INTRODUCTION
In the United States, a number of government agencies are responsible for overseeing the recall of consumer products, from food products to boats and motor vehicles to pesticides. However, fewer agencies, such as the US Department of Agriculture, Food Safety and Inspection Service (FSIS), collect and publicly release recall-specific information on the amount of product recovered following the recall [1]. Data on the amount of recalled product recovered, or the number of product deficiencies corrected, following a recall action can be used to analyse the effectiveness of recalls and examine the consequences of removing unacceptable products from commerce.
FSIS coordinates recalls of meat products in the United States as part of its mission to prevent foodborne illness and ensure the safety of the country's food products regulated by the agency [2]. At the time of a recall, FSIS officials release public notifications through a variety of venues and post notifications and distribution information on the agency's website [1]. Following the recall, the agency additionally posts to its website the amount of recalled product recovered as reported by the recalling establishment.
A 2014 study used FSIS data on the amount of beef products recovered following recall actions due to Shiga toxin-producing Escherichia coli (STEC) O157 to estimate the number of illnesses prevented by the recall [Reference Seys, Sampedro and Hedberg3]. A simulation using a PERT probability distribution estimated that recalls averted 287 reportable STEC O157 illnesses over an 8-year period from 2005 to 2012, which represents an estimated 7500 averted illnesses when accounting for underdiagnosis [Reference Seys, Sampedro and Hedberg3]. Further, the results of a comparison between recalls associated with foodborne illnesses (i.e. illness related) and recalls without notification of illnesses (i.e. non-illness related) suggested that more complete removal of contaminated product from commerce may reduce the risk of illness to consumers [Reference Seys, Sampedro and Hedberg3].
Traceback of food products suspected to be involved in foodborne illnesses is essential for identifying sources and vehicles of transmission, subsequently controlling affected product, and preventing additional illnesses; however, increasing complexity of food supply chains can make product tracing more challenging. FSIS regulatory actions were initiated for 59% of STEC investigation-associated products for which FSIS or state or local public health agencies conducted traceback investigations [Reference Green4]. Integrating results of both the epidemiological investigation and traceback information is critical to identifying the source of illnesses [Reference Sodha5]. In the meat industry, many producers have developed more precise product tracing capabilities due to heightened awareness of the impact of traceability on outbreaks and food safety [Reference Golan6]. However, limitations to effective and efficient traceback investigation of meat products still remain, such as poor record-keeping at retail establishments that grind beef products, which may delay recalls and corrective actions [Reference Gould7].
Rapid detection of foodborne illness clusters by public health surveillance systems is also critical for preventing illnesses. However, resources available at local and state government levels along with staff expertise and interest, substantially impact the variability of jurisdictions’ detection capabilities [Reference Jones8]. In the United States, collaborative efforts such as the Foodborne Diseases Active Surveillance Network (FoodNet) and the Environmental Health Specialists Network (EHS-Net) have built capacity to respond to foodborne diseases in participating sites [Reference Jones, Scallan and Angula9]. However, the majority of jurisdictions in the United States do not receive specific FoodNet or EHS-Net funding and must rely on other local, state, or federal funding sources.
The goal of this study was to assess the factors affecting the amount of product recovered following a recall due to STEC contamination by an FSIS-regulated establishment. STEC O157 and non-O157 together are estimated to cause about 176 000 domestically acquired foodborne illnesses each year [Reference Scallan10]. Consumption of ground beef, and undercooked ground beef in particular, has been identified as a risk factor for illnesses due to STEC O157 and certain non-O157 serogroups [Reference Hadler11–Reference Voetsch16]. Seven serogroups of STEC (i.e. STEC O157, O26, O45, O103, O111, O121, O145) are considered by FSIS to be adulterants in ground beef, and their presence alone will result in a recall action if a positive product has entered commerce [1]. We also explored how the detection of foodborne illness clusters leading to recall actions vary depending on the jurisdictions involved. Our results will lead to a better understanding of the significance of the amount of product recovered following a recall. Furthermore, they will be used to better understand improvements that are needed to more quickly respond to illnesses and more completely remove contaminated product from commerce.
METHODS
Recall data
We obtained FSIS recall data for all STEC recalls carried out by FSIS-regulated establishments from 2000 to 2012. A spreadsheet was edited to contain data fields including the year, FSIS recall number, date of the recall, establishment size, recall class, number of pounds of product recalled, number of pounds of recalled product that was recovered, type of product recalled, and the reason for the recall. Public health alerts, retail-level recalls, and state-initiated recalls were excluded from the spreadsheet. Additionally, recalls not closed at the time of the analyses or with an unknown amount of product recalled were only used in summary statistics but not included in the analyses.
For each of the recalls listed in the spreadsheet, we obtained additional data from recall press releases posted on the FSIS website. The additional data fields included whether the recall was subsequently expanded after the initial recall; the earliest and most recent production dates of recalled product; an estimate of the extent of distribution using the number of states listed in public notifications; whether the product was distributed to hotels, restaurants, or institutions (HRI); whether the recall involved product imported into the United States or exported from the United States; and whether product was fresh, frozen, or both fresh and frozen.
Assessment of recall characteristics
All data were imported into SAS for Windows v. 9.3 (SAS Institute Inc., USA) for data manipulation and analysis. Two new variables were created which represented the percentage of recalled product recovered, the principal outcome variable, as well as the time (i.e. number of days) between the earliest production date of recalled product and the recall date. For nine recalls, the percentage of recalled product reported as recovered by the recalling establishment exceeded 100%. In those instances, we adjusted the amount to reflect a maximum of 100% of product recovered. A P value ≤ 0.05 was considered indicative of statistical significance for all analyses.
To assess the factors associated with product recovery following STEC recalls, we used the Wilcoxon rank-sum (WRS) test to compare the distribution of the percentage of product recovered for several predictor variables, each with two groups. The categorical predictor variables of interest included the size of the establishment (e.g. very small establishments compared against small and large establishments combined), whether the recall included product imported into the United States, whether the recall included product exported from the United States, whether the recalled product was distributed to HRI, whether illnesses were associated with the recall, whether recalled product was fresh or frozen, whether the recall was expanded, and whether the recalled product was distributed on a metro/state (e.g. distributed to a single metropolitan area), regional/sporadic, or national (i.e. distributed to >13 states or reported in the public notifications as national distribution by FSIS) basis. An additional categorical predictor variable was created to better reflect recalled product with a more limited distribution, which was defined as ⩽2 states as reported on the FSIS website.
Specific to an analysis of how the extent of distribution impacted the percent of recalled product recovered, we analysed three levels of distribution for two variables (i.e. limited, regional/sporadic, or national distribution; HRI, both HRI and retail, or retail distribution) using Mantel's test for trend.
Two discrete variables, the number of days between the earliest production date and the recall date and the total pounds of product recalled, were used in two separate approaches. First, they were compared to the categorical predictor variables of interest using the WRS test to specifically examine differences between illness-related vs. non-illness-related STEC recalls. Second, they were dichotomized at the median values for an additional analysis. The variables were evaluated against the percentage of product recovered, also dichotomized using the median value, using Pearson's χ 2 test.
Assessment of foodborne illness detection
To examine the relationship between illness cluster detection and jurisdiction, we created two categorical predictor variables representing whether the state distribution noted in the FSIS public notification included states receiving FoodNet or EHS-Net funding. Additionally, using foodborne outbreak surveillance data from 1998 to 2012 [17–Reference Gould20] we created an additional categorical state tier variable which represented whether product distribution included states that had the highest rates of outbreak reporting during 1998–2012. For recalled product distributed on a nationwide basis, individual states are often not named in the recall public notification. Therefore, for these instances, it was assumed that recalled product was distributed to at least one FoodNet, EHS-Net, and higher outbreak reporting state. These three categorical variables were evaluated against whether STEC recalls were illness related vs. non-illness related using Pearson's χ 2 test.
RESULTS
From 2000 to 2012, a total of 172 recalls by FSIS-regulated establishments occurred due to STEC, principally STEC O157, and were associated predominantly with beef products, with a median of 12 recalls (range 5–22 recalls) each year. An estimated 84 000 000 lb or 38 000 metric tonnes (median 21 000 lb or 10 000 kg per recall) of beef, pork, and bison products were recalled. An estimated 27 000 000 lb or 12 000 metric tonnes (median 2000 lb or 900 kg per recall) of recalled product were recovered. Overall, for the 13-year study period, about 33% of product recalled due to STEC was reported as recovered.
Evaluating all STEC recalls from 2000 to 2012, we excluded one because it had an undetermined amount of product recalled. A total of 171 STEC recalls were included in the analyses. Figure 1 depicts the percentage of product recovered for both the STEC-related and all other recalls. The extreme categories of 0–10% and 90–100% of recalled product recovered accounted for >50% of the STEC recalls.

Fig. 1. Percent of recalled product recovered, Shiga toxin-producing Escherichia coli vs. all other recalls, 2000–2012.
Assessment of recall characteristics
Lower amounts of product being recalled were associated with products distributed on a metro/state basis (WRS test, P < 0·001), on a more limited basis (WRS test, P < 0·001), and exclusively to HRI (WRS test, P < 0·001). Recalls of products distributed nationally (WRS test, P < 0·001) and exclusively to retail (WRS test, P = 0·003) were associated with higher amounts of product recalled.
A higher percentage of recalled product recovered by the establishment was associated with product distributed exclusively to HRI and conversely, a lower percentage with product distributed exclusively to retail facilities (Table 1). Progressively less recalled product was recovered from exclusive HRI to combined HRI and retail to exclusive retail distribution (Table 2). Recalled products distributed on a more limited basis positively affected the percentage of product recovered following a recall due to STEC compared to nationwide distribution (Table 1). Further, recalled products distributed on a metro/state basis positively affected the percentage of product recovered (WRS test, P = 0·06). There were statistically significant trends in the amount of recalled product recovered and limited, regional/sporadic, and national distribution (Table 2). There were no statistically significant associations found with recalled product recovered in the STEC recall analyses for very small (WRS test, P = 0.1), small (WRS test, P = 0.4), or large (WRS test, P = 0.5) establishment size, involvement of imported product (WRS test, P = 0.7), fresh (WRS test, P = 0.4) or frozen (WRS test, P = 0.9) product, international distribution (WRS test, P = 0.2), or recall expansions (WRS test, P = 0.5).
Table 1. Characteristics associated with recovery of recalled products for Shiga toxin-producing Escherichia coli recalls, categorical predictor variables, 2000–2012

HRI, Hotel/restaurant/institution.
* Product distribution: Limited = distribution to ⩽2 states; National = ‘national distribution’ in recall press releases or distribution to >13 states.
Table 2. Trends associated with recovery of recalled products for Shiga toxin-producing Escherichia coli, 2000–2012
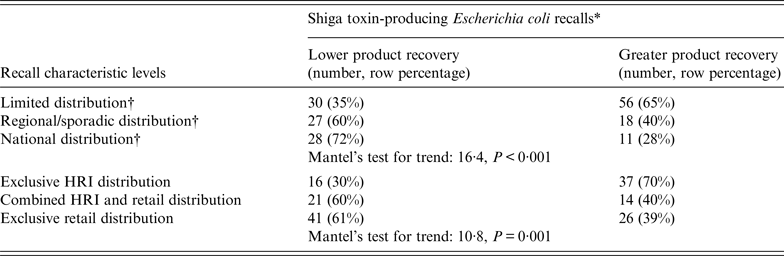
HRI, Hotel/restaurant/institution.
* Product recovery dichotomized at the median value (18%).
† Product distribution: Limited = distribution to ⩽2 states; Regional/sporadic = distribution to >2 but <14 states; National = ‘national distribution’ in recall press releases or distribution to >13 states.
A greater number of days between the earliest production date of recalled product and the recall date was associated with decreased amounts of product recovered for STEC recalls (χ 2 = 27·9, P < 0·001). Additionally, there was an inverse relationship between the amount of product recalled and the percentage of product recovered (χ 2 = 15·2, P < 0·001).
Illness-related recall analysis
Illness-related recalls were associated with an increased recall size (WRS test, P < 0·001), a greater number of days between the earliest production date of recalled product and the recall date (WRS test, P < 0·001), and decreased percentage of product recovered (Table 1). Illness-related STEC recalls were less likely than non-illness-related STEC recalls to be associated with exclusive HRI distribution (χ 2 = 5·6, P = 0·02) and distribution on a metro/state (χ 2 = 12·8, P < 0·001) and more limited (χ 2 = 23·8, P < 0·001) basis. Illness-related STEC recalls were more likely than non-illness-related STEC recalls to be associated with nationwide distribution (χ 2 = 20·7, P < 0·001).
Assessment of foodborne illness detection
FoodNet states were more likely than non-FoodNet states to be listed for recalled product distribution for illness-related STEC recalls compared to non-illness-related STEC recalls as were EHS-Net sites (Table 3). Using the tiered approach a similar trend was observed: states that were never listed in the lowest tier of foodborne outbreak reporting rates from 1998 to 2012 were more likely to be listed for recalled product distribution for illness-related STEC recalls (Table 3).
Table 3. Assessment of foodborne illness detection for Shiga toxin-producing Escherichia coli recalls, 2000–2012

FoodNet, Foodborne Diseases Active Surveillance Network; EHS-Net, Environmental Health Specialists Network; Higher tier states, states that were never represented in the lowest tier of outbreak reporting rates of foodborne outbreak surveillance summaries from 1998 to 2012.
DISCUSSION
Our analyses suggest that the complexity of product distribution is a core predictor of the amount of recalled product recovered following an STEC recall by an FSIS-regulated establishment. HRI exclusive, metro/state, and more limited distribution patterns were all associated with higher recovery of recalled product. Each of these scenarios would probably have a more straightforward distribution chain. This would make product recovery more rapid and more complete than retail and national distribution, which were associated with a lower recovery of recalled product.
The association between product recovery and the number of days from the earliest production date for contaminated product to the recall action is noteworthy. Delays in initiating a recall would correspond with more product being consumed and product being distributed further through the supply chain, both of which would correspond to less recalled product being recovered. In this regard, complexity of distribution and product recovery may increase the likelihood of contaminated product consumption leading to the occurrence of an outbreak. In order to maximize recall effectiveness, and prevention of foodborne illnesses, robust systems need to be in place to facilitate early detection of foodborne illness clusters as well as a timely and successful response. To help accomplish this, the multidisciplinary Council to Improve Foodborne Outbreak Response (CIFOR) has created foodborne outbreak detection and investigation guidelines for government agencies, including performance measures for public health agencies [21].
The association between illness-related recalls and a smaller amount of recovered products is likely to have resulted from a number of factors. The majority of non-illness, pathogen-related recalls in the dataset are due to positive results of FSIS or establishment pathogen-testing programmes at the establishment. This type of recall tends to be more timely and therefore product would not be as thoroughly distributed, have a more straightforward recall scope, and generally a smaller quantity compared to illness-related pathogen recalls. Additionally, traceback is more difficult when product has been more thoroughly dispersed into the food chain, especially when enough product has been disseminated to result in a cluster of illnesses, vs. reacting to a positive pathogen test from an establishment. In 2013, FSIS required establishments to begin controlling products tested by FSIS for adulterants, such as STEC O157, until all test results are received [22]. This action will lead to more contaminated products being withheld from commerce, and thus reduce the total number of product recalls. It is expected that a greater percentage of future recalls will be generated as a result of illness investigations.
Our results suggest that improvements within the entire system, from detection to investigation to recall, are needed to limit the amount of contaminated product reaching consumers. The association between FoodNet, EHS-Net, and higher outbreak-reporting states and illness-related STEC recalls suggests that improvements in detection of foodborne illness clusters, and additional resources for local and state public health agencies to investigate these clusters, are needed. This finding is supported by previous studies which have found that involvement of FoodNet states in a foodborne illness investigation was associated with regulatory action [Reference Green4] and that surveillance can be linked to public health outcomes [Reference Scharff23]. As for illness-related recalls, more efficient foodborne illness investigations would lead to a more rapid recall action, reducing the amount of time between production and recall dates. Agile investigations encompassing coordinated, rapid, and innovative product tracing are needed [Reference Sodha5, Reference Bhat, Hickey and McEntire24–Reference Shah27] along with investments in resources, training, and expertise.
To improve our ability to evaluate the public health impact of recalls, improvements in data quality are needed. The amount of product recovered is reported from the recalling establishment to FSIS. Although a certain amount of uncertainty is inherent, more accurate and complete reporting is needed. While not directly addressed in our analysis, we noted that for a number of recalls, the amount of product recovered was greater than the amount of product recalled. A mechanism is needed for scrutinizing these occurrences more closely, collecting descriptive data, and indicating why the discrepancy occurred (e.g. commingling of both recalled and non-recalled products). Additionally, we would recommend recall case studies with the goal of better understanding the quantities reports and refining the analyses. To assess early detection and surveillance, we were limited to using the states initially highlighted in FSIS public notifications available online. These states could consist only of known distributors and may not represent the entire distribution chain of recalled product. Preserving the full distribution by state once it has been uncovered would be beneficial for future studies of this nature. More systematic and complete collection and maintenance of descriptive recall data are also needed. Additionally, we hypothesize that recalled products, especially ground meat products, produced at the establishment as case-ready products (e.g. in the final consumer package) would have a higher percentage of product recovered compared to products that are subsequently re-processed or relabelled. Those data are currently not readily available.
Recent changes in the production and regulation of ground beef products should reduce public exposure to contaminated product. However, reducing risk is not the same as eliminating risk. A strong system of public health surveillance will become increasingly important for the rapid identification and recall of contaminated products in commerce.
ACKNOWLEDGEMENTS
The authors thank Bonnie Kissler, Kis Robertson, Kirk Smith, and Lisa Volk for sharing their valuable knowledge and expertise.
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
DECLARATION OF INTEREST
None.







