nih.gov/snp). The selection of studied polymorphisms was mainly determined by a potential biological significance − the localization of the SNPs was in the coding or regulatory regions of genes and might have functional meaning for transcription and protein function. The c.*456G > A(rs10988134) polymorphism of KAT1 is localized in 3′ untranslated region and thec.975-7T > C (rs1480544) SNPis localized in intron ofAADAT. The c.-1849C > A (rs3824259) and c.-1493G > C(rs10089084) polymorphisms are located near 5′ end of IDO1.
2.3 DNA extraction
We used the commercially available Blood Mini Kit (A&A Biotechnology, Gdynia, Poland) to extract genomic DNA from venous blood of patients with depression and from all controls. DNA purity and concentration were determined by measuring e absorbance at 260 and 280 nm. The sample of purified isolated DNA was stored at −20C until further analysis.
2.4 Genotyping
The TaqMans SNP Genotyping Assay (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and 2X Master Mix Takyon for Probe Assay − No ROX (Eurogentec, Liège, Belgium) were used to genotype the studied SNPs in the Bio-Rad CFX96 Real-Time PCR Detection System and analysed in the CFX Manager Software (Bio-Rad Laboratories Inc., Hercules, California, USA). Theywere carried out the real-time polymerase chain reactions.
2.5 Statistical analysis
The SigmaPlot version 11.0 (Systat Software, Inc., San Jose, CA, USA), a statistical software package was used to the statistical analyses. The unconditional multiple logistic regression model was used to the evaluation of the correlation between case-control and each studied SNPs. This association was measured by the odds ratio (OR) with 95% confidence interval (95% CI). Additionally, the OR was adjusted for sex, since women have doubled risk of depression in comparison to men Reference Kessler[51]. We also evaluated the correlation between the cases and controls for each studied polymorphisms in male/female population by used to unconditional logistic regression model. Distribution of genotypes according to the year of the first episode of depression and severity of actual depressed episodes are showed as the median ± inter-quartile range. The Shapiro-Wilks test was used to evaluate normality of the distribution. Next, the Mann-Whitney test or Student’s t test was used to estimate the significance of the difference between the analysed values.
3. Results
3.1 Single nucleotide polymorphisms of the gene encoding TRYCATs enzymes (KAT1, AADAT, IDO1) as the risk of the depressive disorder
Table 2 shows the distribution of genotypes and alleles in patients with the DD and in controls, which is in agreement with the Hardy-Weinberg equilibrium. We only found that the A/A genotype of the c.*456G > A − KAT1 (rs10988134) may increase the risk of depression occurrence. No correlation was found between depressed patients and healthy volunteers in terms of distribution of genotypes of the left-over studied polymorphisms.
Table 2 Distribution of genotypes and alleles of c.*456G > A of KAT1 (rs10988134), c.975-7T > C ofAADAT(rs1480544), c.-1849C > A (rs3824259) and c.-1493G > C(rs10089084) of IDO1 and incidence of depression.

p<0.05 along with corresponding Ors are in bold.
a OR adjusted for sex.
3.2 Single-nucleotide polymorphisms of the genes encoding TRYCATs enzymes and the age of the first episode of depression, and the severity classification on the Hamilton Depression Rating Scale
No significant differences was found between distribution of genotypes and the severity classification on the Hamilton Depression Rating Scale. Neither did we observe the defferences in the distribution of genotypes and the age distribution of the first depressive episode (Supplementary Fig. 1 and Supplementary Fig. 2).
3.3 Gene-gene interactions and the risk of depression
Gene-gene interactions as a risk of depressive disorder is showed in Table 3. We observed that the T/T-C/C combined genotype of the c.975-71T > C − AADAT (rs1480544) and the c.-1493G > C − IDO1 (rs10089084) more than doubled the risk of the DD occurrence, while the C/C-C/A genotype of the c.975-7T > C − AADAT (rs1480544) and the c. C > A − IDO1(rs3824259) polymorphism combination reduced this risk. No statistical correlation was found between the combined genotypes of c.*456G > A (rs10988134) − KAT1 and c.-1849C > A − IDO1 (rs3824259), c.*456G > A (rs10988134) − KAT1 and − IDO1 (rs10089084), c.975-7T > C − AADAT(rs1480544) and c.*456G > A − KAT1 (rs10988134) and development of depression.
Table 3 Gene-gene interactions of studied polymorphisms and the risk of DD.
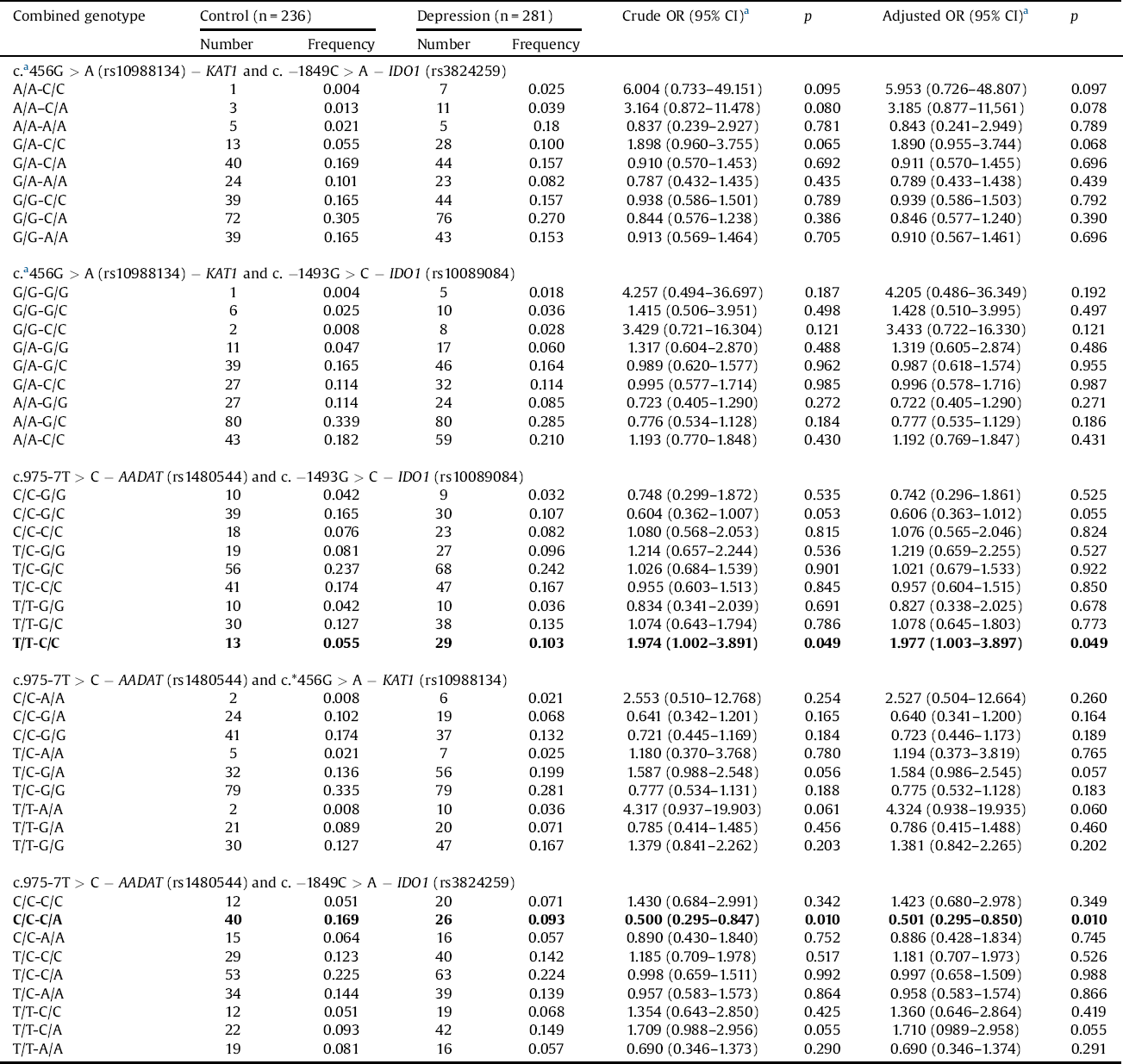
p < 0.05 along with corresponding Ors are in bold.
a OR adjusted for sex.
3.4 Haplotypes and the risk of occurrence of depression
We also analysed if the haplotypes of the studied polymorphisms is associated with occurrence of depression and the results are presented in Table 4. We found that the CC haplotype of the c.-1849C > A − IDO1 (rs3824259) and c.-1493G > C − IDO1 (rs10089084) polymorphism increased the risk of depression occurrence. However, we did not observe a connection between depression and other haplotypes of the studied polymorphisms ofIDO1gene.
Table 4 Haplotypes of IDO1 and the risk of depression.

p < 0.05 along with corresponding ORs are in bold.
3.5 Single-nucleotide polymorphisms of genes encoding enzymes of TRYCATs pathway depression occurrence in male and female population
Kessler Reference Kessler[51] observed that women were exposed to a doubled risk of depression when compared to men. Therefore, we studied the connection between the depression occurrence in male or female groups and all examined polymorphisms (Table 5). On the one hand, we found that the A/A genotype and A allele of the c.*456G > A − KAT1 (rs10988134) were association with an increased risk of depression development in male population, while in female population we did not observe this correlation. On the other hand, the G/G genotype and the G allele of the same polymorphism were linked to a lower risk in male population, while this dependence was not observed in females. Moreover, we investigated the relationship between the distribution of genotypes or alleles and gender in patients with depression but this association was not found for all the studied polymorphisms (data not published).
Table 5 Distribution of genotypes and alleles of the c.*456G > A of KAT1 (rs10988134), c.975-7T > C ofAADAT(rs1480544), c.-1849C > A (rs3824259) and c.-1493G > C (rs10089084) of IDO1 and the risk of DD in male and female population.

p < 0.05 along with corresponding ORs are in bold.
3.6 Single-nucleotide polymorphisms of genes encoding TRYCATs enzymes of and effectiveness of treatmentof the depression
We also study an impact the single-nucleotide polymorphisms of genes encoding TRYCATs enzymes on the effectiveness of antidepressant therapy (Table 6). Therefore, we divided the patients into two groups − those with the total score of Hamilton Rating Scale for Depression after treatment at the maximum of 7 points (marked as the effectiveness of antidepressant therapy) and those with the total score after treatment above 7 points (marked as an ineffective antidepressant therapy). We observed that the T/T genotype and the T allele of c.975-7T > C − AADAT(rs1480544) were related to a low effectiveness of the antidepressant therapy, while the C allele of the same polymorphism was positively correlated with a response to the applied SSRIs treatment. In the remaining cases, no correlation between SNP’s occurrence and effectiveness of antidepressant treatment was found. Moreover, we showed some significant differences in the distribution of studied polymorphism genotypes and the percentage of Hamilton Rating Scale for Depression (Fig. 1). We found a difference in the percentage dispersion of Hamilton Rating Scale for Depression between the A/A and G/A, G/A and G/G, A/A and G/G genotypes of the c. *456G > A KAT1 (rs10988134) polymorphism. Moreover, the disturbances were also observed between the C/C and T/C, T/C and T/T, C/C and T/T genotypes of the c.975-7T > C − AADAT (rs1480544) polymorphism.

Fig 1. Distribution of single-nucleotide polymorphisms of genes encoding KAT1, KAT2 and IDO1 and the percentage of the Hamilton Rating Scale for Depression after an antidepressant therapy. Horizontal lines represent the median, while whiskers show the inter-quartile range.
Table 6 The impact of the single-nucleotide polymorphisms of genes encoding enzymes on the effectiveness of depression treatment.

p < 0.05 along with corresponding ORs are in bold.
4. Discussion
Among various hypotheses explaining the pathogenesis of depression, impairments in TRYCATs pathway are now considered the major contributor to the development of DD. Elevated TRYCATs markers, i.e.quinolinic acid and kynurenine, were found in depressed patients and may be associated with some incorrect actions of the pathway enzymes [Reference Maes, Leonard, Myint, Kubera and Verkerk6, Reference Maes, Galecki, Verkerk and Rief7]. In addition, our recent study demonstrated that SNPs of genes encoding two isoforms of tryptophan hydroxylase − an enzyme involved in the initial and rate-limiting step in the synthesis of serotonin and melatonin − modulated the risk of depression Wigner et al., 2017b. In this paper, we reconfirmed that the TRYCATs pathway may be involved in pathogenesis of depression and we studied the relationship between four SNPs in KAT1, AADAT and IDO1 (two polymorphisms) genes and the risk of DD occurrence.
One of such key enzymes in TRYCATs pathway is kynurenine aminotransferase encoded by gene localized in 9q34.11.The enzyme plays many important roles, including, its cysteine conjugating beta-lyase activity, transaminase activity towards many amino acids and is involved in salvaging of α-keto acids derived from essential amino acids Reference Han, Cai, Tagle and Li[52]. As mentioned in the introduction, KAT catalyzes formation of kynurenic acid, which shows neuroprotective potency Reference Maes, Galecki, Verkerk and Rief[7]. Hence, previous studies showed that the change of KAT level may be associated with neurodegenerative disorders Reference Han, Cai, Tagle and Li[52]. The patients with Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and Huntington’s disease were characterized by a lower concentration of KAT in the central nervous system, while patients with HIV infection, Down’s syndrome, amyotrophic lateral sclerosis, schizophrenia and epilepsy had increased level of the enzyme Reference Han, Cai, Tagle and Li[52]. Up to date, no evidence has been presented to prove a relationship existing between polymorphisms of KAT1 and the development of neurodegeneration; however, it was established that the SNP analysed in the study causes a transition in the 3′UTR region which may affect a KAT1 transcript stability Reference Moore, Boffetta, Karami, Brennan, Stewart and Hung[53], http//:genome.UCSC.edu). Additionally, other studies showed that the SNPs in this region of gene may affect mRNA half-life and degradation and, consequently, may lead to an increase or a decrease of gene expression [Reference Jacobson and Peltz54, Reference Decker and Parker55]. Accordingly, we were the first to show that the A/A genotype of c.*456G > A − KAT1 (rs10988134) may increase the risk of depression occurrence (p < 0.05)(Table 2). Thus, the A/A genotype may be associated with a decreased concentration or activity of KAT1 and may lead to an increase the level of neurotoxic kynurenine. Interestingly, when we stratified the study group by gender, this association was valid only for the male population (p < 0.05) (Table 5.). Although the consequence of this status is unknown, we may speculate that this differences may result from a different enzymes activity or concentration in men and in women group. The next studied polymorphism is located in locus 4q33 encoding AADAT (KAT2). The enzyme demonstrates the activity towards aminoadipate and α-ketoglutarate and catalyzes transamination for a number of amino acids Reference Han, Cai, Tagle and Li[52]. The c.975-71T > C − AADAT (rs1480544) SNP is in a putative exonic splicing silencers (ESSs), thus it may lead to some quantitative changes in the production of canonical mRNAs and peptide Reference Kralovicova and Vorechovsky[56]. So far, only one study, carried out on the Brazilian population, showed that this SNP may have an impact on the phenotype − the C/T genotype of this SNP affected the host’s expression of markers of the immune response to bacterial meningitis Reference de Souza, Fontes, da Silva, Coutinho, Leib and Agnez-Lima[42]. On the other hand, in our study we did not find any significant differences between depressed patients and healthy volunteers (Table 2). However, in gene–gene analyses, we found that the combined genotype of c.975-71T > C − AADAT (rs1480544) and c.-1849C > A (rs3824259) of IDO1 or c.-1493G > C of IDO1 (rs10089084) may modulate the risk of the depression development (p < 0.05) (Table 3). Additionally, we showed that the T/T genotype is associated with poorer outcome of the SSRIs treatment (p< 0.05) (Table 6).This discovery can contribute to choosing the right personalized antidepressant treatment.
Lastly, we evaluated distribution of two SNPs encodes indoleamine 2,3-dioxygenase, which catalyzes the first and rate-limiting step in the TRYCATs pathway, leading to initiation of N-formylkynurenine. Both polymorphism present near 5′ (regulatory region) end of IDO1–gene located on chromosome 8p11. [Reference Dai and Gupta57–Reference Munn and Mellor59]. The previous studies suggested that the SNP in noncoding regions, including introns and regulatory regions, may cause an altered mRNA stability, degradation and expression, resulting in some changes in the activity of the final protein product [Reference Prokunina and Alarcón-Riquelme60–Reference Roden, Altman, Benowitz, Flockhart, Giacomini and Johnson62]. Despite the potential changes caused by these polymorphisms, we did not detect the link between the SNPs occurrence and the development of depression (Table 2). Similarly, in a study of these polymorphisms frequency in patients with interferon-alpha-related depression in hepatitis C as compared to controls Reference Galvão-de Almeida, Quarantini, Sampaio, Lyra, Parise and Paraná[63] its authors did not find any statistically significant results concerning thesetwo polymorphisms. On the other hand, as mentioned earlier, the combined genotype of either of these SNPs and c.975-71T > C − AADAT (rs1480544) may be associated with occurrence of depression (p< 0.05) (Table 3).
The present study had some potential limitations. First of all, the sample size was relatively small and consequently of low power, which could lead to both false negative as well as false positive findings. Therefore these results should be interpreted with caution and considered preliminary. Second, the studied population was from Poland only, which reduces the possibility of confounding from ethnicity, so it does not permit any extrapolation of the results to other ethnic groups.
The findings of this work cast a new light on the pathogenesis of depression; however, some additional larger case-control studies on different population groups and functional experiments are necessary before the final resolution about/findings as to the role of the TRYCATs pathway in the development of this disease.
5. Conclusion
In the current work,we showed that the chosen four SNPs of genes involved in tryptophan catabolites pathway may influence the risk of depression occurrence. Therefore, these polymorphisms may be considered as independent depression markers.
Conflict of interest
None.
Acknowledgment
Source of support: This study was supported by the financing from a scientific research grant from the National Science Centre of Poland (no. UMO-2015/19/BNZ7/00410)
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2018.05.001.










Comments
No Comments have been published for this article.