INTRODUCTION
Toxoplasmosis is a common zoonotic infection caused by the Apicomplexan protozoan, Toxoplasma gondii. Cats, the definitive parasite host, are infected when they consume animal tissue containing T. gondii tissue cysts [Reference Frenkel, Dubey and Miller1]. T. gondii sexual reproduction with oocyst formation, which only occurs in the felid intestinal tract, results in the excretion of large numbers of disinfection-resistant oocysts into the environment [Reference Frenkel, Dubey and Miller1–Reference Dubey4]. Intermediate hosts, including humans, livestock, and other mammals, are infected by ingestion of sporulated oocysts, by ingestion of meat containing tissue cysts, or by congenital transmission [Reference Tenter, Heckeroth and Weiss3, Reference Montoya and Liesenfeld5, Reference Hill and Dubey6]. In the intermediate host the tachyzoite stage is able to invade a wide variety of nucleated cell types where it forms a vacuole, replicates, and escapes by cell disruption [Reference Montoya and Liesenfeld5]. Completing the cycle, tissue cysts containing bradyzoites frequently develop in muscle cells and in central nervous system cells where they remain for the lifetime of the host [Reference Montoya and Liesenfeld5].
Among immunocompetent humans, postnatally acquired infection with T. gondii is generally asymptomatic or results in a mild illness with non-specific symptoms such as lymphadenopathy and fever [Reference Tenter, Heckeroth and Weiss3, Reference Hill and Dubey6, Reference Weiss and Dubey7]. Sequelae involving ocular lesions are known to occur with some frequency [Reference Tenter, Heckeroth and Weiss3, Reference Hill and Dubey6–Reference Jones and Holland8]. In contrast, severely debilitating and sometimes fatal cases of toxoplasmosis can occur when a latent infection is reactivated in an individual due to the loss of immune system function (HIV infection or chemotherapy) or when a newly infected, pregnant woman transmits the parasite to her child in utero (congenital toxoplasmosis) [Reference Tenter, Heckeroth and Weiss3, Reference Montoya and Liesenfeld5, Reference Weiss and Dubey7, Reference Lopez9]. Because of the potentially devastating consequences to the child, considerable resources have been devoted to preventing congenital toxoplasmosis by (1) identifying pregnant women who are newly infected using immunoglobulin (Ig) M assays and IgG antibody avidity tests or (2) identifying women who are at risk for primary infection during pregnancy with an IgG antibody detection assay [Reference Lopez9, Reference Torgerson and Mastroiacovo10].
Approximately 1–2 weeks after primary infection with T. gondii, a parasite-specific IgG antibody response develops that is believed to remain elevated for life [Reference Montoya and Liesenfeld5]. IgG antibody seroprevalence can, therefore, be used as a marker for infection prevalence in population surveys. Numerous studies and reviews have established that Toxoplasma seroprevalence increases with age, is higher in parts of Central America, South America, and West Africa, and is often lower in colder climates [Reference Tenter, Heckeroth and Weiss3, Reference Pappas, Roussos and Falagas11]. In the developing world, seroprevalence values as high as 75% in women of childbearing age are not uncommon [Reference Tenter, Heckeroth and Weiss3, Reference Pappas, Roussos and Falagas11]. In contrast, seroprevalence values in the USA and in some parts of Western Europe are generally <50% and have been in decline over the past 10–15 years [Reference Tenter, Heckeroth and Weiss3, Reference Pappas, Roussos and Falagas11–Reference Chatterton14]. The current estimate for US women of childbearing age, 11%, implies that most women are at risk of primary infection during pregnancy [Reference Jones13]. In fact, prevalence in US-born residents was observed to increase in a linear fashion through the fifth decade of life (40–49 years of age) [Reference Jones13].
To date, most of the published Toxoplasma seroprevalence surveys have been accomplished using a single, Toxoplasma-specific assay, most often a commercial or an in-house ELISA, agglutination, or immunofluorescent antibody assay (reviewed in [Reference Tenter, Heckeroth and Weiss3]). The advent of the multiplex bead assay (MBA) has made possible the simultaneous detection of multiple pathogen-specific antibody responses, including those against Toxoplasma. For example, Binnicker et al. [Reference Binnicker, Jespersen and Harring15] reported the use of a commercial kit that detects T. gondii-, rubella virus-, and cytomegalovirus-specific IgG antibodies in serum, and Griffin et al. [Reference Griffin16] reported a multiplex oral fluid assay for IgG antibodies to T. gondii, Helicobacter pylori, norovirus, and Cryptosporidium. While the T. gondii target antigen in the commercial assay kit was not defined, the oral fluid assay used both a crude parasite antigen preparation and a commercially prepared, recombinant form of the immunodominant SAG1 (p30) protein [Reference Griffin16–Reference Kasper, Crabb and Pfefferkorn18]. If both sensitive and specific, an assay based on a recombinant antigen would be preferable to one that uses a crude parasite preparation because of the expense, biohazards, and potential variability issues associated with parasite growth in culture [Reference Aubert19]. The SAG1 antigen, however, is not an ideal candidate because antibody recognition is conformation dependent, and properly folded antigen is somewhat difficult to express in quantity [Reference Burg17, Reference Kim20].
An alternative immunodominant T. gondii antigen that can be easily expressed in bacterial cultures as a glutathione-S-transferase (GST) fusion protein is the SAG2A (p22) protein [Reference Prince21, Reference Parmley22]. IgG ELISAs using recombinant SAG2A were recently shown to be sensitive (95%) and specific (100%) compared to the crude Toxoplasma antigen ELISA [Reference Santana23, Reference Bela24]. In this work, we demonstrate that a bead coupled with recombinant SAG2A protein can be incorporated into our neglected tropical disease (NTD) MBA panel and used to determine Toxoplasma seroprevalence. We use the incident seroconversions observed in a longitudinal cohort study to estimate the Toxoplasma seroconversion rate in Haitian children and compare that rate to a reverse catalytic model rate estimate from a community-wide seroprevalence survey. Integration of seroprevalence surveys for Toxoplasma into NTD treatment and elimination surveys would allow for better estimates of the potential burden of congenital toxoplasmosis in underserved regions of the world and might provide additional insights into the relative contributions of various transmission pathways to human infection.
MATERIALS AND METHODS
Study location and sample collection
The characteristics of the Haitian study populations as well as the methods of sample collection have been described previously [Reference Lammie25–Reference Priest28]. Study participants resided in a coastal region of Haiti (Leogane Commune) where intense transmission of lymphatic filariasis (LF) was occurring. Beginning in 1990, a longitudinal birth and sibling cohort was enrolled in a study of the impact of maternal infection status on the transmission of LF to children. The children in the subset used in the current study (n = 142) were enrolled between 0 and 6·8 years of age (median 1·4 years) and followed for an average of 5·1 years (median 4·7 years, range 0·5–9·1 years). They donated a total of 771 samples (median 5 samples/child; range 2–9 samples/child). In 1998 residents from a nearby community with ages between 0 and 90 years were enrolled in a study of community-wide LF treatment through the use of diethylcarbamazine (DEC)-fortified salt. Of 441 samples originally collected, 383 remained in sufficient volume for testing in the current study. Both the Institutional Review Board at the Centers for Disease Control and Prevention and the Ethical Committee of L'Hôpital St Croix in Leogane, Haiti, reviewed and approved the study protocols. Study participants gave consent for additional infectious disease testing at a later date.
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Cloning and expression of T. gondii SAG2A antigen
The T. gondii SAG2A antigen coding sequence (GenBank M33572) lacking the 26 residue amino-terminal signal peptide and the 14 residue carboxy-terminal anchor signal [Reference Prince21, Reference Parmley22] was PCR amplified from RH strain genomic DNA (kindly provided by N. Lang-Unnash, University of Alabama at Birmingham, Birmingham, AL) using the following forward and reverse deoxyoligonucleotide primers: 5′-CGC GGA TCC TCC ACC ACC GAG ACG CCA GCG-3′ and 5′-GCG GAA TTC TTA CTT GCC CGT GAG AGA CAC AGG G-3′, respectively. In these sequences, the restriction sites used for cloning are underlined, and the reverse primer included an in-frame stop codon (in italics). Protocols for the PCR amplification of the target sequence using AmpliTaq gold DNA polymerase (Perkin-Elmer Cetus, USA), for directional cloning of the PCR product into the BamHI and EcoRI sites of pGEX 4 T-2 vector (GE Healthcare, USA), for expression of the recombinant GST fusion protein in E. coli BL21 cells (Stratagene, USA), and for the initial purification of the recombinant protein on a 10 ml glutathione Sepharose 4B affinity column (GE Healthcare) have previously been reported [Reference Priest29, Reference Priest30]. Eluted protein was dialysed overnight at 4°C against 300 volumes of 25 mm Tris buffer (pH 7·5) using Spectra/Por 3 dialysis membrane (3500 Da cut-off; Spectrum Laboratories, USA). Final purification was accomplished on a Mono-Q HR 5/5 strong anion exchange column (GE Healthcare) with 25 mm Tris buffer (pH 7·5) and a 20 ml linear gradient from 0 to 0·4 m NaCl. The flow rate was 1 ml/min, and 1 ml fractions were collected. A total of 3·6 mg protein was collected in two 1-ml fractions at ~0·25 m NaCl in the gradient profile. The protein was dialysed against 500 volumes of buffer containing 0·85% NaCl and 10 mm Na2HPO4 (pH 7·2) (PBS) overnight at 4°C (Spectra/Por 3) in preparation for coupling to beads. The recombinant GST/SAG2A fusion protein (rSAG2A/GST) was used in all multiplex assays. Control Schistosoma japonicum GST protein with no fusion partner was expressed and purified as described previously [Reference Priest30]. Protein concentrations were measured with the BCA microassay (Pierce, USA).
Antigen coupling and multiplex bead assays
For multiplex assays of the longitudinal study samples, SeroMap beads (Luminex Corporation, USA) were coupled in PBS buffer (pH 7·2) according to the previously published protocol using 120 μg rSAG2A/GST or GST control protein for 12·5 × 106 beads [Reference Priest30, Reference Moss31]. For assays of the cross-sectional study samples, 12·5 × 106 SeroMap beads (Luminex) were coupled with either 12·5 μg rSAG2A/GST protein or 20 μg GST control protein in buffer containing 50 mm 2-(N-morpholino)-ethanesulfonic acid (MES) and 0·85% NaCl (pH 5·0). For both proteins, the amount required for coupling at pH 5·0 was empirically determined within the range of 10–40 μg in order to optimize peak signal intensity and maintain low backgrounds. Others have demonstrated that less protein is required for efficient bead coupling when a lower pH buffer is used [Reference Griffin16].
The rSAG2A/GST- and GST-coupled beads were included among the 28 beads used in a total IgG antibody multiplex assay of the longitudinal sample set. The assay protocol and results for the LF-, Giardia-, Cryptosporidium-, and malaria-specific markers have been described previously [Reference Hamlin27, Reference Arnold32, Reference Moss33]. rSAG2A/GST- and GST-coupled beads were also included among 16 beads used in a total IgG antibody multiplex assay of the cross-sectional sample set. The assay protocol and results for a malaria-specific marker in the cross-sectional sample have been described previously [Reference Arnold32]. All samples were assayed in duplicate at a dilution of 1:400 in PBS buffer that included 0·8% polyvinylpyrrolidone, 0·5% polyvinyl alcohol, 0·05% Tween-20, 0·5% BSA, 0·02% sodium azide, and 3 μg/ml E. coli extract [Reference Moss31, Reference Waterboer, Sehr and Pawlita34]. After blank subtraction, an average value was calculated from the two median fluorescent intensities for each sample minus background (MFI-bg). Cut-off values and assay performance were determined with the aid of a CDC-defined panel of 100 well-characterized sera [Sabin-Feldman dye test (Palo Alto Research Institute, USA) and IgG immunofluorescence assay (CDC, USA)] that included both confirmed negative samples and positive samples with varying levels of IgG reactivity [Reference Lopez9]. Test sample values falling between the highest confirmed negative and the lowest confirmed positive were treated as indeterminate responses. Values >323 and >797 were considered to be positive for Toxoplasma-specific IgG antibodies for pH 7·2 and pH 5·0 coupled beads, respectively.
Statistical analysis
An alpha level of 0·05 was set for tests of statistical significance. Statistical comparisons were performed using SigmaStat for Windows, version 2.03.0 (SPSS Inc., USA). All statistical modeling was conducted in R version 3.0.1 (www.r-project.org). A Kaplan–Meier model cumulative incidence curve was generated using the R survival analysis package [Reference Therneau and Grambsch35]. Indeterminate responses were not considered in the survival analysis and maternal responses were treated as negatives. We estimated the seroconversion rate in the longitudinal cohort using the number of seroconversions divided by the person-time at risk. When calculating person-time, we treated individuals in the cohort as either interval censored (in the case of seroconversions) or as right-censored at the last point of observation (in the case of persistent negatives); we assumed that interval censored individuals seroconverted at the midpoint between the last age they were classified as negative and the first age they were seropositive. Children who were seropositive at their first measurement were excluded from the analysis. We estimated the rate over the entire 11-year period and for each year of age (we classified children aged 9–11 years in the same group due to sparse data in the older age range). We calculated 95% confidence intervals for the seroconversion rate estimates by bootstrap resampling individuals with replacement with 1000 iterations, and used the 2·5% and 97·5% of the bootstrap distribution as the confidence interval.
We also fitted parametric models to the longitudinal data and the cross-sectional data to estimate seroconversion rates (also called force of infection). Estimating seroconversion rates over different age periods is equivalent to assuming a catalytic model with a constant rate; it is also equivalent to assuming an exponential survival model in which the probability of seroconversion by age t is: P(t) = 1 – exp(–λ*t), and the rate, λ, is constant (or constant within an age category for piecewise exponential models) [Reference Muench36, Reference Hens37]. While a constant rate assumption may be reasonable at young ages, T. gondii seroconversion rates are thought to be higher at young ages and then decline at older ages in low- and middle-income countries [Reference Jones38, Reference Fernandes39]. For this reason, we also fit a more complex, damped exponential linear catalytic model that allowed the underlying seroconversion rate (also called force of infection) to vary by age in the cross-sectional survey data that included individuals aged 0–90 years [Reference Fernandes39, Reference Farrington40]. We used a parametric bootstrap method to calculate 95% confidence intervals for model-based estimates of the force of infection [Reference Wasserman41]. We fitted all models using maximum likelihood assuming a binomial error structure.
RESULTS
MBA performance
From an analysis of the CDC Toxoplasma reference serum set, the sensitivity and specificity of the MBA for Toxoplasma-specific IgG antibodies were both determined to be 100% regardless of the pH used for bead coupling (data not shown). Consistent with the theory of more efficient coupling at lower pH, the median value of the SAG2A-specific IgG antibody responses from the CDC serum set was significantly higher for beads coupled at pH 5·0 (7365) than for beads coupled at pH 7·2 (2308) (Wilcoxon signed rank test, P < 0·001), and the indeterminate range was wider for beads coupled at pH 5·0 (152–797 MFI-bg) than for beads coupled at pH 7·2 (125–323 MFI-bg). However, antibody responses determined using the two bead sets were correlated with a Spearman's rank order correlation coefficient of 0·963.
A subset of the CDC samples (n = 8) having a wide range of response values (66– 30 411MFI-bg) was used to demonstrate that responses remained stable regardless of the number of different bead classifications included in the assay. SAG2A-specific IgG values obtained in the 28-plex assay format were not significantly different from values obtained when only the rSAG2A/GST and GST beads were used in an assay (Wilcoxon signed rank test, P = 0·742; Pearson correlation coefficient 0·998) (data not shown). We also saw no evidence of antibody cross-reactivity between the rSAG2A/GST beads and GST-only control beads regardless of the pH used for coupling. IgG antibody responses to the GST-only control beads in samples from the longitudinal serum set were consistently low (median 13, range 0–202) and reached the Toxoplasma indeterminate range for only three samples (0·4%) (data not shown). One of these three samples was negative for antibodies to the rSAG2A/GST bead while the other two had positive responses in excess of 25 000 MFI-bg units. In the cross-sectional serum study with beads coupled at pH 5·0, IgG responses to the GST-only control bead were higher (median 32, range 0–5053) with 21 values in the indeterminate range (5·4%) (data not shown). Three samples (0·8%) had GST-only responses above the cut-off for Toxoplasma antibody positivity (1006, 2145, and 5053 MFI-bg), but the rSAG2A/GST responses for these three samples were either negative (n = 1) or indeterminate (n = 2).
Toxoplasma-specific IgG responses in the longitudinal cohort children
The characteristics of the children enrolled in the longitudinal Haitian cohort have been described previously [Reference Hamlin27]. All of the indeterminate (n = 4) and positive (n = 4) responses detected in children aged <8 months were a result of maternal IgG antibody transfer: the children were antibody negative at one or more follow-up time points and many demonstrated similar patterns of reactivity with other markers such as the Cryptosporidium parvum P2 antigen [Reference Priest28] (data not shown). In subsequent analyses, these maternal responses were treated as negatives. The nine children aged >1 year who were antibody positive at enrolment (median age 3·8 years, range 1·4–5·1 years) were significantly older than children who were negative at enrolment (n = 133, median 1·3 years, range 0–6·8 years) (Mann–Whitney rank sum test, P < 0·001). At the end of their participation in the study 59 children were positive and 83 children remained negative for Toxoplasma-specific antibodies.
Example anti-SAG2A IgG antibody profiles for six of the study participants are shown in Figure 1. In most instances, children who were positive at enrolment or who seroconverted during the study maintained high levels of antibodies (>10 000 MFI-bg units) throughout the follow-up period. However, as exemplified by two profiles in Figure 1 (solid circle and open triangle), 9/59 (15·3%) antibody-positive children demonstrated a slow decline in antibody response over the course of the follow-up period, and the peak antibody responses of some children (Fig. 1, solid circle) appeared weak in comparison. However, as would be expected with a parasite that establishes a lifelong infection, no instances of reversion to seronegative status were observed in the study participants (median duration of post-infection follow-up 2·7 years, range 0–7·9 years).

Fig. 1. Multiplex bead assay (MBA) detection of Toxoplasma-specific antibodies in serum from Haitian children. Antibody responses to the Toxoplasma SAG2A antigen were determined by MBA as previously described. Responses are plotted vs. age for six children enrolled in the longitudinal study who seroconverted during follow-up.
The age-specific SAG2A antibody response distributions for all of the samples collected during the longitudinal cohort study (n = 771) are shown in Figure 2. Responses in children aged <3 years were generally low, and median antibody responses increased with age (Fig. 2). Because some children had more than one sample collected in a given year of life (e.g. at 6·02 and 6·7 years), only the first sample from each year was used to calculate the age-specific seroprevalence values shown in Table 1. Of the five indeterminate responses observed in children aged >1 year (Table 1), three were followed by a strong positive response, and two represented the last sample collected from the child (data not shown).

Fig. 2. Age-specific SAG2A antibody response distributions for children enrolled in a longitudinal study in Haiti. Antibody values determined by multiplex bead assay are plotted vs. the age at the time of sample donation for all of the samples assayed from the longitudinal study (n = 771). Boxes include values between the 25th and 75th percentiles, whiskers include values between the 10th and 90th percentile, and outliers are indicated by data points. The median values are indicated within the box by a line.
Table 1. Age-specific Toxoplasma SAG2A IgG seroprevalence values for children enrolled in a Haitian longitudinal cohort study 1990–1999
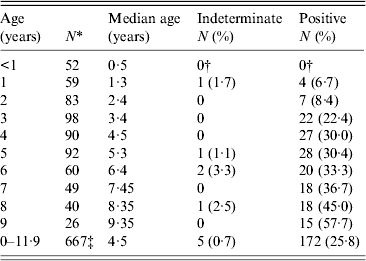
* Only the first sample collected in each year of life was included in the age-specific prevalence calculation. Results from 104 of the 771 samples were, therefore, excluded.
† Four indeterminate and four positive responses observed in children aged <1 year were determined to be of maternal origin and were treated as negatives.
‡ Includes 12 children who were aged 10 years and six children who were aged 11 years.
Toxoplasma-specific IgG responses in the cross-sectional population
As described previously, donors enrolled in a community-wide, DEC-fortified salt treatment campaign for LF ranged in age from 0 to 90 years [Reference Freeman26, Reference Priest28], and 383 samples remained in sufficient volume for analysis in the current study. The age-specific SAG2A antibody response distributions for all of the samples assayed during the cross-sectional study are shown in Figure 3. Only one (9·1%) of 11 children aged <1 year was found to have a detectable response to the SAG2A antigen, and this response fell within the indeterminate range (data not shown). Because follow-up samples were unavailable, we were unable to determine if this weak response was a result of maternal antibody transfer or was early evidence of an acute infection. Females (n = 224) outnumbered males (n = 155) in the study and were significantly older (median age 16 years vs. 12 years, respectively; Mann–Whitney rank sum test, P = 0·002), but median antibody responses were not significantly different between the sexes (21 MFI-bg units vs. 21 MFI-bg units, respectively; Mann–Whitney rank sum test, P = 0·672).
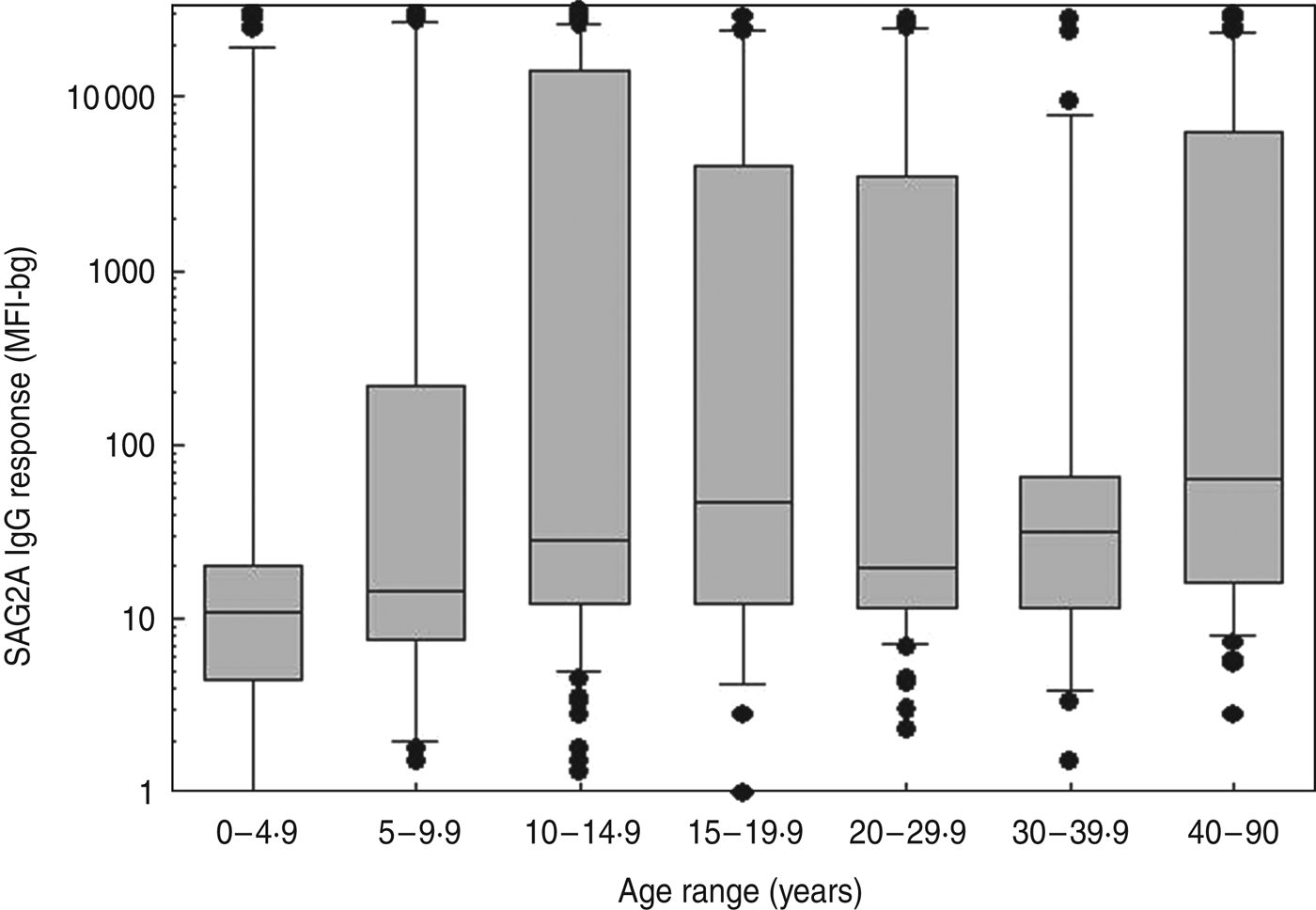
Fig. 3. Age-specific SAG2A antibody response distributions for residents of a community in Haiti. Antibody values determined by multiplex bead assay are plotted vs. the age at the time of sample donation for all of the samples assayed from the cross-sectional community study (n = 383). Boxes include values between the 25th and 75th percentiles, whiskers include values between the 10th and 90th percentile, and outliers are indicated by data points. The median values are indicated within the box by a line.
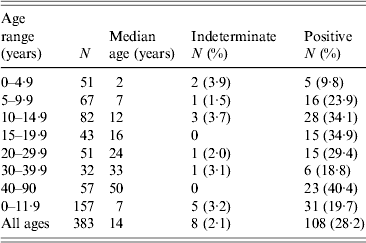
The age-specific seroprevalence values for the cross-sectional study are shown in Table 2. Seroprevalence peaked at 34·9% between ages 15 and 19·9 years and declined in the next two age categories. The increase in seroprevalence values observed in the youngest three age categories in Table 2 was statistically significant (χ 2 test, P = 0·007), but changes in seroprevalence after age 10 years were not significant (χ 2 test, P = 0·313). Children aged <12 years who were enrolled in the cross-sectional study (Table 2) had a significantly higher median age than children enrolled in the longitudinal study (7 years vs. 4·5 years, respectively; Mann–Whitney rank sum test, P < 0·001), but their seroprevalence values were not significantly different (19·7% vs. 25·8%, respectively; χ 2 test with Yates correction, P = 0·139). A direct comparison of anti-SAG2A antibody response values from participants in the cross-sectional study to values from participants of the longitudinal study was not possible because the assays were conducted with beads coupled under different conditions.
Table 2. Age-specific Toxoplasma SAG2A IgG seroprevalence values for residents enrolled in a Haitian community-wide, cross-sectional study, 1998
Seroconversion rate estimates and survival curve
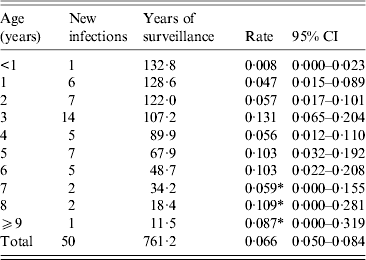
Serum donation intervals (n = 481) ending with an antibody response value in the negative range (<125 MFI-bg units) and interval-censored values from children who seroconverted accounted for 761·2 child-years of follow-up in the longitudinal study (Table 3). There were 50 incident seroconversions among the 133 children who were negative for SAG2A-specific antibodies at enrolment, and the average incidence rate was 0·066 infections/year at risk (95% CI 0·050–0·084). However, as shown in Table 3, the incidence rate was not constant with age over the first 11 years: children aged <2 years had a lower risk of infection per year than did children aged ⩾2 years and 3-year-olds experienced the peak rate of 0·131 infections/year. Rate estimates for older children aged >6 years should be interpreted with caution because of the limited years of surveillance. Figure 4 shows the Kaplan–Meier cumulative incidence curve for children enrolled in the longitudinal study. The median time to T. gondii seroconversion was estimated to be 9·7 years (95% CI 7·6–∞). A piecewise exponential model that allowed rates to vary by year corresponding to estimates in Table 3 (Fig. 4, open triangles) provided a better fit of the data compared to a model with a single rate over the entire 11-year period (Fig. 4, exponential model) (likelihood ratio test, P < 0·001).

Fig. 4. Toxoplasma cumulative incidence curve and model predictions for children enrolled in a longitudinal study in Haiti. The Kaplan–Meier cumulative incidence curve for SAG2A-specific IgG antibody responses vs. age (–––) as well as the upper and lower 95% confidence intervals (- - -) were determined from censored data as described in Materials and Methods section. The median time to seroconversion was estimated to be 9·7 years (95% confidence interval 7·6–∞). Only children who were negative at study enrolment (n = 133) were considered in the analysis. Indeterminate responses were dropped and maternally derived responses were treated as negative. The curve generated using the single rate exponential model (– - – -) and the points generated using the piecewise exponential model with age-specific incidence rates (△) are shown.
Table 3. Numbers of Toxoplasma infections and infection rates by year of life in a subset of the longitudinal cohort children (N = 133) who were antibody negative upon enrolment
CI, Confidence interval.
* Limited years of surveillance suggest a cautious interpretation of rates.
The seroconversion rate estimated with a model from current status seroprevalence data in the cross-sectional study was lower overall compared to the rates estimated in the longitudinal cohort. Based on the exponentially damped linear catalytic model [Reference Fernandes39], the average seroconversion rate for ages 0–11 years was 0·034 infections/year (95% CI 0·027–0·041), and it was highest at age 2·6 years (0·057 infections/year, 95% CI 0·033–0·080) (Fig. 5). Because seroprevalence increased approximately linearly over ages 0–11 years in the cross-sectional study, a simple model that assumed a constant rate over the age range generated a comparable rate estimate to the more complex model (0·032 infections/year, 95% CI 0·022–0·045) for ages 0–11 years.
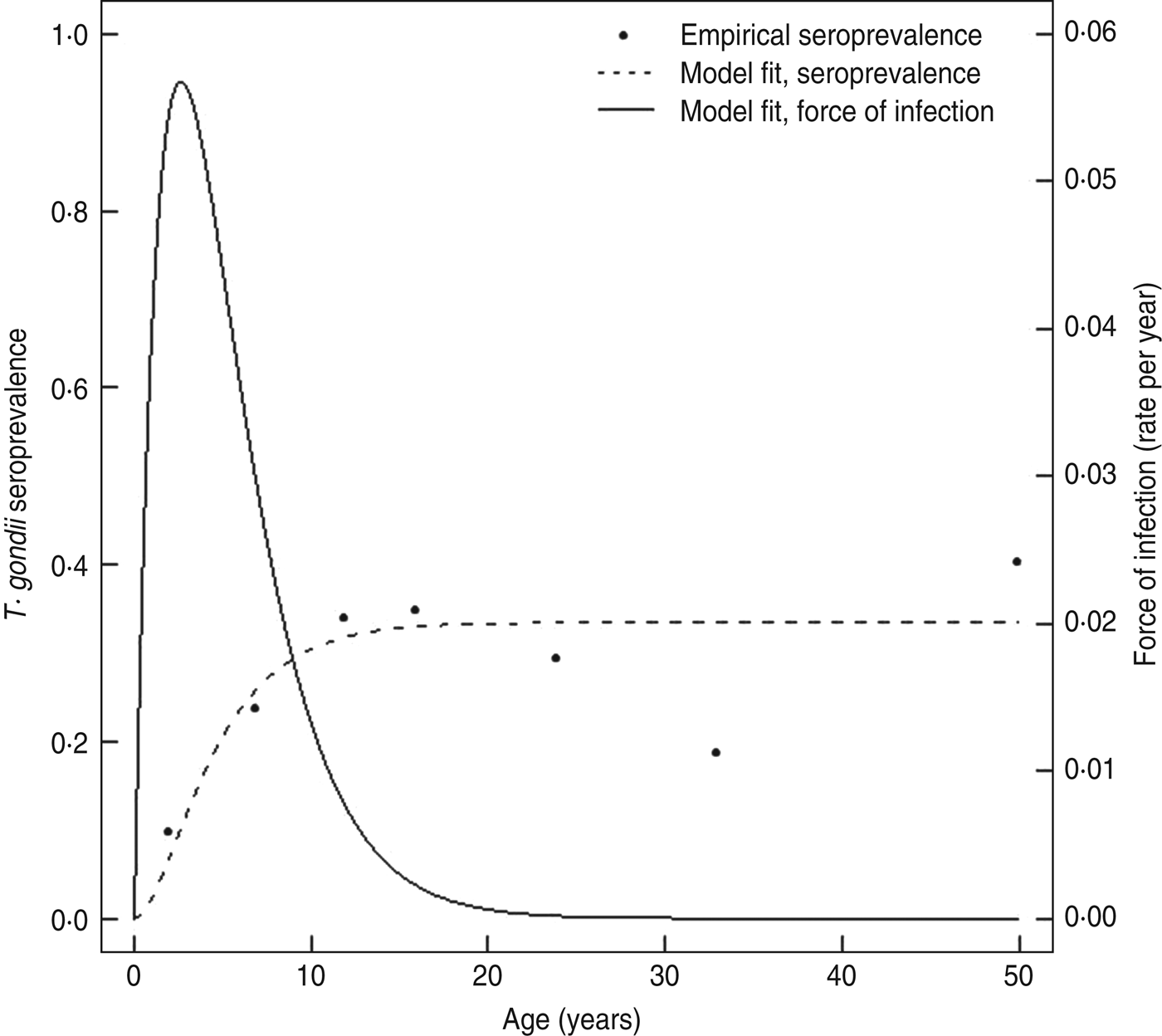
Fig. 5. Predicted Toxoplasma seroprevalence curve and force of infection curve for the Haiti cross-sectional community survey. A damped exponential linear catalytic model that allowed the underlying seroconversion rate to vary with age (–––) was used to generate a predicted prevalence curve (- - -) from the observed seroprevalence and median age data from Table 2 (•) as described in the Materials and Methods section.
DISCUSSION
Population estimates of T. gondii infection prevalence in Haiti are somewhat limited. Using the gold-standard Sabin–Feldman dye test, the 1956 work of Feldman & Miller determined that 36% of a small set of urban Port-au-Prince residents (n = 104; age range 0 to ⩾50 years) were seropositive for IgG and/or IgM complement-fixing antibodies to T. gondii [Reference Sabin and Feldman42, Reference Feldman and Miller43]. A peak seroprevalence of 46% was observed in the 5–9 and 10–19 years age groups [Reference Feldman and Miller43]. In 1979 Raccurt et al. [Reference Raccurt, Mojon and Boncy44] used an indirect immunofluorescent assay with an undefined sensitivity and specificity to show that only 5·9% of residents in seven rural communities on the southern Haiti peninsula had antibodies to T. gondii (n = 544, age range 0 to ⩾50 years) and that seroprevalence varied substantially between communities (range 0–15%) [Reference Raccurt, Mojon and Boncy44]. Using a new recombinant protein-based IgG MBA that is 100% sensitive and 100% specific compared to the Sabin–Feldman dye test in an analysis of the CDC Toxoplasma serum reference panel, we have shown that Toxoplasma seroprevalence in one rural Haiti coastal community is 28·2% overall. While our seroprevalence values are higher than that reported for the rural southern peninsula and lower than that observed in the intensely urban setting of Port-au-Prince, a direct comparison of data generated with different assays over a 40-year time-frame is difficult.
Except for the 1991 study of Etheredge & Frenkel where no age effect was noted in rural Panama [Reference Etheredge and Frenkel45], many contemporary cross-sectional studies in Central and South America have shown that Toxoplasma seroprevalence values increase rapidly in preschool and school-age children and then plateau at geographically distinct peak seroprevalence values in older children and young adults. The 1999 and 2003 surveys of children in rural Guatemala by Jones et al. [Reference Jones38] found that seroprevalence was only 12·5% in children aged <3 years but that it increased markedly to 43% by age 5 years and remained above 40% to age 10 years. Fernandes et al. [Reference Fernandes39] sampled children and adults in an urban setting in Brazil in 1990 and reported that seroprevalence increased from 6·1% in 3-year-olds to 27·8% in 5- to 9-year-old children and peaked at 76·9% in the 15–19 years age group. In rural Amazonia, Brazil, Ferreira et al. [Reference Ferreira46] noted a similar profile: 35% infected between ages 5 and 14 years, 75% infected by age 30 years. The age-specific seroprevalence in their study peaked at approximately 90% in adults. Carme has named this type of age vs. seroprevalence profile the ‘Tropical model’ and has proposed that it is indicative of a location where children are exposed to an oocyst-contaminated environment at an early age and where adults do not have significant exposure to cyst-contaminated meat in their diet [Reference Carme47]. The key feature of the model is that the incidence rate must vary with age. Empirical evidence of age-dependent incidence rates was reported by Frenkel et al. [Reference Frenkel48] after a 5-year longitudinal study of 571 children in Panama. In this environment, the risk of infection was shown to increase from 0·014 infections/year in 1-year-old children to 0·040 infections/year in 3-year-old children before declining to 0·023 infections/year in 5-year-old children. Similarly, Fernandes et al. used a catalytic model to determine force of infection from their prevalence data and calculated that children in the Brazilian study area were most at risk between ages 5 and 10 years with rates of approximately 0·06 infections/year [Reference Fernandes39]. Our observed age-specific incidence rates in Haitian children from the longitudinal study (range 0·008–0·131 infections/year) and our calculated force of infection curve determined from the cross-sectional community survey are also consistent with this model. Both study methods suggest that the peak of infection pressure in this area of Haiti occurs in the 2–3 years age range.
The complete spectrum of epidemiological factors that fix the plateau seroprevalence in a particular population has yet to be determined. Carme [Reference Carme47] hypothesized that density and infection rates in cats combined with cat faeces contamination and oocyst stability in the environment were the most important factors, but supporting data are limited to a few studies [Reference Etheredge and Frenkel45, Reference Frenkel and Ruiz49]. What is clear is that average seroprevalence values vary widely between populations in Central and South America and even between similar communities in the same nation. Etheredge & Frenkel found large differences in seroprevalence between children of different villages in Panama (range 0–42·5%) [Reference Etheredge and Frenkel45], and Bahia-Oliveira et al. showed that average seroprevalence was significantly impacted by socioeconomic status within a single community [Reference Bahia-Oliveira50]. The overall Haitian community prevalence (28·2%) is lower than that reported in the literature for many study areas: 65·8% in Amazonia, Brazil [Reference Ferreira46]; 64% in Honduras [Reference Feldman and Miller43]; 84% for the lower socioeconomic population in Campos, Brazil [Reference Bahia-Oliveira50]; and 60% in Guadeloupe, West Indies [Reference Barbier, Ancelle and Martin-Bouyer51]. Whether these seroprevalence differences reflect the inherent differences of the assays used, are impacted by the age distributions and collection strategies of the various sample sets, or are indicative of true variations in the populations surveyed could be addressed by testing a representative sample of each population with a single assay after the fashion of the US and Mexican national surveys [Reference Jones13, Reference Caballero-Ortega52]. We are currently working to confirm the sensitivity and specificity of our MBA using a larger panel of sera and to incorporate it into a Haiti national survey. As we have previously described, the strength of the assay format is that additional infection markers can be added to a MBA survey panel with minimal additional incurred costs [Reference Lammie53].
Our study does have several limitations. As mentioned above, our sample sets were collected from two adjacent communities in the Leogane Commune and may not be representative of any other region of Haiti. Second, the sample sets we analysed were collected as part of two vector-borne disease studies of LF. Thus, we have no environmental, household, or diet information on any of the risk factors relevant to an epidemiological analysis of T. gondii transmission or infection. Third, we have some evidence of clustered family seroconversion in the longitudinal cohort children that may have skewed our seroprevalence and incidence numbers. We observed three households where siblings seroconverted in the same collection interval as would be expected in the case of a common infection source. However, households where only one sibling seroconverted were also observed, and our median number of enrolled children per household was 1. Our overall incidence rate (0·066 infections/year) is lower than that reported by Ferreira et al. in rural Amazonia, Brazil (0·09 infections/year) [Reference Ferreira46]. Finally, because adults between 20 and 40 years of age in our cross-sectional survey had lower (but not significantly different) seroprevalence values than teen-aged children, it is unclear whether the SAG2A-specific IgG response wanes during chronic infection to the point of reversion to seronegative status. This potentially confounding question can most easily be addressed in a setting where peak seroprevalence is reached by young adulthood and where older adult seroconversions are rare.
Despite these limitations, we believe this work represents a proof-of-principal for the addition of a Toxoplasma-specific marker to MBA format surveys for seroprevalence. The rSAG2A/GST assay should provide a convenient and cost-effective tool for future epidemiologic studies on the prevalence of T. gondii infection around the world and will provide additional data to allow better estimates of the risk of infection during pregnancy.
ACKNOWLEDGEMENTS
The authors express their gratitude to the families who participated in the projects, to the staff of L'Hôpital St Croix, and to the past members of the filariasis research team. We thank J. Jones (CDC) for helpful suggestions on the manuscript and N. Lang-Unnash (University of Alabama at Birmingham) for parasite DNA.
Financial support was provided by the Centers for Disease Control and Prevention, the National Institutes of Health, and the United Nations Development Programme/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases (grant nos. 920 528and 940 441). K.L.H. was supported by a CDC/APHL Emerging Infectious Diseases Fellowship.
Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.










