Flavonoids have various bioactivities such as antioxidative, anti-inflammatory and anticarcinogenic activities, and their physiological functions are expected to occur when they are absorbed in the body( Reference Hertog, Hollman and Katan 1 , Reference Hertog, Kromhout and Aravanis 2 ). Recently, the absorption and metabolism of flavonoids such as quercetin and catechin present in many vegetables and fruits have been studied, and it has been determined that the bioavailability of flavonoids are dependent on their basic chemical structures, degree of polymerisation, types of glycosylated moiety, and molecular weights( Reference Scalbert and Williamson 3 – Reference Bravo 5 ). Moreover, it has been made clear that the absorption rate of flavonoids is generally low( Reference Manach, Williamson and Morand 6 ). Meanwhile, glucosidase activities and transport efficacy in the digestive tract as well as metabolic modification by intestinal microbial flora have also been shown to affect the bioavailability of flavonoids( Reference Tamura, Hirayama and Itoh 7 – Reference Konishi, Kobayashi and Shimizu 10 ). Therefore, attention has also been focused on whether the absorption of flavonoids can be influenced by other food constituents such as lipid emulsions, fatty acid patterns, difructose anhydride III, phytic acid and milk( Reference Azuma, Ippoushi and Ito 11 – Reference Roura, Andre's-Lacueva and Estruch 15 ).
However, few researchers have so far examined the influences of dietary fibre and indigestible oligosaccharides, which are the main matrices in vegetables and fruits, on the absorption of flavonoids. Pectin, a water-soluble fibre, is widely distributed in the cell walls of fruits and vegetables, especially citrus and apple. It is well known that pectin has lipid-lowering properties( Reference Basu and Ooraikul 16 , Reference González, Rivas and Caride 17 ), prolongs gastric emptying( Reference Fleming and Lee 18 ), and alters intestinal microflora( Reference Dongowski, Lorenz and Proll 19 ). Thus, it is expected that indigestible polysaccharides also affect the absorption and metabolism of flavonoids.
Because flavonoids are generally localised in many fruits and vegetables that contain pectin, investigating the effect of pectin on flavonoid absorption is important. A previous study has shown that the absorption of quercetin was increased after a single oral administration in rats receiving diets containing apple pectin for 6 weeks, and the crypt depth and villus thickness of the ileum in pectin-fed rats were greater than those in rats fed diets without pectin( Reference Nishijima, Iwai and Saito 20 ). Thus, the increase in quercetin absorption might be attributed to the pectin-induced improvement of the small intestine's morphological properties. This finding shows the possibility of pectin possessing a new bioactivity as a water-soluble fibre. However, most inhibitory effects of water-soluble fibres on nutrient absorption have occurred when fibres and other nutrients are ingested at the same time( Reference Fernandez 21 , Reference Hexeberg, Hexeberg and Willumsen 22 ).
Therefore, the objective of the present study was to investigate whether the simultaneous ingestion of quercetin with apple pectin enhances the absorption of quercetin in healthy men. Moreover, to elucidate the enhancing mechanism, the effects of dose dependency and degree of apple pectin methylation on the absorption of quercetin were also investigated.
Materials and methods
Subjects and ethics
Healthy men were recruited as volunteers. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethical Committee of Fuji Women's University. The contents and methods were explained in full to all prospective subjects, and written informed consent was obtained from all subjects.
A group of nineteen men (mean age 31·9 (sd 2·6) years, body weight 71·7 (sd 6·8) kg and BMI 23·9 (sd 2·3) kg/m2) were subjected to the study on the simultaneous ingestion of quercetin with apple pectin (Expt I), and six men (mean age 28·5 (sd 3·0) years, body weight 73·7 (sd 7·2) kg and BMI 23·7 (sd 3·0) kg/m2) were subjected to the study on the dose dependency of apple pectin (Expt II) and the study on the degree of apple pectin methylation (Expt III).
Samples and reagents
For the ingestion study, UNIPECTIN HM-1 as a high-methoxy apple pectin (HM pectin), and UNIPECTIN LMSN325 as a low-methoxy apple pectin (LM pectin) were purchased from Unitec Foods Company, Limited, and quercetin supplement, which contained 70 % (w/w) quercetin aglycone, was purchased from Jarrow Formulas.
Quercetin and β-glucuronidase/sulfatase (analytical grade) from Helix pomatia were purchased from Sigma, isorhamnetin, tamarixetin and kaempferol were purchased from Extrasynthese. Other reagents and solvents were of special grade or liquid chromatographic grade.
Expt I (study on the simultaneous ingestion of quercetin with pectin)
The composition of test drinks for the simultaneous ingestion study is shown in Table 1. The NP drink contained 0·5 mg/ml of quercetin without pectin, and the PEC drink contained 0·5 mg/ml of quercetin with 10 mg/ml of HM pectin. Pectin and sugar solution, and tartaric acid and flavour solution were prepared separately and sterilised, and were then mixed. Quercetin was added into this mixture and sonicated. Just before ingestion, 200 ml of both drinks were sonicated and filled sanitarily in plastic cups.
Table 1 Composition of the test drinks (100 ml)
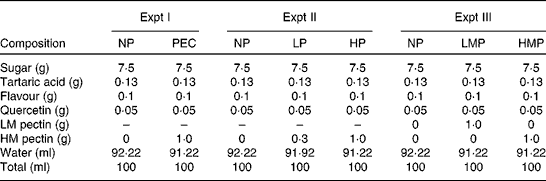
Expt I, study on the simultaneous ingestion of quercetin with pectin; Expt II, study on the dose dependency of apple pectin; Expt III, study on the degree of apple pectin methylation; NP, quercetin drink without pectin; PEC, quercetin drink with 10 mg/ml of apple pectin; LP, quercetin drink with a low content (3 mg/ml) of pectin; HP, quercetin drink with a high content (10 mg/ml) of pectin; LMP, quercetin drink with low-methoxy pectin; HMP, quercetin drink with high-methoxy pectin; LM pectin, low-methoxy apple pectin; HM pectin, high-methoxy apple pectin.
All subjects avoided the ingestion of flavonoid-rich foods such as fruits and vegetables starting from the evening meal on the day before the study, and they fasted for 14 h. Before ingestion of the test drinks, subjects' adherence to non-ingestion of quercetin was confirmed by analysing their urine for quercetin. Subjects were randomly allocated to two groups of nine and ten persons, and they ingested 200 ml of the NP or PEC drink at a time. After ingestion of the drinks, the same meals without vegetables and fruits at lunch and dinner were supplied to all subjects, and they drank mineral water ad libitum for 24 h. All urine samples were collected into plastic bottles (250 ml) containing 125 mg ascorbic acid at every excretion within 24 h, and urine was weighed and stored at − 80°C until measurement. Urine collection at 24 h after ingestion marked the end of the first cycle of the simultaneous ingestion study; after an interval of over 1 week, the second cycle of the study was carried out with a cross-over.
Expt II (study on the dose dependency of pectin)
Table 1 shows the composition of the test drinks. All the drinks contained 0·5 mg/ml of quercetin, and the NP, LP and HP drinks contained 0, 3 and 10 mg/ml of apple pectin, respectively. These drinks were prepared by a similar procedure to Expt I, and ingestion volume was established as 200 ml for each subject.
Dietary and drinking conditions of the subjects during the experiment were also controlled in similar conditions to Expt I. After prior confirmation of the non-ingestion of quercetin by analysis of the urine, six subjects ingested 200 ml of the NP drink (0 mg/ml of pectin) at a time. All urine samples were collected at every excretion within 8 h, because the urinary excretion of all deconjugated metabolites of quercetin (quercetin, isorhamnetin and tamarixetin) did not increase at or after 8 h in Expt I. The urine samples were collected and stored according to the procedure described in Expt I. The ingestion test was carried out in order of the NP drink, followed by the LP drink and the HP drink, and each test was performed at intervals of over 3 d.
Expt III (study on the degree of pectin methylation)
All test drinks contained 0·5 mg/ml of quercetin, and the NP, LMP and HMP drinks contained 0 mg/ml of pectin, 10 mg/ml of LM pectin and 10 mg/ml of HM pectin, respectively (Table 1). They were prepared in a similar procedure to that described in Expt I, and ingestion volume was established as 200 ml for each subject.
Dietary and drinking conditions during the experiment were also controlled in similar conditions to that in Expt I, and the non-ingestion of quercetin by the subjects was confirmed by the analysis of the urine before ingestion of the test drinks. For this study, six subjects ingested 200 ml of the NP drink at a time, and all urine samples were collected and stored at every excretion within 8 h according to the procedure of Expt I. The ingestion test was carried out in order of the NP, HMP and LMP drinks, and each test was performed at intervals of over 3 d.
Determination of quercetin and its metabolites in the urine
Quercetin and its metabolites in the urine were quantitatively determined by HPLC. Briefly, 50 μl of urine were placed in a microtube, and 5 μl of 0·2 m-ascorbic acid solution and 50 μl of 33·3 μKat/ml of β-glucuronidase (type H5) containing 3·3 μKat/ml of sulfatase in 0·2 m-acetate buffer (pH 5·0) were added; the solution was mixed immediately and incubated at 37°C for 2 h. After incubation, 5 μl of 0·1 mm-kaempferol in dimethyl sulfoxide as an internal standard and 200 μl of ethyl acetate were immediately added to the mixture, and the mixture was thoroughly shaken for 5 min and centrifuged at 3000 g for 10 min. Metabolites were extracted twice into the ethyl acetate phase, and the organic layer was then evaporated to dryness. The residue was redissolved in 50 μl of acetonitrile/0·5 % (v/v) phosphoric acid solution (35:65, v/v) and centrifugally filtered using an Ultrafree MC microcentrifuge filter (0·45 μm; Merck Millipore Limited) for HPLC analysis. The recovery of this method was checked (>90 %) using authentic quercetin, isorhamnetin, tamarixetin and kaempferol.
The HPLC apparatus consisted of a G1379B degasser, a G1312B pump, a G1329B autosampler, a G1316B column oven, and a G1315C diode array detector (Agilent Technologies, Inc.). HPLC analysis was performed under the following conditions: column, Zorbax Stable Bond C18 (1·8 μm, 50 mm × 4·6 mm inner diameter; Agilent Technologies, Inc.); mobile phase, acetonitrile and 0·5 % (v/v) phosphoric acid solution; flow rate, 1 ml/min; column temperature, 40°C; detection, UV at 370 nm (detection range 210–450 nm); injection volume, 5 μl. The elution profile was as follows: 0–2 min, isocratic elution acetonitrile/0·5 % (v/v) phosphoric acid solution (25:75, v/v); 2–9 min, linear gradient from 25:75 (v/v) of acetonitrile/0·5 % (v/v) phosphoric acid solution to 45:55 (v/v); 9–12 min, initial condition.
Urinary levels of deconjugated quercetin, isorhamnetin and tamarixetin were determined by an external standard method. The quantification limits for quercetin, isorhamnetin and tamarixetin were 57·1, 56·3 and 62·9 ng/ml, respectively, and these detection limits were 19·4, 18·0 and 20·9 ng/ml, respectively. The quantitative concentration of each metabolite was converted to the excretion amounts, and the absorption rate (%) was calculated from the sum of all excreted metabolites.
Statistical analysis
Results are expressed as means and standard deviations. In Expt I (the simultaneous ingestion study), statistical analysis was carried out using Student's t test for each corresponding subject, and P <0·05 was considered significant. In Expt II and III (dose-dependency study and degree of pectin methylation study), statistical analysis was carried out using the multiple comparison test of Dunnett after a two-way factorial ANOVA by StatView version 5 (SAS International), and P <0·05 was considered as significant.
Results
Simultaneous ingestion of quercetin with pectin
All subjects completed the study. Fig. 1 shows the time-course changes in the cumulative urinary excretion (sum of quercetin and its metabolites) in a subject after ingestion of the test drinks. The excretion levels after ingestion of the PEC drink were higher than those after ingestion of the NP drink, and both levels reached a plateau at 8 h.

Fig. 1 Typical time-course changes in the cumulative urinary excretion of quercetin and all metabolites in a subject within 24 h of ingesting the quercetin drinks with or without pectin. ![]() , 0·5 mg/ml of quercetin drink without pectin;
, 0·5 mg/ml of quercetin drink without pectin; ![]() , 0·5 mg/ml of quercetin drink with 10 mg/ml of apple pectin.
, 0·5 mg/ml of quercetin drink with 10 mg/ml of apple pectin.
The mean urinary excretion levels of quercetin and its metabolites (isorhamnetin+tamarixetin) in nineteen subjects within 24 h after ingestion of the NP and PEC drinks are shown in Fig. 2. The excretion levels of quercetin and its metabolites after ingestion of the PEC drink were increased, and the sum of both excretion rates after ingestion of the PEC drink (1·05 %) was significantly higher than that after ingestion of the NP drink (0·46 %).

Fig. 2 Sum of urinary excretion of quercetin and its metabolites within 24 h after ingestion of the quercetin drinks with or without pectin. NP, 0·5 mg/ml of quercetin drink without pectin; PEC, 0·5 mg/ml of quercetin drink with 10 mg/ml of apple pectin. Values are means (n 19), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the NP drink (P <0·05). ![]() , Quercetin;
, Quercetin; ![]() , isorhamnetin+tamarixetin.
, isorhamnetin+tamarixetin.
Although there were differences in the values of excretion among individual subjects, the rate of increase in excretion after ingestion of the PEC drink compared with the NP drink was over 2·0 in thirteen subjects, and the average rate of increase in nineteen subjects was 2·5. Moreover, the lowest rate of increase in an individual subject was 1·01, and the decrease in the urinary excretion of quercetin and its metabolites was not induced by the ingestion of the PEC drink. The ratios of quercetin excretion to total excretion after ingestion of the NP and PEC drinks were 85·6 (sd 11·7) and 81·7 (sd 8·3) %, respectively, and there were no significant differences between the two ratios.
Dose-dependent effect of pectin on quercetin absorption
All six subjects showed that the sum of the urinary excretion of all metabolites after ingestion of the HP drink (10 mg/ml of pectin) was higher than that after ingestion of the NP drink (0 mg/ml of pectin), and five subjects showed higher excretion levels after ingestion of the LP drink (3 mg/ml of pectin) than after ingestion of the NP drink. Furthermore, four subjects showed higher excretion levels after ingestion of the HP drink than after ingestion of the LP drink. For the subjects who did not show a dose-dependent increase in quercetin excretion, the differences in excretion levels were in the error range.
Fig. 3 shows the mean excretion rates of quercetin and its metabolites (isorhamnetin+tamarixetin) in the urine within 8 h after ingestion of the test drinks. The excretion rate of quercetin after ingestion of the HP drink was significantly higher than that after ingestion of the NP drink. The sum of the excretion rates of all metabolites after ingestion of the NP, LP and HP drinks was 0·43, 0·60 and 0·71 %, respectively. A significant increase in quercetin absorption by pectin ingestion was found, and the rates of increase for ingestion of the LP and HP drinks v. the NP drink were 1·68 and 2·23, respectively. Although there was no significant difference in the sum of the excretion levels between the LP and HP drinks, they were significantly higher than those after ingestion of the NP drink.

Fig. 3 Sum of urinary excretion of quercetin and its metabolites within 8 h after ingestion of the quercetin drinks with or without the various contents of apple pectin. NP, 0·5 mg/ml of quercetin drink without pectin; LP, 0·5 mg/ml of quercetin drink with a low content (3 mg/ml) of apple pectin; HP, 0·5 mg/ml of quercetin drink with a high content (10 mg/ml) of apple pectin. Values are means (n 6), with standard deviations represented by vertical bars. Mean value was significantly different from that of the NP drink: *P <0·05, **P <0·01. ![]() , Quercetin;
, Quercetin; ![]() , isorhamnetin+tamarixetin.
, isorhamnetin+tamarixetin.
The ratios of quercetin excretion to the sum of metabolite excretion after ingestion of the NP, LP and HP drinks were 95·6, 94·5 and 94·1 %, respectively; there was no change in the ratios by the dose of pectin.
Effect of the degree of pectin methylation on the enhancement of quercetin absorption
All six subjects showed higher excretion levels of quercetin and its metabolites after ingestion of the HMP drink than those after ingestion of the NP drink. In comparing the sum of the excretion levels between the ingestion of the LMP and HMP drinks for each subject, four subjects showed higher levels of excretion after ingestion of the HMP drink than after ingestion of the LMP drink, one subject showed equal levels of excretion for both drinks, and one subject showed lower levels of excretion for the HMP drink than for the LMP drink.
Fig. 4 shows the urinary excretion rates of quercetin and its metabolites (isorhamnetin+tamarixetin) within 8 h after ingestion of the NP, LMP and HMP drinks. The excretion levels of quercetin and its metabolites after ingestion of the LMP drink were higher than those after ingestion of the NP drink, and the excretion levels after ingestion of the HMP drink were also higher than those after ingestion of the LMP drink. The ingestion of the HMP drink showed a significantly higher excretion level of quercetin than the ingestion of the NP drink. The sum of the excretion of quercetin and its metabolites after ingestion of the HMP drink was 0·93 %, and it was 2·13-fold significantly higher than that after ingestion of the NP drink (0·44 %). Although the sum of the excretion levels after ingestion of the LMP drink (0·70 %) did not significantly correspond to that after ingestion of the NP drink (P= 0·06), a tendency of increase was found and the rate of increase was 1·69. The ratios of quercetin excretion to sum of excretion in all groups ranged between 91 and 95 %, and there was no significant difference between the presence of pectin and its degree of methylation.

Fig. 4 Sum of urinary excretion of quercetin and its metabolites within 8 h after ingestion of the quercetin drinks with varied degrees of apple pectin methylation. NP, 0·5 mg/ml of quercetin drink without pectin; LMP, 0·5 mg/ml of quercetin drink with low-methoxy apple pectin; HMP, 0·5 mg/ml of quercetin drink with high-methoxy apple pectin. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the NP drink (P <0·05). ![]() , Quercetin;
, Quercetin; ![]() , isorhamnetin+tamarixetin.
, isorhamnetin+tamarixetin.
Discussion
After absorption, metabolised quercetin was excreted into the bile( Reference Petri, Tannergren and Holst 23 ), and a portion of them was reabsorbed and excreted into the urine( Reference Hollman 24 ). Hollman et al. ( Reference Hollman, van Trijp and Buysman 25 ) reported that urinary excretion of quercetin for 24 h predicted the AUC of time-course plasma concentration of quercetin very well. The results from the present study on urinary excretion of quercetin are correlated with a study reporting the AUC of plasma concentration in rats after a single oral administration of quercetin( Reference Nishijima, Iwai and Saito 20 ). Accordingly, urinary excretion of quercetin and its metabolites within 24 h was used as an index of total absorption of orally administered quercetin in order to alleviate a burden on the volunteers in repeated blood sampling. Thus, in the present study, the significant increase in the excretion levels of quercetin and its metabolites after ingestion of the PEC drink showed that the simultaneous ingestion of apple pectin enhanced the absorption of quercetin in human subjects.
Quercetin is mainly metabolised to conjugates such as glucuronide and sulphate, and is methylated in the liver and small intestine( Reference Murota and Terao 26 , Reference Williamson, Barron and Shimoi 27 ). There was no difference in the ratios of quercetin excretion to the sum of excretion between the ingestion of the NP and PEC drinks. In the present study, quercetin and its metabolites were determined after a deconjugation treatment, so the effect of pectin on conjugation metabolism is unclear. However, the ratios of quercetin level to methylated metabolite (isorhamnetin+tamarixetin) level were not altered in the ingestion of both drinks; thus, it was assumed that pectin did not particularly affect the metabolism of quercetin. Consequently, it was thought that the enhancing effect of apple pectin on the absorption of quercetin occurred at luminal absorptive sites in the small intestine. Furthermore, it is well known that the catechol structure in the B ring of quercetin is very important for antioxidant activity( Reference Yamamoto, Moon and Tsushida 28 , Reference Moon, Tsushida and Nakahara 29 ). Therefore, the increase in quercetin absorption without the increase in the ratio of methylated metabolites was considered to be beneficial for receiving the antioxidant activity of quercetin. This result demonstrates that the enhancing effect of apple pectin on the absorption of quercetin also occurs in human subjects. Soluble dietary fibre such as pectin can have inhibitory activity on the intestinal absorption of nutrients( Reference Hexeberg, Hexeberg and Willumsen 22 , Reference Rock and Swendseid 30 ); however, the present results show the possibility that pectin enhances absorption according to the property of the nutrient. Because the enhancing effect of pectin occurred during the simultaneous ingestion with quercetin, the increasing mechanism through the morphological alteration of the epithelium in the jejunum and ileum by chronic ingestion of pectin( Reference Nishijima, Iwai and Saito 20 ) was difficult to elucidate; it is likely that pectin itself participates in the increase of quercetin absorption.
Accordingly, the dose dependency of pectin in enhancing the absorption of quercetin was examined. As the content of pectin in apples has been reported to be approximately 1 %( Reference Thakur, Singh and Handa 31 ), the dose of pectin in the present study was examined at the true range of pectin levels in apples. A significant increase in quercetin absorption by pectin ingestion and the effects of the dose dependency of pectin on the increase in quercetin absorption were clear. Although there were subjects who were not affected by the ingestion of apple pectin, it was concluded that the ingestion of pectin, nonetheless, did not lower the absorption of quercetin in human subjects. Similar to the simultaneous ingestion study, a certain rate of quercetin in the sum of metabolites was found regardless of the pectin concentrations. It is suggested that apple pectin may not affect the metabolism of quercetin in the body and provides benefits for receiving the physiological activities of quercetin. Moreover, because even 3 mg/ml of pectin enhanced quercetin absorption, it was estimated that apples having a similar level of pectin could possess this enhancing ability.
Pectin is a polysaccharide that consists of polygalacturonic acids linked by α-1,4 glycoside bonds, and carboxylic acids in galacturonic acids are partially methyl esterified. Pectin is classified into HM pectin that has over fifty degrees of methylation, and LM pectin that has less than fifty degrees of methylation. Natural pectin, which is widely distributed in the cell walls of vegetables and fruits, is predominantly a HM type, and the degree of methylation affects the characteristics of pectin such as solubility, viscosity and gelation. HM pectin has high viscosity, while LM pectin has low viscosity in solution( Reference Thakur, Singh and Handa 31 ). Because the enhancing effect of apple pectin on quercetin absorption in human subjects was dose dependent, it was hypothesised that the physical properties of pectin such as viscosity could participate in the enhancing effect. So, the effect of the degree of pectin methylation on the enhancement of quercetin absorption in human subjects was investigated. The ingestion of the LMP drink showed a tendency of increasing the sum of quercetin excretion in comparison with the ingestion of the NP drink, and the HMP drink increased the sum of excretion significantly higher than the NP drink. Thus, it was concluded that the enhancing activity of LM pectin was weaker than that of HM pectin. This result showed that the addition of HM pectin increased the absorption of quercetin over 2-fold. We have found that high-viscous guar gum increased the absorption of quercetin in rats, but low-viscous indigestible dextrin did not exhibit this enhancing activity (data not shown). Therefore, the alteration of quercetin absorption induced by the degree of pectin methylation suggested that the increase in pectic solubility and viscosity could participate in the absorption of quercetin. There was no difference in the ratios of quercetin excretion to all metabolite excretion in the degrees of pectin methylation study. Therefore, it is also suggested that pectin does not affect the metabolism of methylated quercetin.
From the above-mentioned results, it is clear that the enhancing effect of apple pectin on the absorption of quercetin also occurs in humans, and its effect is exhibited immediately through the simultaneous ingestion with quercetin. Thus far, soluble dietary fibres are well known to inhibit the absorption of other nutrients such as carotenoids, fats and sugars( Reference Rock and Swendseid 30 , Reference Flourie, Vidon and Florent 32 , Reference Hyun, Vahouny and Treadwell 33 ). Specifically, there have been reports that the simultaneous ingestion with pectin only inhibited the absorption of other nutrients. Hollands et al. ( Reference Hollands, Hart and Dainty 34 ) reported that epicatechin consumed from whole apple purée is less bioavailable than epicatechin-rich extract, suggesting that the apple matrix, notably pectin, inhibits the absorption of epicatechin. In contrast, Aprikian et al. ( Reference Aprikian, Duclos and Guyot 35 ) described that combining ingestion of apple pectin and a polyphenol-rich fraction was more effective than separate ingestion on large-intestinal fermentation and lipid metabolism, and they have suggested the synergic effect of fibre and polyphenols in apple. The present results show the possibility of a new reverse physiological activity of pectin that increases the absorption of nutrients according to its characteristics. Therefore, these findings suggest that the effect of pectin differs according to the species of flavonoids. It is necessary to investigate the relationship between the enhancing effect of pectin and the structures and properties of flavonoids in order to elucidate that the ingestion of plant food components including both flavonoids and dietary fibre, not supplements, would be of benefit to human health.
Morphological alterations such as increases in crypt depth and villus thickness in the ileum of rats that were induced by the chronic ingestion of apple pectin have been suggested to be a primary factor in the mechanism of the enhancing effect of pectin in a previous study( Reference Nishijima, Iwai and Saito 20 ). However, in the present study, a single and simultaneous ingestion of quercetin with apple pectin also increased the absorption of quercetin. It was clear that the enhancing activity varied in a pectin dose-dependent manner, and HM pectin had stronger activity than LM pectin. Because there was no finding that the differences in the degree of pectin methylation influenced the crypt depth and villus thickness of the ileum, it was thought that the morphological alterations in the small intestine by the ingestion of pectin were not predominant, and that the mechanism was directly an effect of pectin on the absorption of quercetin. The physical property of pectin was largely altered according to the degree of methylation; especially, HM pectin had stronger viscosity than LM pectin, and it lowered the fluidities of digestive contents( Reference Schneeman 36 ). From the present study, HM pectin was thought to enhance the absorption of quercetin through its high-viscous influence on the fluidity and solubility of quercetin in the digestive tract. Additionally, it has been found that trimethyl chitosan chloride increased the absorption of polymers such as peptides by increasing tight junction permeability in absorptive epithelium cells( Reference Thanou, Verhoef and Junginger 37 ). Trimethyl chitosan chloride is a methylated chitosan, an indigestible polysaccharide, and it has a point in common with pectin. So, the possibility was also considered that the increase in tight junction permeability by pectin, similar to trimethyl chitosan chloride enhanced the absorption of quercetin. In future, its mechanisms could be revealed by conducting experiments on the physical effects of pectin on the behaviour of quercetin and related flavonoids, and by performing in vitro studies using an absorptive cell model.
Quercetin is metabolised to glucuronide and sulphate, and methylation occurs mainly in the liver and small intestine at the same or immediate time of absorption( Reference Murota and Terao 26 , Reference Williamson, Barron and Shimoi 27 ). It almost always exists as metabolites in the body( Reference Graf, Ameho and Dolnikowski 38 ). The antioxidant activities of metabolites are weaker than that of unchanged quercetin; however, this activity could be retained according to the conjugated formula( Reference Moon, Tsushida and Nakahara 29 ). To intensify the antioxidant activity in the body that is administered with quercetin, increasing not only the absorption of quercetin but also the existence of strong antioxidative conjugated quercetin in the body will be effective. In the present study, quercetin and its methylated metabolites, isorhamnetin and tamarixetin, were detected in the urine by deconjugation treatment. It is necessary to elucidate the effects of apple pectin on the metabolic form of quercetin, and future studies should investigate whether the increase in quercetin absorption induces the intensification of antioxidant activities in the body.
Quercetin is not present in the aglycone form in plant foods; however, the investigation on the effect of pectin was narrowed down to a quercetin aglycone in the present study based on our previous finding( Reference Nishijima, Iwai and Saito 20 ) demonstrating that apple pectin did not enhance the absorption of rutin, a glycoside of quercetin. On the basis of the possibility that the physical properties of pectin participate strongly in the enhancement of quercetin absorption, the mechanism of the enhancing ability of pectin was expected to be made clear by elucidating the increase in the absorption of quercetin glycosides, which are the main forms of quercetin found in many foods.
Acknowledgements
The present study received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors' contributions are as follows: T. N. designed and performed the research, was responsible for the ethics application, analysed the data and wrote the manuscript; Y. T. recruited and liaised with volunteers, and performed the HPLC analysis; Y. S. recruited and liaised with volunteers and performed the studies; T. I. was responsible for the ethics application; K. I. designed the research, analysed the data, was responsible for overall supervision of the research and wrote the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.








