Honey has a very long history of safe use and an equally long history as a traditional medicine for its antimicrobial activity, including protection from pathogens and external wound healing(Reference Lusby, Coombes and Wilkinson1, Reference Molan, Lila and Mawson2). Other beneficial functions that have been attributed to honey include antioxidant, anti-tumour, anti-inflammatory, antimutagenic and antiviral properties, with the observed physiological effects dependent on the nutritional composition of the honey consumed. That composition is a product of the botanical origin of the honey as well as pollen sources, environmental conditions and processing steps(Reference Viuda-Martos, Ruiz-Navajas and Fernandez-Lopez3). The presence in honey of active ingredients such as H2O2, polyphenols and other aromatic compounds is thought to be responsible for the beneficial aspects of honey and other bee-related products such as propolis and royal jelly. Furthermore, honey obtained from the manuka shrub in New Zealand (Leptospermum scoparium J. R. Forst & G. Forst) may contain an additional antibacterial property known as the unique manuka honey factor (UMF®). This is a phytochemical-derived property from the nectar of the flower rather than antimicrobial activity attributed to H2O2, which results from the action of bee-derived glucose oxidases on water(Reference Mavric, Barth and Henle4). The UMF® varies between batches of manuka honey and across seasons. Accordingly, the UMF® of each batch is tested after processing and rated according to an industry-adopted scale relating to the antimicrobial efficacy (from 0 (low efficacy) to 20 (high efficacy) and over), with the higher rating indicating higher antibacterial potency. UMF rating is based on a well diffusion assay where the area of exclusion of bacterial growth that the honey causes relative to the phenol control is measured (http://www.umf.org.nz/Unique-Manuka-Factor.cfm). For example, a UMF® rating of 10 has equivalent antimicrobial potency to a 10 % phenol solution. In 2007 a group of scientists in Dresden, Germany, demonstrated the correlation between the UMF® of manuka honeys and levels of a methylglyoxal (MGO)(Reference Mavric, Barth and Henle4). Whilst MGO certainly accounts for the majority of UMF® activity, we believe it does not account for all(Reference Rosendale5). MGO is a by-product of several essential biological processes such as glycolytic bypass, acetone metabolism and amino acid breakdown. It is also a potentially toxic metabolite that accumulates in various cell types. It can react with protein metabolites during processes such as cooking, leading to the formation of Amadori rearrangement products. These products are unstable reaction intermediates that can degrade to advanced glycation endproducts (AGE). They are implicated in a number of serious diseases, including renal disease, diabetes, neurodegenerative disease and heart disease(Reference Ames6). Although AGE comprise a whole class of compounds, there is one, N ɛ-(carboxymethyl)-lysine (CML), that is not only abundantly present in food but has also been closely studied in relation to disease risk. Manuka honey has sufficient MGO (approximately 800 mg/kg in UMF® 20+) to impart desirable antimicrobial activity, but it is not known whether this concentration of MGO might induce undesirable effects such as the accumulation of AGE in consumers. Measurements of levels of CML, a by-product of MGO activity following honey ingestion, will test this relationship.
IgE is one of five immunoglobulins that form an important part of the humoral immune response, and is a pivotal effector molecule of type 1 hypersensitivity allergic reactions. Serum IgE is frequently measured in the diagnosis of allergic responses in atopic individuals with IgE-mediated allergies. These individuals can display serum IgE levels up to ten times the levels in non-atopic individuals(Reference Sicherer and Leung7). When an atopic individual comes into contact with a food allergen for the first time, the allergen is recognised by a B cell and plasma cells then produce large amounts of IgE specific to that allergen. These specific IgE molecules attach to the body's mast cells and when the food allergen is next encountered, the IgE-primed mast cells release granules containing various cytokines and histamine, which cause symptoms characteristic of an allergic response (such as inflammation, excess mucus production, itching and congestion).
Studies have demonstrated that honey(Reference Sanz, Polemis and Morales8, Reference Shamala, Jyothi and Saibaba9) exhibits a prebiotic effect by increasing the populations of bifidobacteria and lactobacilli in the gut. This may be beneficial not only in terms of maintaining a healthy gut microbiota, but may provide some protection against the development of IgE-associated allergic disease. Previous research(Reference Borchers, Selmi and Meyers10–Reference Lahtinen, Boyle and Kivivuori12) has suggested that supplementation with probiotics in the prenatal or early months of life may reduce levels of IgE-associated allergic disease amongst children.
Maintaining a healthy balance of the major bacterial groups is an important part of gut health and gut homeostasis. The human gastrointestinal tract has a huge surface area (over 100 times that of the skin) and, with its high moisture content, stable temperature and abundant nutrients, is the ideal environment for micro-organisms. It has been proposed that over 500 different species of bacteria are present in the colon alone, most of which would be obligate or facultative anaerobes along with some aerobes(Reference Conway13). The populations of micro-organisms resident in the gut colonise its surface and play an essential role in normal digestion and gut health. However, a variable number and proportion of transient populations of micro-organisms interact with and sometimes even displace the populations normally resident, affecting the host's gut health.
In the present study we hypothesised that consuming UMF® 20+will not result in any allergic response, either to the plant or bee proteins contained in the honey, and that any prebiotic effects (reflected in increases in lactobacilli and bifidobacteria) will not result in increased IgE levels, providing some protection against an IgE-associated allergic response. Our aims were, therefore, to investigate the safety of UMF® 20+by measuring several of the most relevant health endpoints for a strongly antimicrobial food containing MGO and potentially residual allergenic bee products. We did this by establishing whether UMF® 20+caused an allergic response (as measured by IgE levels), changed major commensal and beneficial bacterial groups in the gut representative of a normal healthy microbiota and/or affected levels of CML, one of the most common AGE.
Experimental methods
Study design
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Canterbury Upper South A Ethics Committee, New Zealand; a written informed consent was obtained from all subjects. A randomised, double-blind cross-over dietary design was used for the present study, which was approved by the Canterbury Ethical Committee. A total of twenty healthy individuals (fifteen female, five male) were recruited from within Christchurch, with an age range of 42–64 years, and BMI ranging from 20·5 to 50·1 kg/m2. A sample size of twenty was chosen based on sample sizes used in other published clinical trials where the same end-point parameters such as quantification of microbial groups were used. A total sample size of twenty subjects in a cross-over design would enable effect sizes greater than 0·7 to be detected as statistically significant (two-tailed α = 0·05) with 80 % power.
Screening criteria included healthy subjects between 34 and 64 years of age, with no diabetes or chronic illness, as assessed by means of a questionnaire. The subjects 2were also not taking any medications. Allergies and non-atopic status (for example, eczema, asthma, irritable bowel disease, bee allergies) were determined by a questionnaire. Mild cases as determined by participants were included. The study ran for 12 weeks, during which time the participants continued their normal diets with the inclusion of the allocated honey. For the first 2 weeks all honey was excluded from participants' diets, after which they consumed 20 g honey per d in two doses of 10 g each. UMF® 20·3 was taken by ten participants and the multiflora control honey (UMF® < 8·2) was taken by the other ten participants. After 4 weeks there was another 2-week ‘washout’ and then the two groups swapped to the other type of honey for 4 weeks. Compliance was accepted on an honesty basis with missed doses recorded on the questionnaire as well as empty containers being returned to the trial coordinators.
Faecal and blood samples
Fasting blood samples were collected at the beginning of the study, starting with the first sample after the initial 2-week washout, and then weekly during the 4-week interventions with honey. No samples were collected during the 2-week ‘washout’ period. Blood was collected into plain vacutainers and the serum separated by centrifugation on the same day as collection. Samples were stored at − 80°C until the end of the trial. Spot faecal samples were collected in the morning before visits to the clinic in a screw-capped bottle at the beginning and end of each 4-week intervention cycle. Subjects were asked to immediately store their samples in an icebox provided and once they had been delivered to the laboratory samples were stored at − 80°C until processing.
IgE measurement
IgE measurement was carried out on frozen serum collected weekly during each of the honey interventions. The ImmunoCAP® testing system (ImmunoCAP®; Phadia AB, Uppsala, Sweden) was used to quantify total IgE levels in serum, and samples were run over 2 d in chronological order in batches of forty. Samples from a single individual were run in the same batch. The total IgE test is a sandwich immunoassay. The unique part of the test system is the solid phase, ImmunoCAP, which consists of a cellulose derivative enclosed in a capsule. ImmunoCAP solid phase is the best solid phase for allergy diagnostics. It ensures low non-specific binding of all relevant antibodies, regardless of antibody affinity. This provides high sensitivity (detection limit of 0·1 kU/l) and allows detection of very low concentrations of IgE antibodies (in the range 2–5000 kU/l). A calibration curve was run initially with six standards: 2, 10, 50, 200, 1000 and 5000 kU/l. Samples were run once and compared with the standard curve, as well as internal standards (quality controls): total IgE high (388 kU/l), total IgE medium (99·5 kU/l) and total IgE low (19·8 kU/l).
Nɛ-(carboxymethyl)-lysine measurement
CML was measured using an ELISA kit containing a monoclonal anti-CML antibody KM-2A9, a CML-bovine serum albumin antigen-coated plate, a CML-human serum albumin standard and a second antibody detection system of peroxidase-conjugated anti-mouse IgG polyclonal and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate reagent to visualise the results (CircuLex CML/N ɛ-(carboxymethyl)-lysine ELISA kit, 96 assays, product code CY-8066; MBL International, Woburn, MA, USA). CML measurement was carried out on baseline samples and after participants had consumed each of the honeys for 4 weeks. A sample of 30 μl serum was used and this was diluted 1:4 with buffer according to the kit instructions. Samples were carried out in duplicate along with seven standards between 0 and 20 ng CML-human serum albumin per ml. Protein concentrations of each sample were also determined by an analytical laboratory (MedLab, Palmerston North, New Zealand) using standard methodology and CML levels corrected for protein concentration.
Faecal extraction
Three subsamples of approximately 200 mg were taken from the outer edge (where possible) of each stool sample while still frozen. This was in order to maximise the integrity of the DNA since it has been shown that DNA damage increases towards the centre of a stool sample. Genomic DNA was extracted from faecal samples using the Qiagen DNA Stool Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions for isolating gram-positive bacteria. DNA concentration and quality were measured on a NanoDrop™ spectrophotometer (NanoDrop Products, Wilmington, DE, USA).
Bacteria culture and conditions
The following bacteria were collected as standard reference strains for each group being investigated: Lactobacillus rhamnosus, Bifidobacterium adolescentis, B. fragilis, Escherichia coli O157:H7 and Clostridium perfringens. These were obtained from the New Zealand Reference Culture Collection (Environmental Science and Research Ltd, Porirua, New Zealand) or from internal laboratory stocks. The bacteria were all grown at 37°C in anaerobic chambers (Fort Richard Laboratories, Auckland, New Zealand) in anaerobic atmospheric conditions (7 % CO2, 30 % water, 63 % N2, < 0·1 % O2). Media were: Lactobacillus and bifidobacteria in de Man–Rogosa–Sharpe (MRS) medium supplemented with 0·05 % cysteine (w/v); E. coli in tryptic soy broth (TSB); Bacteroides and Clostridia in brain–heart infusion (BHI) broth. An overnight culture was counted on a haemocytometer and DNA extracted from 1 × 109 cells using a Qiagen™ DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions for either Gram-positive or -negative bacteria extraction. The concentration and quality of the DNA were measured on a NanoDrop™ spectrophotometer (NanoDrop Products, Wilmington, DE, USA).
Primers
Primers covering five bacterial groups were used for real-time PCR analysis of faecal DNA. These were tested for specificity and cross-reactivity against all of the reference samples using conventional PCR and agarose gel electrophoresis.
Real-time PCR
Real-time PCR was carried out in a ninety-six-well plate format using an ABI Lightcycler 7500 (Applied Biosystems (Pty) Ltd, Melbourne, Vic, Australia). Reactions were carried out in 20 (Lactobacillus) or 25 μl volumes with 1X PowerSYBR master mix (Applied Biosystems (Pty) Ltd), 0·5 μm primers (0·625 μm for Lactobacillus) and 1 μl template DNA. A 10-fold standard curve was included on each plate at a 1:10 dilution. Faecal samples were loaded neat. All samples were loaded in triplicate.
The standard amplification program was used, which involved an initial incubation at 50°C for 2 min, then denaturation at 95°C for 10 s. Following this, forty cycles of denaturation were repeated at 95°C for 15 s and annealing at 60°C for 1 min. A dissociation curve was performed with denaturation at 95°C for 15 s, annealing at 60°C for 1 min and a final denaturation at 72°C for 15 s.
Analysis
In real-time PCR a cycle threshold (Ct) value is obtained for each sample tested, which relates to the amount of starting DNA present in that sample. In brief, the amount of product is plotted on a graph of fluorescence (y axis) v. time in PCR cycles (x axis). An arbitrary level of fluorescence is chosen as a cut-off value and then the time (or cycle number) taken for each sample to cross that threshold is the Ct value.
An average of the triplicate Ct values was used to plot the standard curve of the serial dilution of reference bacteria (109–104) and only R 2 values >0·99 were accepted. The regression line formula (y = mx +c) was then used to determine the approximate number of cells/mg faeces for each sample.
Statistical analysis
For each participant, the mean of duplicate baseline IgE measurements was used as the individual baseline. For the two treatment cycles the differences from the baseline IgE level were calculated for each individual, and then the mean of these differences was used in further statistical analysis. Two-way ANOVA was used to compare the mean changes in IgE levels relative to the individual baselines for the honey type and the intervention period. Individual subjects were treated as blocks in the analysis. The geometric means and 95 % CI for IgE levels (calculated by back-transforming the means and CI of log-transformed data) were also determined to describe the IgE levels during the dose periods. To compare the effects of each honey on bacterial populations, the measure used for analysis was the ratio of bacteria counts before and after consuming the honey – to indicate the proportional change from baseline. The ratios were log-transformed before ANOVA to equalise the variances, and after the ANOVA the means were back-transformed. All CML measurements were carried out in duplicate and the differences between baseline and post-treatment values for each honey were compared by two-way ANOVA with the individuals treated as blocks. Following ANOVA, 95 % CI were calculated for the mean of each honey type. Statistical analysis was carried out using GenStat for Windows 10th edition software (VSN International Ltd, Hemel Hempstead, Herts, UK).
Results
The geometric mean of serum total IgE at baseline was 18·3 kU/l for UMF® 20+and 17·2 kU/l for multiflora honey. The geometric means (95 % CI) are shown in Table 1 for both honey treatments. Mean total IgE levels were calculated on a weekly basis by treatment group for all twenty participants and remained below 50 kU/l for the whole study, which is in line with published results for non-atopic individuals (Table 1). The mean difference in IgE levels compared with baseline levels is presented in Table 2. ANOVA showed that IgE levels did not change significantly with either honey type (P = 0·86) or with intervention period (P = 0·86). CI (Table 2) showed that IgE levels did not change significantly (the CI limits include zero) with either UMF® 20+or multiflora honey.
Table 1 Total IgE levels (kU/l) for all twenty participants at baseline and at the end of each week during the manuka UMF® 20+honey and multiflora honey treatment (Geometric mean values and 95 % geometric confidence intervals)
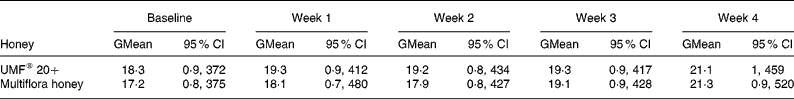
GMean, geometric mean; UMF, unique manuka honey factor.
Table 2 Mean difference in IgE level (kU/l; relative to each subject's mean baseline) for two honey types given in two intervention periods*
(Mean values and 95 % confidence intervals)

UMF, unique manuka honey factor.
* sem 3·52. Sample size was ten for each mean.
There was no effect on the number of bacteria in each of the five major bacterial groups measured by PCR of the DNA with either UMF® 20+(Fig. 1) or multiflora honey (Fig. 2). There was no significant effect of honey type on the numbers of bacteria present: Bacteroides (P = 0·82), E. coli (P = 0·63), Clostridia (P = 0·60), Lactobacillus (P = 0·88) and bifidobacteria (P = 0·70).
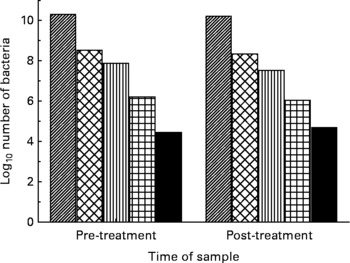
Fig. 1 Log10 number of bacteria pre- and post-treatment with manuka honey, UMF® 20+, in the five bacterial groups: (▨), Bacteroides; (![]() ), Bifidobacterium; (▥), Lactobacillus; (
), Bifidobacterium; (▥), Lactobacillus; (![]() ), Escherichia coli; (■), Clostridium.
), Escherichia coli; (■), Clostridium.

Fig. 2 Log10 number of bacteria pre- and post-treatment with multiflora honey in the five bacterial groups: (▨), Bacteroides; (![]() ), Bifidobacterium; (▥), Lactobacillus; (
), Bifidobacterium; (▥), Lactobacillus; (![]() ), Escherichia coli; (■), Clostridium.
), Escherichia coli; (■), Clostridium.
The mean CML level, a measure of AGE, at baseline for UMF® 20+was 2·9 (95 % CI 2·7, 3·2) ng/ml and at the end of 4 weeks was 2·8 (95 % CI 2·6, 3·1) ng/ml. For the multiflora honey at baseline the mean CML level was 3·0 (95 % CI 2·7, 3·2) ng/ml and after 4 weeks was 2·8 (95 % CI 2·5, 3·0) ng/ml. The mean difference in CML levels for UMF® 20+after the 4-week intervention was − 0·08 (95 % CI − 0·46, 0·30) ng/ml and for the multiflora honey − 0·19 (95 % CI − 0·57, 0·19) ng/ml. There was no significant difference in the mean change in CML levels between the two honey types (P = 0·67). The mean (and 95 % CI for the mean) percentage difference in CML levels for UMF® 20+after the 4-week intervention was − 0·8 (95 % CI − 14·0, 12·5) % and for the multiflora honey − 2·8 (95 % CI − 16·1, 10·4) %. There was no significant difference in the mean change in CML levels between the two honey types (P = 0·82).
Discussion
The aim of the present study was to investigate the safety of consumption of manuka honey compared with multiflora honey. Allergic responses to honey are relatively uncommon and have been attributed mainly to the presence of components of bee origin in the products(Reference Helbling, Peter and Berchtold14). Using IgE as a marker of humoral immune response, the present study established that following ingestion of UMF® 20+and multiflora honey for 4 weeks the total IgE levels of the twenty participants did not significantly increase or decrease. Levels of IgE also remained at a level consistent with a non-atopic response during the study. Therefore, it can be concluded that at this level of consumption, UMF® 20+and multiflora honey had no significant effect on allergic status. In addition, results demonstrated that there were no significant changes in numbers of any of the five groups of bacteria tested following the consumption of either UMF® 20+or multiflora honey.
The antimicrobial properties of honey are well documented and have largely been demonstrated in in vitro experiments(Reference Bogdanov, Jurendic and Sieber15). The mechanisms suggested for these antimicrobial properties centre around the action of H2O2 produced from glucose oxidase or around non-peroxide activity, which depends upon flower and nectar sources. Other factors, such as pH, osmolarity and the action of flavonoids, have also been reported, suggesting that these properties may be the result of a combination of factors(Reference Viuda-Martos, Ruiz-Navajas and Fernandez-Lopez3). It has been proposed that the higher the UMF® factor, the greater the antibacterial potency(16). This is proposed to be due to its ability to stimulate the growth of probiotic bacteria such as lactobacilli and bifidobacteria which would then outcompete pathogenic strains as much as its direct antibacterial effect against pathogenic bacteria(Reference Shamala, Jyothi and Saibaba9). However, in the present study the high UMF® 20+honey did not have a significant effect on levels of any the bacterial groups measured. This investigation examined the effects of honey when introduced to the normal diet of the participants. Dietary intakes were not evaluated during the treatment periods. Interactions between the honey and other dietary components consumed during the study may have masked effects on gut microbiota. Storage of the test products may also have been a factor, as it is well established that duration of storage and heat reduce the antibacterial properties of honey(Reference Badawy, Shafii and Tharwat17). Participants were given the honey samples on a weekly basis and asked to store the product in the fridge to prevent heat damage. Furthermore, in many of the human studies demonstrating biological effects, intakes of honey were at the higher dosage of 50–80 g honey per d(Reference Bogdanov, Jurendic and Sieber15). We used a lower dose, as it is more like the amount likely to be consumed on a daily basis. The lack of antimicrobial effect may therefore be due to insufficient honey being consumed. Anecdotal evidence suggests that manuka honey can help to alleviate gut-related conditions, perhaps by re-establishing the optimum balance of gut microflora. Although no beneficial changes occurred in the five gut bacterial groups that were quantified in the present study, no adverse effects were reported from consuming the honey. Honey consumption has been shown to be beneficial in other areas of gut health, particularly in relation to stomach infections. A reduction in the severity of Helicobacter pylori infection and other intestinal disorders such as peptic ulcer has been reported, along with a reduction in the severity of diarrhoea or gastroenteritis(Reference Bogdanov, Jurendic and Sieber15). Participants in the present study were not asked to comment on other perceived beneficial effects from honey consumption.
The present study investigated the levels of CML, an AGE identified in disease risk. Formation of AGE following the reaction of MGO with protein has been postulated to be a potential risk for many diseases, including diabetes, renal disease and CVD. There were no significant differences in the levels of CML detected during consumption of either UMF® 20+honey or multiflora honey in the present study, suggesting that consuming the amount of honey given in this trial has no detrimental effect in relation to AGE. Although a considerable amount of literature has been published regarding the relationship between AGE compounds and disease(Reference Bengmark18), the basis of their pathogenicity has still not been fully elucidated. Studies have shown that the bioavailability of CML is low with rapid and high excretion rates in urine and faeces being reported, suggesting that interaction with body protein is low. There is also evidence that CML may be degraded by colonic microbiota(Reference Ames6). Thus, these compounds may be too rapidly excreted to have a detrimental effect in healthy individuals but it was still important to demonstrate the absence of these compounds in the present study population following relatively high levels of manuka honey consumption.
The ability of AGE to induce oxidative stress by generating free radicals and thus facilitate oxidative damage to molecules such as carbohydrates, proteins, nucleic acids and lipids, is thought to be one mechanism that may adversely affect human health(Reference Ames6). Honey is known to contain many significant antioxidant properties, including phenolic acids (caffeic, coumaric, ferrulic, ellagic, chlorogenic) and flavonoids (chrysin, pinocembrin, pinobanksin, quercetin, kaempferol, luteolin, galangin, apigenin, hesperetin, myricetin)(Reference Viuda-Martos, Ruiz-Navajas and Fernandez-Lopez3). Manuka honey has also been shown to contain the specific phenolic compound, methyl syringate, which has been demonstrated to specifically scavenge superoxide anion radicals, providing a high antioxidant activity(Reference Henriques, Jackson and Cooper19, Reference Inoue, Murayama and Seshimo20). Since these antioxidant compounds are actively absorbed in the small intestine, any observed effects are likely to occur systemically. It is possible that free radical production may reduce the formation of AGE. Some studies have shown that antioxidant substances can inhibit the formation of AGE by inhibiting the glycation process and the conversion of the intermediary Amadori products to AGE(Reference Bengmark18).
Presently, available data do not support a positive relationship between intake of dietary AGE and disease in healthy individuals, although for certain groups, such as the young, diabetics or individuals with compromised gut health, there may be some risk. Many common foods in the diet are major sources of AGE, including dairy products, meat, grains and coffee. Thus our daily exposure to these compounds is vast. More dietary intervention studies are required in this area to establish if minimising consumption of these products whilst consuming manuka honey might provide significant benefits to health(Reference Ames6).
Conclusion
There is evidence in the literature that honey has many beneficial health effects such as antibacterial, antioxidant, anti-tumour, anti-inflammatory and anti-viral activity. The principal active ingredient responsible for these effects in manuka honey is MGO. This compound, however, is associated with increases in AGE that may trigger inflammatory processes involved in chronic disease states, such as CVD and diabetes. Results from the present study suggest that both manuka honey and multiflora honey are safe to consume at the levels tested here in terms of several biomarkers (IgE and CML) and also that the gut microbiota homeostasis was not detrimentally affected.
Acknowledgements
The present study was funded by the Foundation for Research, Science and Technology through the ‘Food for Helicobacter pylori’ research programme (contract no. CO2X0402) and by Comvita New Zealand Ltd (Te Puke, New Zealand).
R. S., A. W., J. S. and J. W. were involved in the study design; S. E., J. W. and A. W. were involved in the data collection; M. M., H. M., M. R. and J. S. were involved in the data analysis; A. McL. carried out the statistical analysis; A. W., J. S. and S. E. wrote the manuscript.
The authors have no conflicts of interest to declare.






