Introduction
Habitat loss is the primary driver for the loss of biodiversity worldwide (Gaston et al. Reference Gaston, Blackburn and Goldewijk2003, Pimm et al. Reference Pimm, Raven, Peterson, Şekercioğlu and Ehrlich2006, Brook et al. Reference Brook, Sodhi and Bradshaw2008), and is of special concern for species that have small ranges and therefore small populations, such as those restricted to islands (BirdLife International 2000). More than 90% of bird species extinctions since 1800 have occurred on islands (Johnson and Stattersfield Reference Johnson and Stattersfield1990, Banko et al. Reference Banko, Camp, Farmer, Brinck, Leonard and Stephens2013). Moreover, islands account for 48% of all Endemic Bird Areas, with more than 600 globally threatened bird species being confined to them (Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998, Ricketts et al. Reference Ricketts, Dinerstein, Boucher, Brooks, Butchart, Hoffmann, Lamoreux, Morrison, Parr, Pilgrim, Rodrigues, Sechrest, Wallace, Berlin, Bielby, Burgess, Church, Cox, Knox, Loucks, Luck, Master, Moore, Naidoo, Ridgely, Schatz, Shire, Strand, Wettengel and Wikramanayake2005, Dallimer and King Reference Dallimer and King2007). An understanding of the habitat requirements of threatened island-endemic species is therefore crucial to our ability to provide effective management for their long-term conservation.
The Caribbean islands harbour a rich biodiversity and were identified as one of 25 globally important biodiversity hotspots (Myers et al. Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000). Habitat conversion and invasive species threaten native taxa, many of them endemic to these islands, yet little is known about their habitat requirements. The Forest Thrush Turdus lherminieri is restricted to forests on four islands in the Lesser Antilles: Montserrat, Guadeloupe, Dominica and St Lucia (Collar Reference Collar, Del Hoyo, Elliott and Christie2005, Eraud et al. Reference Eraud, Arnoux, Levesque, Van Laere and Magnin2012), with each island assumed to hold distinct subspecies, T. l. montserrati, T. l. lherminieri, T. l. dominiciensis and T. l. sanctaeluciae, respectively (Clement and Hathway Reference Clement and Hathway2000, Zuccon Reference Zuccon2011). The subspecies on St. Lucia may have recently become extinct (last sighting 2007; BirdLife International 2013). Owing to a dramatic overall decline in its population over the last 15 years, the species is listed as globally ‘Vulnerable’ (BirdLife International 2013), but the causes of its precarious status on each island are largely a matter of speculation.
Generally, however, the Forest Thrush is believed to be threatened by habitat loss, nest predators and hunting on Guadeloupe (Eraud et al. Reference Eraud, Arnoux, Levesque, Van Laere and Magnin2012, BirdLife International 2013). On Guadeloupe the species occurs in areas of continuous rainforest from 110 to 1,400 m above sea level and wet coastal forests (Eraud et al. Reference Eraud, Arnoux, Levesque, Van Laere and Magnin2012, Arnoux et al. Reference Arnoux, Eraud, Thomas, Cavallo, Garnier and Faivre2013). While previous studies have mainly investigated morphological and genetic differentiation (Arnoux et al. Reference Arnoux, Eraud, Thomas, Cavallo, Garnier and Faivre2013, Reference Arnoux, Eraud, Navarro, Tougard, Thomas, Cavallo, Vetter, Faivre and Garnier2014), no study has quantified the habitat preferences of the Forest Thrush. A better understanding of the species’s habitat requirements is needed to assess the extent of habitat loss and guide conservation management.
Animals of dense tropical forests are generally difficult to detect. Many studies of habitat associations in birds ignore the fact that some individuals may have been missed during surveys (Kéry et al. Reference Kéry, Royle and Schmid2005, Reference Kéry, Dorazio, Soldaat, van Strien, Zuiderwijk and Royle2009, Kéry Reference Kéry2008). Imperfect detection may affect abundance estimates and habitat inferences if aspects of habitat structure with potential to affect species presence or abundance also affect the ability of an observer to detect the species (Kéry Reference Kéry2008). Two common approaches to account for imperfect detection are distance sampling and repeat surveys using multiple visits or observers (Nichols et al. Reference Nichols, Thomas, Conn, Thomson, Cooch, Evan and Conroy2009). Of these, distance sampling is problematic in tropical forests where dense vegetation precludes accurate determination of radial distances, leading to inaccurate density estimations (Alldredge et al. Reference Alldredge, Simons and Pollock2007, Simons et al. Reference Simons, Alldredge, Pollock, Wettroth and Dufty2007, Oppel et al. Reference Oppel, Cassini, Fenton, Daley and Gray2014a). However, repeat surveys combined with binomial mixture models allow the estimation of detection probability and provide an excellent basis for analysing habitat preferences (Royle Reference Royle2004, Kéry et al. Reference Kéry, Royle and Schmid2005, Kéry Reference Kéry2008).
Here we use binomial mixture models to elucidate the habitat associations of the Forest Thrush while accounting for the imperfect detection process in a dense tropical forest. We conducted this study on Montserrat, which holds a significant proportion of the Forest Thrush world population. We use the information about habitat preferences of the Forest Thrush to make recommendations for the conservation of the species and to inform future land management on Montserrat.
Methods
Study area
Montserrat (16°45'N, 62°12'W) is an island of 104 km2, located at the northern end of the Lesser Antilles in the Greater Caribbean Basin. It experiences a moist tropical climate, with an annual rainfall of 1,000–2,500 mm per year (Hilton et al. Reference Hilton, Atkinson, Gray, Arendt and Gibbons2003). There are three main hill ranges: Silver Hills, Soufrière Hills and Centre Hills, the latter two rising to 700–900 m asl (Young Reference Young2008). A range of habitats occur on the island, including dry, mesic and wet forests, and elfin woodland on exposed ridges at high elevations. Following volcanic activity in the Soufrière Hills that started in 1995, almost 60% of the island’s forest habitat was lost, and now the largest remnant exists in the Centre Hills, with an area of 11 km2 (Young Reference Young2008).
Bird monitoring
Bird surveys were carried out during the breeding season of the Forest Thrush in April 2013. We conducted 10-minute point counts between 06h00 and 14h00 Atlantic Standard Time (AST), during which all visual and acoustic detections of the Forest Thrush were recorded. Point counts were carried out three times per point over a three-week period at 88 survey points located randomly within the Centre Hills forest, with a minimum of 200 m between points (for details see Dalsgaard et al. Reference Dalsgaard, Hilton, Gray, Aymer, Boatswain, Daley, Fenton, Martin, Martin, Murrain, Arendt, Gibbons and Olesen2007, Oppel et al. Reference Oppel, Cassini, Fenton, Daley and Gray2014a).
Habitat data
Based on previous work on related species (Jones et al. Reference Jones, Linsley and Marsden1995, Dallimer and King Reference Dallimer and King2007, Rangel-Salazar et al. Reference Rangel-Salazar, Martin, Marshall and Elner2008), we recorded geographical and habitat variables that might influence Forest Thrush abundance at each point (Table 1). Land cover type at the survey point, distance to permanent water sources and distance to forest edge or clearing (roughly 10 m2 or larger) were obtained from high-resolution aerial images available on a GIS web-platform (http://landinfo.gov.ms/, accessed 14 April 2013) (Table 1).
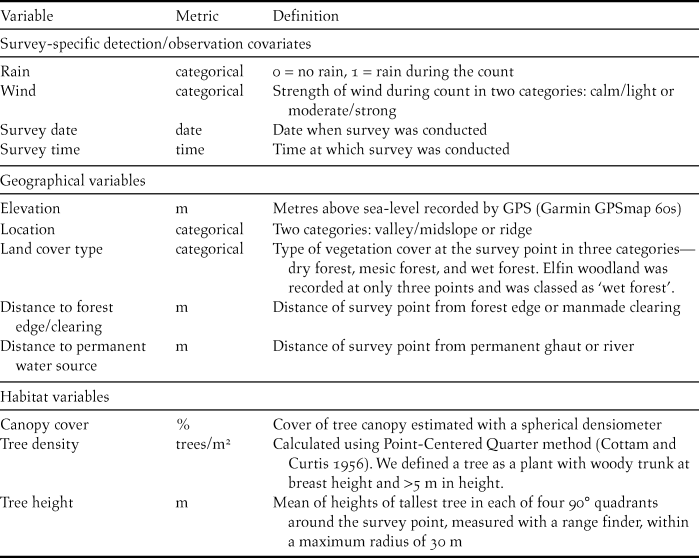
Table 1. Definitions of survey-specific detection covariates, geographical and habitat variables recorded at 88 survey points across the Centre Hills on Montserrat in 2013.
Data analysis
We aimed to relate the abundance of the Forest Thrush to various habitat variables. While habitat or landscape variables usually influence species occurrence and abundance, some habitat features and other variables (e.g. wind, rain, sampling time) may affect an observer’s ability to detect the species (Royle and Dorazio Reference Royle and Dorazio2008, Nichols et al. Reference Nichols, Thomas, Conn, Thomson, Cooch, Evan and Conroy2009). To account for imperfect detection in our data we used binomial mixture models (BMM), which are a class of hierarchical models that require spatial as well as temporal replication of counts (Royle and Nichols Reference Royle and Nichols2003, Kéry et al. Reference Kéry, Royle and Schmid2005, Royle et al. Reference Royle, Nichols and Kéry2005); they have already been used with forest bird monitoring data on Montserrat (Oppel et al. Reference Oppel, Cassini, Fenton, Daley and Gray2014a). A critical assumption for BMMs is that the population is demographically closed over the period during which the repeat surveys are conducted. To satisfy this assumption, repeat surveys of the same survey points were conducted within 2–3 weeks, during which demographic closure is highly likely.
BMMs consist of a hierarchy of two Generalised Linear Models (GLMs), the first of which models the observation process to estimate detection probability while the second models true abundance in relation to environmental variables, given that not all birds are detected (Kéry and Schaub Reference Kéry and Schaub2012). We modelled the abundance component as a random Poisson process to estimate the size of the local ‘superpopulation’ of birds whose home range overlaps the radius around a sampling station. We used a Poisson rather than a negative binomial distribution, as the negative binomial distribution generally yields larger and less precise estimates for count data of common species (Kéry et al. Reference Kéry, Royle and Schmid2005, Joseph et al. Reference Joseph, Elkin, Martin and Possingham2009, Hunt et al. Reference Hunt, Weckerly and Ott2012). The observation model component was conditional on the number of birds estimated at each sampling station, and calculated the probability of detection based on repeated counts at a given site using binomial trials for each bird.
We constructed multiple biologically plausible candidate models in three hierarchical steps. First, we tested models describing the detection process, while keeping abundance constant. We considered that wind, survey time, survey date, rain and location of the survey point might affect detection probability, and explored all possible combinations of these variables, including quadratic effects of survey date to account for non-linear change in detection probability (Schmidt et al. Reference Schmidt, McIntyre and MacCluskie2013). In the second step, we explored all possible combinations of site-specific habitat covariates (elevation, tree height, tree density, canopy cover) as well as a quadratic effect of elevation on Forest Thrush abundance in a suite of models that included all detection covariates from the most parsimonious detection model. For sedentary forest species such as the Forest Thrush local habitat is usually the primary factor affecting abundance, while landscape-scale variables have a weaker secondary effect (Dolman Reference Dolman2012). Hence, in a third step, we added landscape effects (distance to forest edge, distance to permanent water source, land cover type) to the most parsimonious local habitat model, while retaining the detection covariates. Because some landscape variables were strongly correlated with elevation (r > 0.5; land cover type and distance to forest edge), we omitted combinations of these correlated variables in the third step. Across the three steps, we constructed a total of 841 models to test detection covariates and habitat relationships of the Forest Thrush. We used an information-theoretic approach to select the most parsimonious model from all three steps (Burnham and Anderson Reference Burnham and Anderson2002) and used the most parsimonious model for inference to avoid overestimation of variable importance based on model weights (Galipaud et al. Reference Galipaud, Gillingham, David and Dechaume-Moncharmont2014).
All numeric site and survey-specific covariates were standardised by rescaling so that all predictors had zero mean and unit variance, in order to enhance the convergence of the numerical optimisation algorithm (Kéry et al. Reference Kéry, Royle and Schmid2005). All categorical variables were structured as ordered factors, except for location of point and land cover type, which were structured simply as factors. All models were fitted using the ‘pcount’ function in the ‘unmarked’ package (Fiske and Chandler Reference Fiske and Chandler2011), and models with combinations of terms were tested using the ‘dredge’ function in the ‘MuMIn’ package (Barton Reference Barton2014) in R version 3.1.0 (R Development Core Team 2014).
The goodness of fit of the most parsimonious model was assessed with Pearson’s χ 2 statistic via 200 simulations using parametric bootstrap. If the observed statistic is not too extreme (beyond the 0.05 percentile of the reference distribution), then the model fits (Sillett et al. Reference Sillett, Chandler, Royle, Kéry and Morrison2012). In addition, lack of model fit was quantified using the overdispersion parameter ĉ as follows:
where
![]() $\bar X_B^2$
is the average of the test statistics obtained from the parametric bootstrap. If the model is an adequate description of the data, then ĉ should be approximately 1 (MacKenzie and Bailey Reference MacKenzie and Bailey2004).
$\bar X_B^2$
is the average of the test statistics obtained from the parametric bootstrap. If the model is an adequate description of the data, then ĉ should be approximately 1 (MacKenzie and Bailey Reference MacKenzie and Bailey2004).
Although the average home range size of 30 radio-tracked male Forest Thrushes during the breeding season on Guadeloupe was 0.08–0.2 km2 and the maximum distance covered in a day was < 200 m (Eraud et al. Reference Eraud, Arnoux, Levesque, Van Laere and Magnin2012), the distance between points may not have been sufficient to ensure complete spatial independence of point counts, which would affect the inference drawn from models. We therefore examined spatial independence of samples by calculating Moran’s I of the most parsimonious model residuals using the function ‘moran.test’ in the R-package ‘spdep’.
Results
We counted 480 Forest Thrushes at the 88 survey points during the three repeat surveys, with 0–6 birds encountered during each point count and most detections (> 90%) being aural. The best detection model included wind, time, location of point and survey date, and indicated that detection probability was generally low (mean ± SD = 0.16 ± 0.06, range 0.05–0.30) and decreased later in the morning, with stronger wind, and when point count stations were located on ridges, but increased in the middle of the month-long field survey (Figure 1, Table 2). Including these detection covariates, there was overwhelming support for elevation and canopy cover as determinants of Forest Thrush abundance (Tables 2, 3). Predicted Forest Thrush abundance ranged from 1–15 birds per point (mean ± SD = 11 ± 3 birds), increased with canopy cover, and was highest at intermediate elevations (Figure 1). The bootstrap P value for the most parsimonious model based on Pearson’s χ 2 statistic was 0.71, suggesting that it provided an adequate fit to the data. The overdispersion parameter ĉ = 0.94 indicated that there was no evidence for a lack of fit of this model. There was no spatial autocorrelation of the model residuals (Moran’s I = -0.011, P = 0.49).
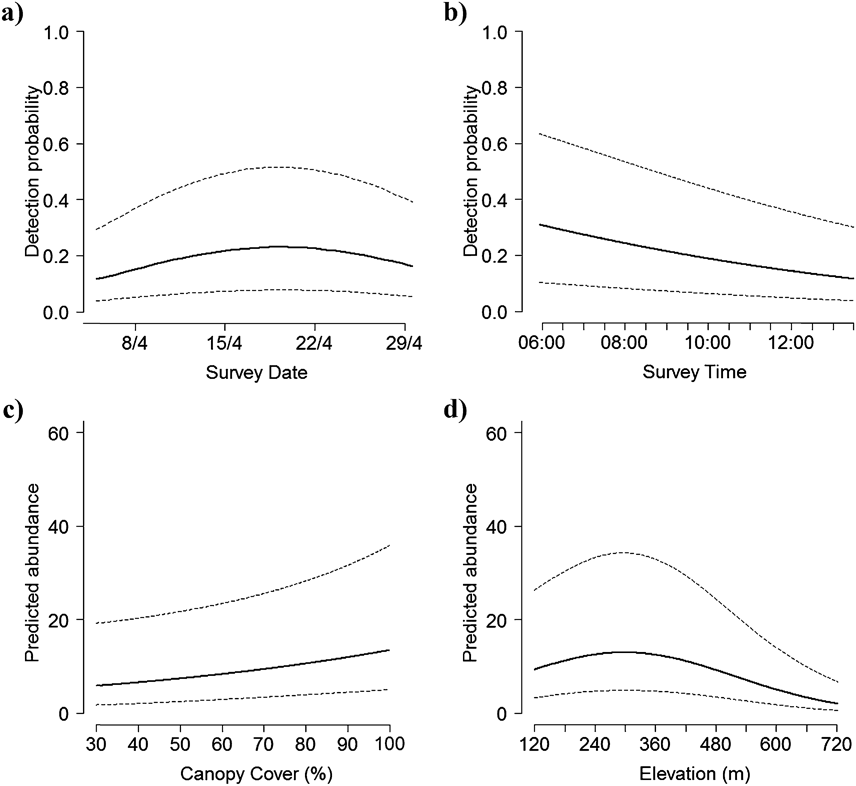
Figure 1. Factors influencing detection probability (a-b), and habitat variables influencing Forest Thrush abundance (c-d) in the Centre Hills of Montserrat, 2013, from the most parsimonious binomial mixture model. Dotted lines represent 95% confidence intervals.
Table 2. Top ten candidate binomial mixture models across all three steps of the model selection process, describing the detection and ecological processes involved in explaining abundance of Forest Thrush at 88 survey points in the Centre Hills of Montserrat in 2013. Covariates influencing abundance (λ) and detection probability (p) in each model are listed. Squared terms indicate quadratic effects.
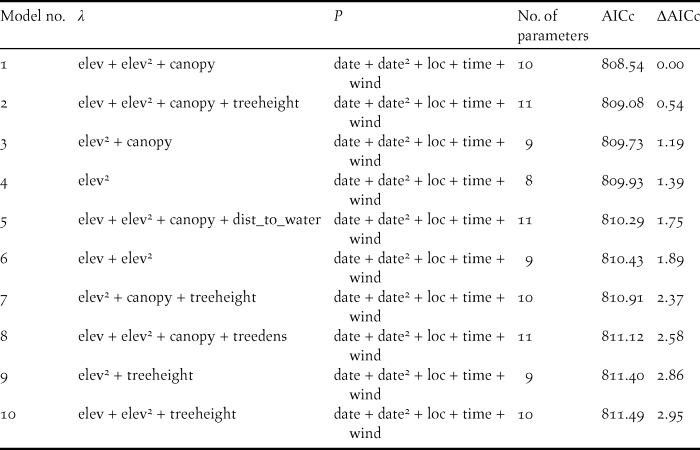
AICc = Corrected Akaike information criterion.
canopy = % canopy cover, date = survey date, dist_to_water = distance to permanent water source, elev = elevation, loc = location of survey point, time = survey time, treedens = tree density, treeheight = tree height, wind = strength of wind (see Table 1 for details of measurements).
Table 3. Parameter estimates from the most parsimonious binomial mixture model explaining abundance of forest thrush at 88 survey points in the Centre Hills of Montserrat in 2013. All variables were standardised (to unit mean and variance) for comparability of parameters.
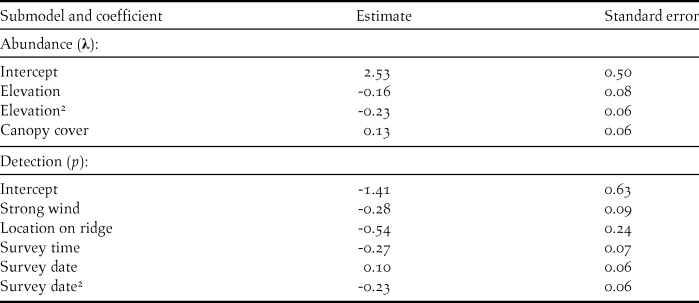
There were ten models across the three steps of the model selection process, which were within three AICc units of the most parsimonious model (Table 2). These models provided some evidence that tree height and distance to a permanent water source could affect the abundance of Forest Thrush. While tree height had a positive effect on abundance (abundance parameter estimate 0.096 ± 0.066 SE), Forest Thrushes appeared to be marginally less abundant in areas farther from a water source (-0.0524 ± 0.057 SE), but the effect size of these two variables was lower than for the variables in the most parsimonious model (Table 3).
Discussion
Canopy cover and elevation influence Forest Thrush abundance on Montserrat, with birds being more abundant at mid-elevations, dominated by closed canopies. Since land cover type and habitat type change along an elevational gradient, elevation was used as a proxy to make inferences about the type of forest that the species prefers. On Montserrat, the elevational habitat gradient is probably driven by moisture, as higher elevations receive more rainfall (Barclay et al. Reference Barclay, Johnstone and Matthews2006). Forest Thrush abundance was greatest at elevations of 200–400 m, which are dominated by mature secondary mesic forests, and declined slightly with further increase in elevation. There was also some evidence that Forest Thrushes were more abundant in areas with taller trees and closer to permanent water sources, but these effects were less pronounced than canopy cover and elevation. Overall, our findings provide quantitative support for qualitative descriptions of Forest Thrush distribution and habitat elsewhere (Eraud et al. Reference Eraud, Arnoux, Levesque, Van Laere and Magnin2012).
Many forest- and woodland-dwelling thrush species are strongly adapted to forage terrestrially in leaf-litter, but generally breed and sometimes feed in the canopy (Collar Reference Collar, Del Hoyo, Elliott and Christie2005). Because leaf-litter is thickest and supports a greater abundance of animal life in relatively level ground under a dense canopy, where low light conditions discourage obstructive undergrowth (Canham et al. Reference Canham, Denslow, Platt, Runkle, Spies and White1990, Bartels and Chen Reference Bartels and Chen2013), the greater abundance of Forest Thrushes in such habitat probably reflects better foraging habitat. Dense canopy cover and reduced lighting may also increase nesting success by reducing visual detection by nest predators (in this case, Pearly-eyed Thrashers Margarops fuscatus), as has been found in Ruddy-capped Nightingale-thrushes Catharus frantzii in Mexico, which enjoy higher nesting success under closed canopies (Rangel-Salazar et al. Reference Rangel-Salazar, Martin, Marshall and Elner2008).
Unsurprisingly, detection probability in our study decreased with increasing strength of wind, and use of ridges as survey points: birds are less audible in windy conditions (Simons et al. Reference Simons, Alldredge, Pollock, Wettroth and Dufty2007), and ridges hold inappropriate foraging substrates while their steep slopes reduce audibility. If not accounted for, this artifact of the observation process might have been mistaken for low Forest Thrush abundance on ridges. It therefore highlights the value of using binomial mixture models to study habitat preferences (Kéry Reference Kéry2008). We also found that detection probability decreased with time of day, which is a well-known phenomenon among songbirds, which often change their vocal activity through the day (Bibby et al. Reference Bibby, Burgess, Hill and Mustoe2000, Schmidt et al. Reference Schmidt, McIntyre and MacCluskie2013). However, as singing intensity increased in the second half of April we observed an intermittent increase in detection probability during the breeding season. Because the monitoring programme on Montserrat was originally designed for a different species with a slightly earlier breeding period, the first round of point count surveys recorded very few singing Forest Thrushes and it is therefore not surprising that detection probability increased over time.
The estimated abundance of birds per survey point in our study was quite large (> 15 birds/point, Fig. 1), a fact that has been recognised in other applications of binomial mixture models (Hunt et al. Reference Hunt, Weckerly and Ott2012). Binomial mixture models estimate the size of a ‘super-population’, which is the number of birds that use the habitat around a counting station (Nichols et al. Reference Nichols, Thomas, Conn, Thomson, Cooch, Evan and Conroy2009, Kéry and Schaub Reference Kéry and Schaub2012). Thus, overlapping home ranges of several pairs, which has been recorded for radio-tracked individuals on Guadeloupe (Eraud et al. Reference Eraud, Arnoux, Levesque, Van Laere and Magnin2012), will lead to several pairs using the same forest area and would thus explain the high estimated abundance per point. Although we are not able to convert our estimates of abundance into density and thus total population size of Forest Thrushes on Montserrat, we are confident that these estimates are reliable to infer habitat associations and to model population changes over time (Parashuram Reference Parashuram2013).
Our study has important implications for the conservation of the Forest Thrush. The preferred forest habitat for the species includes tall mesic forests with closed canopies, which have matured after forest clearance for agriculture in previous centuries and more recent natural disasters (Lugo et al. Reference Lugo, Schmidt and Brown1981, Arendt Reference Arendt1990, Dalsgaard et al. Reference Dalsgaard, Hilton, Gray, Aymer, Boatswain, Daley, Fenton, Martin, Martin, Murrain, Arendt, Gibbons and Olesen2007). Both natural and semi-natural forests are still threatened by human development on all islands in the range of the Forest Thrush (BirdLife International 2014). In addition, intense grazing pressure from feral livestock may alter the forest structure and inhibit regeneration, with potentially negative consequences for the Forest Thrush (Peh et al. Reference Peh, Balmford, Birch, Brown, Butchart, Daley, Dawson, Gray, Hughes, Mendes, Millet, Stattersfield, Thomas, Walpole and Bradbury2014). For the long-term survival of this and other island species, it is critical to retain as much forest cover as possible to allow for population shifts in response to stochastic environmental events such as hurricanes and volcanic eruptions, which are frequent in the Caribbean (Tanner et al. Reference Tanner, Kapos and Healey1991) and have led to major distributional shifts of birds, especially on Montserrat, in the last two decades (Arendt et al. Reference Arendt, Gibbons and Gray1999, Oppel et al. Reference Oppel, Hilton, Ratcliffe, Fenton, Daley, Gray, Vickery and Gibbons2014b). While our study has clarified the habitat associations of the Forest Thrush on Montserrat, urgent work is now needed to establish forest management programmes that protect significant areas of mature forest from damage from overgrazing and losses to human development.
Acknowledgements
We thank L. Martin, J. Boatswain, A. Homer and L. Bambini for assistance with fieldwork and data entry. L. Rogers of the Montserrat Physical Planning Unit kindly granted access to the high- resolution aerial images and GIS resources of the island. The Montserrat Department of Environment provided invaluable logistical support.






