Lactoferrin (or lactotransferrin, Lf) is a glycoprotein from the transferrin family of proteins. It displayed a structural organisation shared among members of the family. That is, it is formed by a single polypeptide chain and folded into two globular lobes, which are called the N-terminal and C-terminal lobes based on their localisation(Reference Lambert, Perri and Meehan1). It is expressed and secreted by epithelial cells in many exocrine secretions including saliva, tears and milk(Reference Liao, Alvarado and Phinney2,Reference Rosa, Cutone and Lepanto3) . Lf is the second most abundant protein in human milk, with a concentration varying from 1 to 7 gm/l (in colostrum)(Reference Liao, Alvarado and Phinney2). The best known Lf property is its strong Fe binding ability. However, Lf function is not limited to Fe homoeostasis because Lf can display strong antimicrobial activity. Importantly, its localisation on the mucosal surface represents one of the first defence systems against microorganism invasion(Reference Jenssen and Hancock4).
A growing body of evidence suggests that Lf plays a key role in host defence by modulating the innate and adaptive immune response(Reference Telang5). The effects of Lf on the innate immune response are related in part to its ability to bind to conserved structures termed pathogen-associated molecular patterns (e.g. lipopolysaccharide (LPS) on gram-negative bacteria and peptidoglycans on gram-positive bacteria), that are critical for the activation of innate immunity. Lf is also capable of enhancing the activation of immune cells. Macrophages function as antigen-presenting cells (APC) to stimulate the development of antigen-specific CD4+ T cells, and Lf enhances their ability function as APC by stimulating the production of cytokines, such as IL-12, responsible for modulating development of Th1 cells(Reference Actor, Hwang and Kruzel6,Reference Trinchieri7) . In addition, human milk-derived Lf is observed to cause maturation of CD4+CD8+ murine T cells, with a preference towards CD4 expression(Reference Zimecki, Mazurier and Machnicki8). In pigs, Lf decreases the recruitment of eosinophils to the duodenum through the intestinal Lf receptor(Reference Cooper, Nonnecke and Lonnerdal9). When administered orally, Lf has the ability to restore the host T-cell compartment, evident by an increase in splenic cellularity and enrichment of CD3+ CD4+ T cells(Reference Artym, Zimecki and Kruzel10), suggesting that Lf may act as an immunostimulatory factor on the mucosal immune system.
Interestingly, Lf peptide fragments generated by proteolysis may be absorbed and capable of conserving the biological activity(Reference Bellamy, Takase and Wakabayashi11). Experiments have emphasised that a similar process may participate in the bioactivity of orally administered Lf(Reference Dallas, Guerrero and Khaldi12). Indeed, recent experiments have suggested that these peptides play a beneficial role in microbial defence and cancer(Reference Yin, Wong and Ng13,Reference Yin, Wong and Xia14) . So far, the biological functions and mechanisms of action of these peptides are not fully understood.
LFP-20, an antimicrobial peptide located in the N terminus of porcine lactoferrin (pLf), displayed higher antimicrobial activity compared with their native protein counterpart(Reference Bellamy, Takase and Wakabayashi11,Reference Bellamy, Wakabayashi and Takase15,Reference Han, Gao and Luan16) . LFP-20 exhibited anti-microbial effects on Escherichia coli, Staphylococcus aureus and Candida albicans (Reference Han, Gao and Luan16). Our previous study investigated that LFP-20 protects intestinal barrier through maintaining tight junction complex and modulating inflammatory response(Reference Zong, Hu and Song17,Reference Zong, Song and Wang18) . However, the mechanism of its anti-inflammation action is in molecular or cellular layer is unknown. Here, an LPS-triggered systemic inflammatory response model was established. We elucidated the cellular mechanisms of the anti-inflammation effects of LFP-20, a peptide fragment of pLf, during LPS stimulation, thereby emphasising the importance of the LFP-20 in protecting from infection.
Methods
Peptide synthesis
LFP-20 (KCRQWQSKIRRTNPIFCIRR) was chemically synthesised by GL Biochem (Shanghai) Ltd, achieving 98 % purity of the synthetic peptide. The peptide was dissolved in endotoxin-free water and stored at –80°C.
Animal model
A total of seventy-two C57/BL6 male mice weighing 18–21 g (6–8 weeks) were obtained from Zhejiang Provincial Center for Disease Control and Prevention (Zhejiang CDC). Animals were individually housed and maintained on a 12 h light–12 h dark cycle under specific pathogen-free conditions. All animals were provided with food and water ad libitum during the experimental period (1 week). Animal studies were approved by the Institutional Animal Care and Use Committee of Zhejiang University and performed in accordance with the institutional guidelines.
After 1 week of adaptation, the mice were randomly divided into six groups of twelve each: control, 5 mg/kg LFP-20 treatment; LPS treatment, 2·5 mg/kg LFP-20 pre-treatment followed by LPS treatment (LFPL + LPS); 5 mg/kg LFP-20 pre-treatment followed by LPS treatment (LFPM + LPS) and 10 mg/kg LFP-20 pre-treatment followed by LPS treatment (LFPH + LPS). The different concentrations of LFP-20 were injected intra-peritoneally once daily for 6 d, whereas the control and LPS-treated groups were intra-peritoneally injected with an equal volume of sterile saline. On day 6, mice in LPS group and LFP-20 (12·5, 25 and 50 µg/ml) followed by LPS-treated groups were intra-peritoneally injected with LPS (10 mg/kg mouse weight) 1 h after LFP-20 or saline treatment, and the other groups were injected with an equal volume of saline. All mice were euthanised by cervical dislocation 6 h after intraperitoneal injection of LPS or saline, and tissues and blood were collected(Reference Zong, Song and Wang18). The spleen and thymus were weighted as spleen or thymus index.
Histopathology
Intestinal tissues of the ileum were fixed in 4 % paraformaldehyde solution immediately after the mice were euthanised. Tissues were embedded in paraffin and cut into 5-mm-thick sections. For the evaluation of histopathology changes, the tissues were stained with haematoxylin–eosin (H&E) and observed under a microscope (Leica NEWDM 4500BR). Histopathological changes were graded on the histological injury scale described by Jang et al.(Reference Jang, Hyam and Jeong19).
Immunohistochemistry
The distal ileum were fixed in 4 % paraformaldehyde solution, embedded in paraffin and cut into slices longitudinally, subsequently permeabilised, stained and fixed. For the immunohistochemical analysis of CD68, anti-CD68 antibodies (Proteintech) were added at a dilution of 1:100 and incubated overnight at 4°C after blocking with 1 % (w/v) bovine serum albumin for 1 h. After washing with PBS, samples were treated with horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG (HuaAn) at a ratio of 1:100. Samples were incubated at 4°C for 1 h and washed with PBS three times. 3,3’-Diaminobenzidine (DAB) (50–100 µl; Dako) was added, and the slices were counterstained with haematoxylin. Finally, the samples were dehydrated in an ethanol (70–100 %) gradient and treated with xylene to increase the transparency of slides. Neutral balsam was used for mounting.
Analysis of apoptotic death (terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labelling assay)
The cell apoptosis rate in the jejunum was detected using the In Situ Cell Death Detection Kit Fluorescein (Roche), according to the manufacturer’s instructions. Proteinase K digestion was performed with 20 µg/ml Proteinase K (Qiagen). Healthy and apoptotic cells were counted in four to six randomly selected fields at 200× magnification. The percentage of apoptotic cells was taken as the percentage of the total number of terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labelling (TUNEL)-positive cells.
Analysis of cytokines and myeloperoxidase in ileum
Concentrations of several cytokines (interferon (IFN)-γ, TNF-α, IL-6, IL-4, IL-5 and IL-12p70) were assessed using commercially available ELISA kits (R&D Systems).
The activities of myeloperoxidase (MPO) were assessed by ELISA kit (Boster Wuhan), according to the manufacturer’s instructions. Results were expressed in U/(mg protein).
Antibodies were determined by ELISA
The concentration of antibodies was measured by ELISA kit. Anti-mouse IgA, IgM, IgE, IgG1, IgG2a, IgG2b and IgG3 antibody ELISA kits were purchased from eBiosciences. Plates were coated with 4 µg/ml anti-mouse IgA, IgM, IgE, IgG1, IgG2a, IgG2b or IgG3 antibodies overnight at 4°C. Then, diluted sera were added in triplicate to the plate for 1 h at 37°C. Then after washing, 4 µg/ml HRP-conjugated anti-mouse IgA, IgM, IgE, IgG1, IgG2a, IgG2b or IgG3 antibodies were added to the plate and were incubated for another hour at 37°C. Finally, the colour was developed by incubation with o-phenylenediamine. The optical density was read at 492 nm with an ELISA reader (Bio-Rad). Standard curves were established to quantitate the amounts of the respective cytokines.
Flow cytometry-based immunophenotyping
Cytometric analysis has been described in our previous studies( Reference Kong, Wang and Yang20 – Reference Zhu, Wang and Xiao22 ). Analysing the distribution of immune cell populations in lymphoid organs, single-cell suspensions were prepared from peripheral blood, mesenteric lymph nodes and spleen. Before analysis, cells were depleted from peripheral blood, mesenteric lymph nodes and spleen by ammonium chloride lysis. After blocking of non-specific binding using TruStain fcX™ (anti-mouse CD16/32) antibody, the immune cell population was stained with fluorochrome-labelled antibodies against phycoerythrin-Cy5 (PE-Cy5)anti-mouse CD3e, fluorescein isothiocyanate (FITC) anti-mouse CD4, phycoerythrin (PE) anti-mouse CD8a, FITC anti-mouse CD45R, and PE anti-mouse pan-natural killer (NK) cells. Isotype control antibodies (PE-Cy5-IgG2a, FITC-IgG2b and PE- IgG) were used as a negative control. All antibodies were purchased from eBiosciences. Normalisation was done with respect to unstained cells. The samples were filtered immediately before analysis to remove any clumps. Data collection and analyses were performed on an FACS Calibur flow cytometer using CellQuest software.
Statistical analysis
Each experiment was performed on at least six animals per group. Statistical analyses were performed by one-way ANOVA, followed by the Duncan post hoc test with GraphPad Prism version 5.01 (GraphPad Software Inc.). Data are presented as means with their standard errors. An α value of P<0·05 was considered statistically significant.
Results
Histopathological symptom analysis
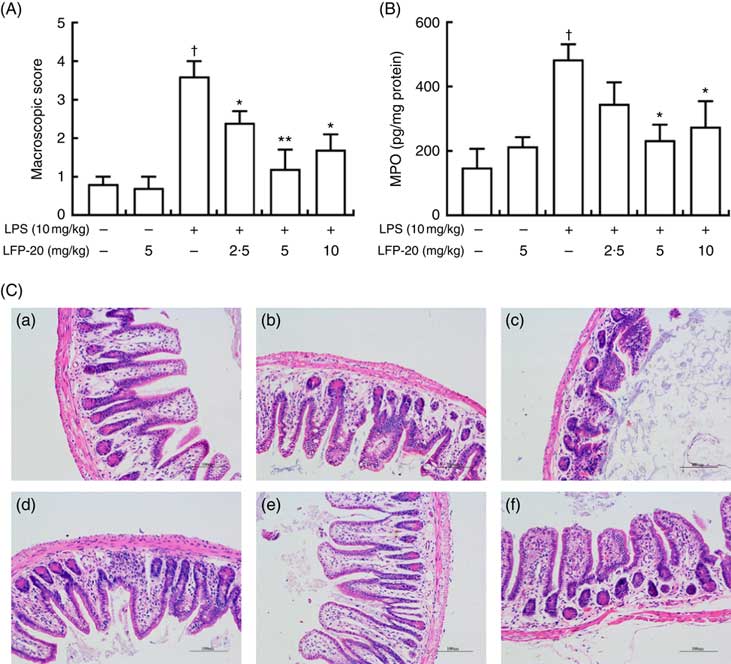
To assess the protection effects of LFP-20 on LPS-induced immune disorders, the distal ileum tissue was stained with H&E. As shown in Fig. 1(A) and (C), histological examination of ileum tissue from the LPS-induced group revealed considerable tissue injury and immune disorder with extensive ulceration of the epithelial layer, oedema, crypt damage to the bowel wall and infiltration of granulocytes and mononuclear cells into the mucosa. In contrast, LFP-20 pre-treatment reduced the LPS-induced symptom in ileum.
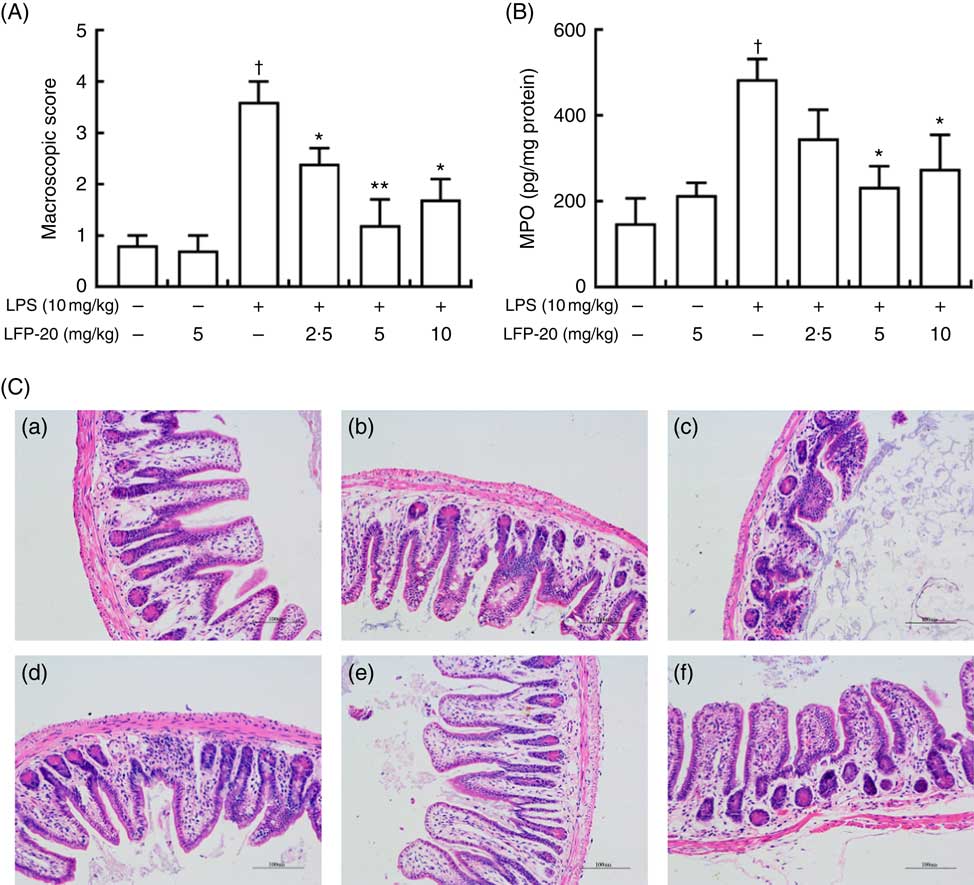
Fig. 1 Histopathological analysis of protection effects of LFP-20 on lipopolysaccharide (LPS)-induced lesions in ileum. (A) Levels of histopathological change quantified by mucosal damage grading. (B) Enzymatic activities of myeloperoxidase (MPO). (C) Representative haematoxylin–eosin-stained section from (C-a) control, (C-b) LFP-20, (C-c) LPS, (C-d) LFPL + LPS, (C-e) LFPM + LPS and (C-f) LFPH + LPS. Original magnification 200×. Values are means, with their standard errors represented by vertical bars. *P<0·05 compared with the LPS-stimulated group, n 6, biological replicates. †P<0·05 compared with the respective control within columns.
Evaluation of macrophage and neutrophil infiltration in ileum mucosa
MPO activity is an indicator for evaluating the degree of infiltration of neutrophils in tissues( Reference Zhang, Xia and Han23 ). As shown in Fig. 1(B), MPO activity in ileum tissue from LPS-treated mice was markedly increased compared with control mice, whereas 5 or 10 mg/kg LFP-20 pre-treated mice showed significantly decreased MPO activities compared with the LPS-treated alone mice. Furthermore, the infiltration of CD68 cells into ileum tissue was detected by immunohistochemistry. In contrast to minimal infiltration of macrophages into the ileum of control mice, we observed increased infiltration of CD68 macrophages into the lesion area of ileum after LPS stimulation. Pre-treatment with LFP-20 reduced the infiltration of macrophages compared with the mice treated with LPS alone (Fig. 2). Together, LFP-20 pre-treatment appeared to attenuate immune disorders in ileum during LPS stimulation.
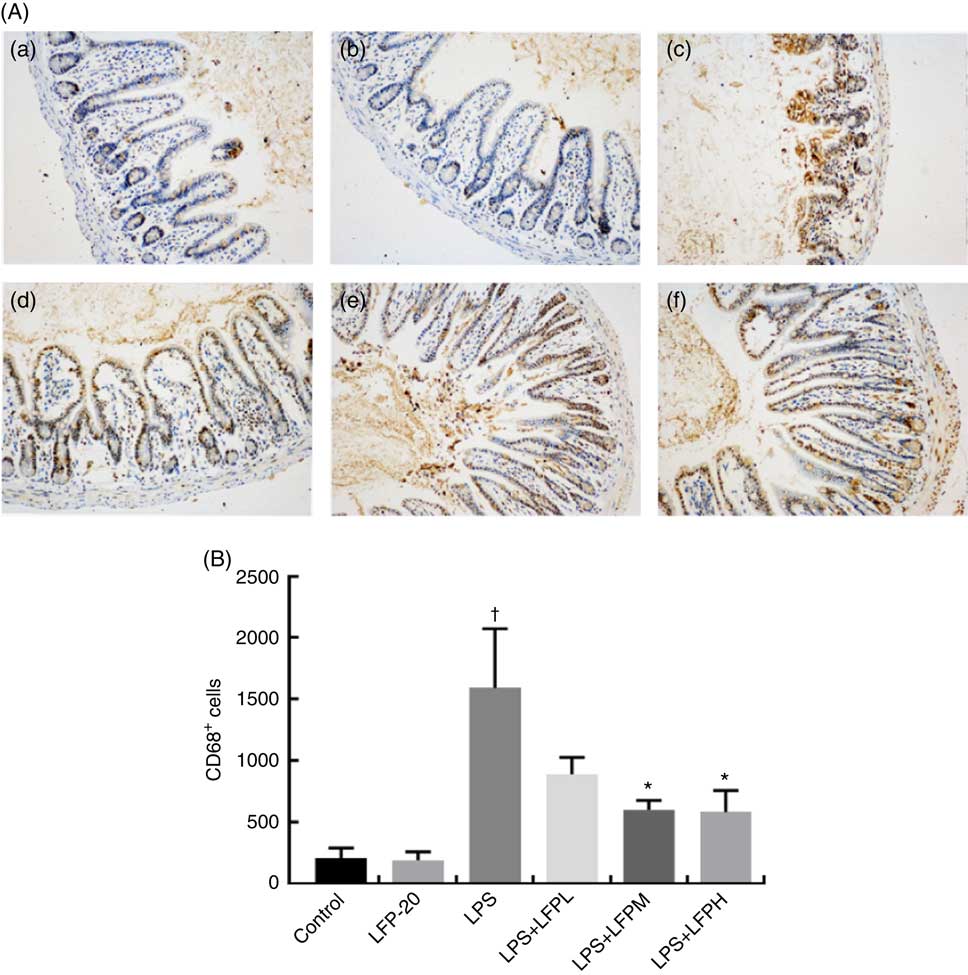
Fig. 2 Protective effects of LFP-20 on macrophage infiltration in ileum. (A) Infiltration of macrophage was represented by CD68+ cells. (a) Control, (b) LFP-20, (c) lipopolysaccharide (LPS), (d) LFPL + LPS, (e) LFPM + LPS and (f) LFPH + LPS. (B) The number of CD68+ cells counted according to the positive colour of brown and the average calculated. Original magnification 200×. Values are means, with their standard errors represented by vertical bars. *P<0·05 compared with the LPS-stimulated group, n 6, biological replicates. †P<0·05 compared with the respective control within columns.
Level of apoptosis in intestinal tissue
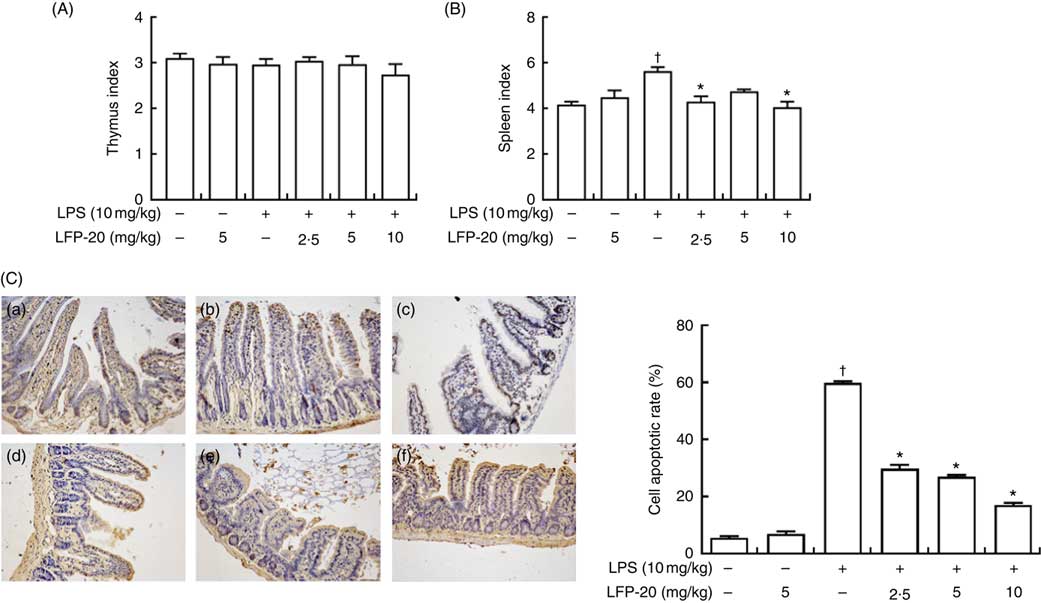
The TUNEL assay indicated that LPS treatment resulted in the apoptosis of epithelial cells in jejunum (brown signals, Fig. 3(C)), which was quantified by measuring the apoptosis index (Fig. 3(C)). Notably, compared with the apoptosis index of only the LPS-treated group, pre-treatment of LPS-administered mice with LFP-20 significantly reduced the apoptosis index in a dose-dependent manner, though the apoptosis indexes in mice of LFP-20 group was similar to that in the control group. Meanwhile, the spleen and thymus are very important immune organs in body. The changes in thymus index and spleen index directly reflect the state of immune function. We found pre-treatment of LFP-20 significantly attenuated the LPS-increased spleen index, while the thymus index was not affected (Fig. 3(A) and (B)).

Fig. 3 Effect of LFP-20 on small-intestinal cell apoptosis and immune organ index. (A) Thymus index. (B) Spleen index. (C) Representative terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labelling images for cell apoptosis (brown signals, original magnification 200×) from (C-a) control, (C-b) LFP-20, (C-c) lipopolysaccharide (LPS), (C-d) LFPL + LPS, (C-e) LFPM + LPS, (C-f) LFPH + LPS. The right panel shows the apoptosis index of jejunal tissues. Values are means, with their standard errors represented by vertical bars. *P<0·05 compared with the LPS-stimulated group, n 6, biological replicates. †P<0·05 compared with the respective control within columns.
Analysis of organ-specific immune cell subpopulation
To address the hypothesis that the effect of LFP-20 on LPS-induced immune disorders in ileum was due to organ-specific immune homoeostasis, lymphoid and myeloid immune cell populations in peripheral blood, mesenteric lymph nodes and spleen were analysed. As shown in Table 1, compared with the control group, LPS stimulation markedly increased the percentages of CD3+CD4+ T cells and B cells in the peripheral blood. Conversely, the percentages of CD3+CD8+ T cells and NK cells in the peripheral blood were smaller in only the LPS-treated group compared with those in the control group. In contrast, LFP-20 pre-treatment prevented the increase of CD3+CD4+ T cells and B cells in peripheral blood during the LPS stimulation. As expected, the LPS-induced decreased percentages of CD3+CD8+ T cells and NK cells in peripheral blood were also suppressed in LFP-20 pre-treatment. Immune cell subpopulation in mice treated with LFP-20 alone was similar to those of the control group. The similar effects were found in mesenteric lymph nodes and spleen, which further substantiated the immunomodulation effects of LFP-20 (Tables 2 and 3). Collectively, these results indicate that LFP-20 pre-treatment facilitated the balance of immune cells during LPS stimulation.
Table 1 Effects of LFP-20 on immune cell subpopulations in peripheral blood (Mean values with their standard errors)

NK, natural killer; LPS, lipopolysaccharide.
* P<0·05 compared with the respective control within columns.
† P<0·05 compared with the LPS-stimulated group. n 6, biological replicates.
Table 2 Effects of LFP-20 on immune cell subpopulations in spleen (Mean values with their standard errors)
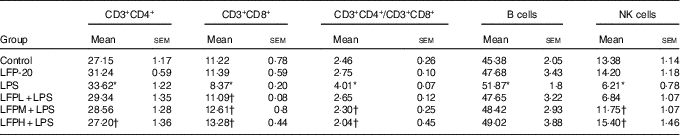
NK, natural killer; LPS, lipopolysaccharide.
* P<0·05 compared with the respective control within columns.
† P<0·05 compared with the LPS-stimulated group. n 6, biological replicates.
Table 3 Effects of LFP-20 on immune cell subpopulations in mesenteric lymph nodes (Mean values with their standard errors)

NK, natural killer; LPS, lipopolysaccharide.
* P<0·05 compared with the respective control within columns.
† P<0·05 compared with the LPS-stimulated group. n 6, biological replicates.
Secretion of Th1- and Th2-cell-specific cytokines in ileum
T lymphocytes mainly secrete cytokines and mediate inflammation-related immune responses( Reference Lythe and Molina-Paris24 ). To evaluate the protective effects of LFP-20 against inflammation in LPS-stimulated mice, cytokines were detected by ELISA, including TNF-α, IL-6, IFN-γ, IL-12p70, IL-4 and IL-5. As shown in Fig. 4(a)–(f), LPS treatment resulted in a significant increase in the concentration of TNF-α, IL-6, IFN-γ, IL-12p70, IL-4 and IL-5 in ileum, compared with untreated mice. Pre-treatment with LFP-20 markedly reduced LPS-induced cytokine secretion, which indicated that LFP-20 altered T lymphocyte functions and sustained a balanced Th1 and Th2 cell response. The concentration of cytokines in mice treated with LFP-20 alone was similar to the control group.
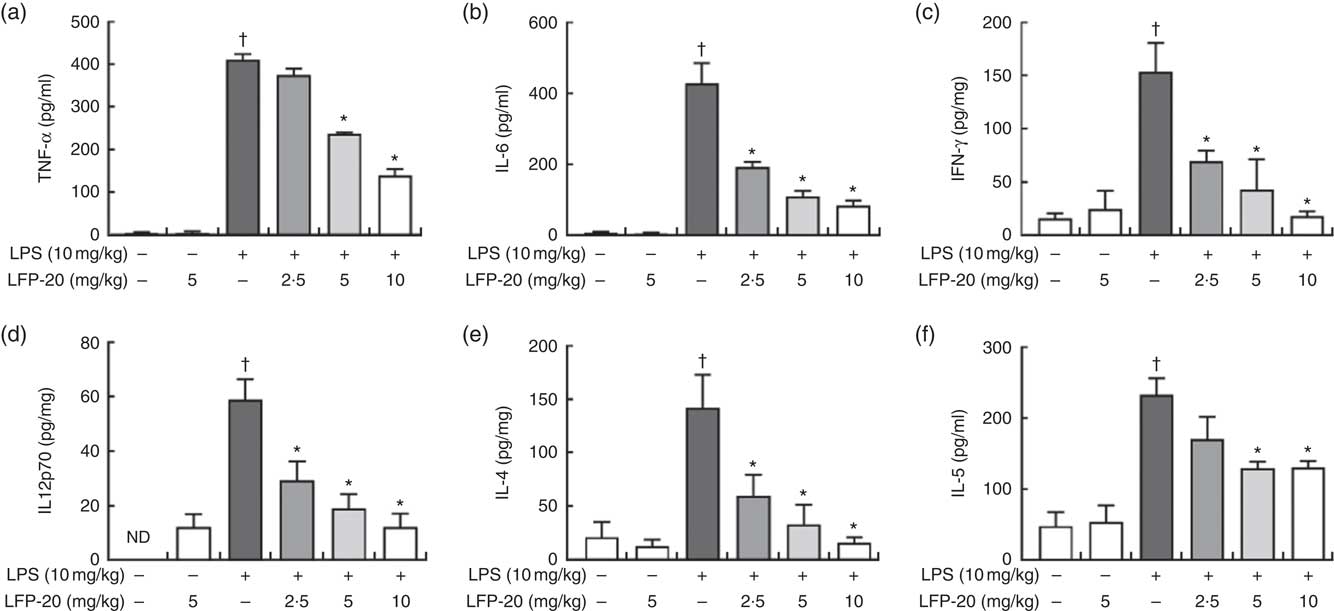
Fig. 4 Concentration of cytokines in ileum tissues. (a) TNF-α, (b) IL-6, (c) interferon (IFN)-γ, (d) IL-12p70, (e) IL-4 and (f) IL-5. Values are means, with their standard errors represented by vertical bars. *P<0·05 compared with the lipopolysaccharide (LPS)-stimulated group, n 6, biological replicates. †P<0·05 compared with the respective control within columns. ND, not detected.
Evaluation of antibody production in B cells after lipopolysaccharide stimulation
Activation of B cells is usually accompanied by antibody production( Reference Zhu, Wang and Xiao22 ). We hypothesised that the LFP-20 sustained Th1 and Th2 response induced B cells to produce antibodies during LPS stimulation. To address this hypothesis, we investigated the level of antibody such as IgA, IgE, IgM, IgG1, IgG2a, IgG2b and IgG3. As shown in Fig. 5(a)–(d), during LPS stimulation, the level of IgM, IgA, IgG2a and IgG2b was not changed but the level of IgE, IgG1 and IgG3 increased. Pre-treatment with LFP-20 could decrease the elevated level of IgE, IgG1 and IgG3 dose dependently in LPS (Fig. 5(a)–(d)). Moreover, the increased ratio of IgG1/IgG2a in LPS, which represented Th2 response, was also blocked in LFP-20 pre-treated mice (Fig. 5(e)). The data demonstrated that LFP-20 regulated B-cell function through Th2 cell response.
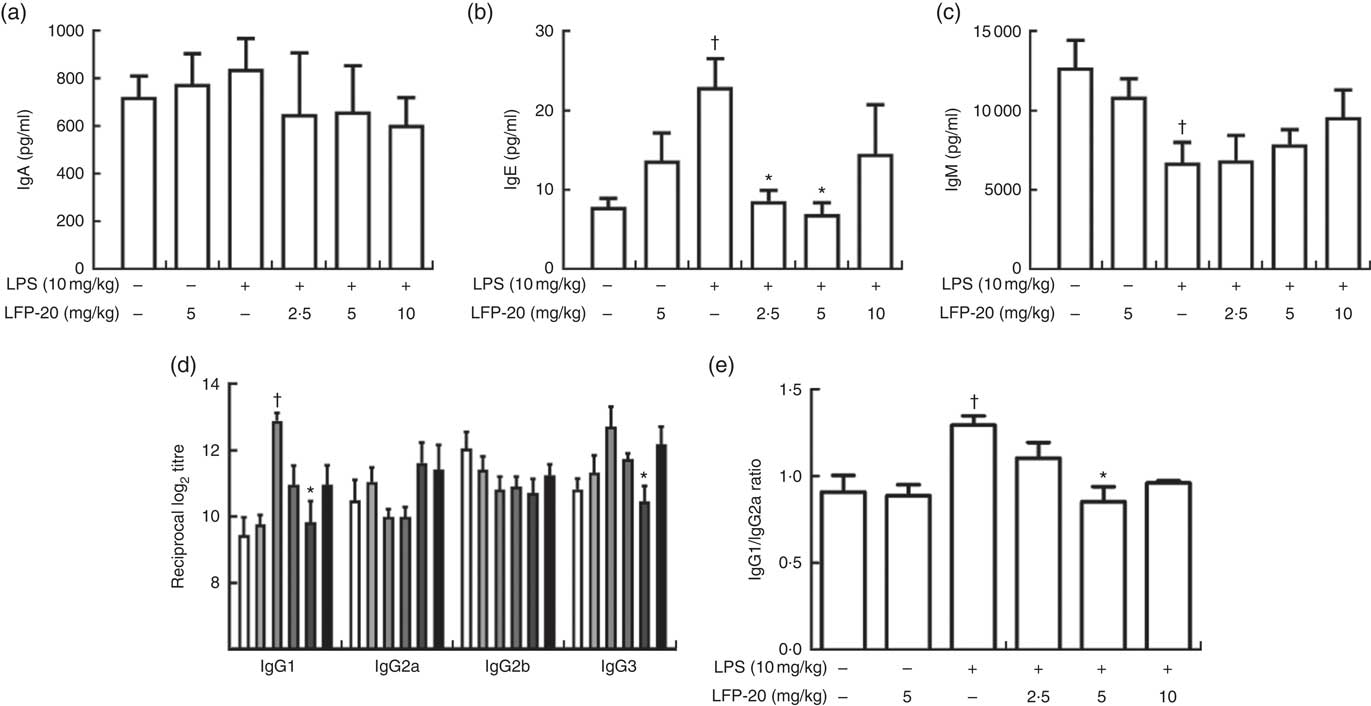
Fig. 5 Effects of LFP-20 on antibody production in lipopolysaccharide (LPS)-stimulated mice. (a) IgA, (b) IgE, (c) IgM, (d) IgG subclasses and (e) the ratio of IgG1/IgG2a. Values are means, with their standard errors represented by vertical bars. *P<0·05 compared with the LPS-stimulated group, n 6, biological replicates. †P<0·05 compared with the respective control within columns. ![]() , Control;
, Control; ![]() , LFP-20;
, LFP-20; ![]() , LPS;
, LPS; ![]() , LPS + LFPL;
, LPS + LFPL; ![]() , LPS + LFPM;
, LPS + LFPM; ![]() , LPS + LFPH.
, LPS + LFPH.
Discussion
LFP-20, an Lf peptide fragment, is the nutritional active factor of Lf. Experiments have emphasised that this proteolysis generates peptide fragments capable of conserving the biological activity such as lactoferricin( Reference Bellamy, Takase and Wakabayashi11 ). The model of LPS endotoxaemia mimics the endotoxin translocation from the intestine to blood circulation.
This study assessed the protective effects of LFP-20 injected intra-peritoneally on inflammation response and intestinal lesions in LPS-stimulated mice. Further, by analysing the distribution and status of immune cells, we explored the cellular not molecular mechanisms.
The gut as a sensory organ could sense the invasion of pathogenic micro-organisms( Reference Furness, Rivera and Cho25 ). Intestinal mucosa is the first layer to defence against pathogens. Macrophages and dendritic cells are largely responsible for the innate immune response in the mucosa( Reference Santaolalla and Abreu26 ). Consistent with the previous studies( Reference Song, Zong and Zhang27 , Reference Han, Zhang and Xia28 ), histopathological analysis revealed LPS stimulation marked atrophy and blunting of the villi, with discontinuous brush borders and disarrayed epithelium. Simultaneously, the infiltration of granulocytes and mononuclear cells into the mucosa was observed, which indicated that LPS stimulation triggered the innate immune response. Pre-treatment with 10 mg/kg LFP-20 reduced the histological evidence of LPS-induced symptom in ileum in a dose-dependent manner. MPO activity is directly proportional to neutrophil concentration in the inflamed tissue and is thus an index of neutrophil infiltration and inflammation( Reference Choudhary, Keshavarzian and Yong29 ). The results indicated that LPS-induced increased MPO activity was also significantly suppressed by LFP-20 pre-treatment. However, MPO is not produced exclusively by neutrophils and is also produced by monocytes and macrophage. In addition, the enzymatic activity of MPO can vary considerably between individuals even if the amount of MPO present is similar( Reference Bustos, Greco and Yapur30 ). To substantiate this finding further, immunohistochemistry with CD68 antibody was conducted to assess infiltration of activated macrophages. As expected, pre-treatment with LFP-20 restrained the infiltration of macrophage in ileum. Collectively, LFP-20 pre-treatment appears to attenuate exaggerated immune response triggered by LPS stimulation.
The observed basal differences in immune cell population located in non-lymphoid organs might result from an altered immune cell repertoire in lymphoid organs and/or immune cell traffic( Reference Pohlmann, Scheu and Ziegler21 ). In line with this thought, flow cytometry analysis of main immune cell subpopulation (T cells, B cells and NK cells) uncovered distinct changes in CD3+CD4+ T cells, CD3+CD8+ T cells, B cells and NK cells counts in peripheral blood during LPS stimulation. In the presence of LFP-20, the mice revealed a reduced CD3+CD4+ T cell and B cell population as well as an elevated amount of CD3+CD8+ T cells and NK cells in the blood, as compared with LPS stimulation alone. To our surprise, the similar immunomodulation effects of LFP-20 on immune cell subpopulation were observed in mesenteric lymph nodes and spleen. This might reduce systemic inflammatory responses as these lymph nodes are drained by antigen presenting cells upon intra-peritoneal LPS treatment to promote the activation of adaptive immune responses by T and B cells.
Mechanistically, lymphocyte are immune effector cells, migrating from peripheral blood to tissues rapidly during infection via chemokine receptors and recognise the pathogens by pathogen recognition receptors( Reference Zhao, Zou and Du31 ). T-cell-derived cytokines bound to B-cell cytokine receptors to promote B-cell proliferation, Ig class switching and somatic hypermutation as well as guide differentiation( Reference Crotty32 ). We found LFP-20 modulated the secretion of both activated Th1-cell-related IL-12p70, IFN-γ, TNF-α and Th2-cell-related IL-4, IL-5, IL-6, which suggested that LFP-20 contributed to a balanced Th1 and Th2 cell response. However, pre-treated with only LFP-20 suppressed LPS stimulation and up-regulated the secretion of antibody such as IgE, IgG1 and IgG3 but did not affect the level of IgM, IgA, IgG2a. Indeed, bovine and human Lf is capable of binding to surface receptors on the human T-cell line( Reference Legrand, van Berkel and Salmon33 , Reference Manzoni, Stolfi and Messner34 ). Lf can promote Th1 responses while inhibiting Th2 responses, and it causes T-cell receptor cross linking, which leads to the inhibition of T-cell activation, reduces the release of inflammatory factors such as IL-5 and IL-17 and further alleviates the degree of inflammation( Reference Wang, Deng and Ren35 ). Consistently, previous studies have shown that orally administered Lf has been demonstrated to increase the pool of CD4+ T cells, the secretion of IgA and IgG in murine mucosa with intestinal secretion( Reference Sfeir, Dubarry and Boyaka36 , Reference Manzoni, Meyer and Stolfi37 ). These data suggested that Lf acted on B cells, which are well-known antigen presenters, to allow for their subsequent interaction with T cells, which favours elevation of the antibody response( Reference Sfeir, Dubarry and Boyaka36 , Reference Siqueiros-Cendon, Arevalo-Gallegos and Iglesias-Figueroa38 , Reference Debbabi, Dubarry and Rautureau39 ). In addition, in a chemotherapy-induced immune suppression murine model, Lf administered intra-peritoneally was able to decrease the suppression of antibody-forming cells and facilitate the restoration of the immune response( Reference Artym, Zimecki and Kuryszko40 ). Collectively, our data demonstrated that LFP-20 regulated B-cell function through Th2 cell response. However, further study is needed to illustrate the signalling pathway of antibody secretion in cytokine-activated B cells.
Altogether, this study provided compelling evidence demonstrating that LFP-20 sustained immune homoeostasis through regulating cytokines and antibody secretion to defend LPS-triggered systemic inflammatory response. The insights obtained from this study further advance our understanding of the nutritional value of Lf and highlight the importance of its peptide in the development of immune response.
Acknowledgements
The authors would like to thank Wang lab members for helpful discussion.
This study was funded by a grant from the National Natural Science Foundation of China, number 3163000269 and a grant from the Modern Agroindustry Technology Research System, number CARS-36.
X. Z. and Y. W conceived the project, designed the experiments. X. Z., H. W. and J. Z. performed the experiments. X. Z wrote the manuscripts. All authors discussed the results and edited the manuscript.
Authors declare that they have no conflict of interest in relation to this study.