The prevalence of obesity among women of reproductive age has increased in most parts of the world in recent years( 1 , Reference Lovelady 2 ). Pregnancy is a period naturally associated with weight gain. Excessive gestational weight gain, however, often results in weight retention after birth, which in turn is associated with maternal overweight and increased risk of adverse health outcomes in later pregnancies( Reference Skouteris, Hartley-Clark and McCabe 3 – Reference Villamor and Cnattingius 6 ). For this reason, reducing postpartum weight retention (PPWR) is important in order to manage the growing health problem of obesity.
PPWR is highly variable and its major risk factors include high pre-pregnancy weight and high gestational weight gain( Reference Krause, Lovelady and Peterson 7 – Reference Mamun, Kinarivala and O'Callaghan 10 ). Strategies for reducing the risk of PPWR often include modification of lifestyle factors like diet and physical activity( Reference Amorim, Linne and Lourenco 11 , Reference Birdsall, Vyas and Khazaezadeh 12 ). Breast-feeding also is usually suggested as a means for women to reduce PPWR. Observational studies show that length of breast-feeding is inversely associated with maternal PPWR( Reference Krause, Lovelady and Peterson 7 , Reference Baker, Gamborg and Heitmann 13 – Reference Janney, Zhang and Sowers 15 ). This effect has often been attributed to the energy cost of exclusive lactation, which is approximately 2100 kJ/d( Reference Lovelady 2 ).
Breast-feeding and obesity also exhibit a reverse association. Existing obesity makes breast-feeding more difficult and studies have shown that women with obesity tend to breast-feed their children for a shorter period of time than normal-weight mothers( Reference Baker, Michaelsen and Sorensen 16 , Reference Wojcicki 17 ). This may be due to biological factors directly associated with the obesity, such as delayed onset of milk secretion, but also to socio-economic and cultural factors not causally related to the overweight and to psychosocial factors like feelings of depression or anxiousness( Reference Pedersen, Baker and Henriksen 18 , Reference Amir and Donath 19 ). Since socio-economic status also is associated with obesity, it is important to evaluate socio-economic status as a potential confounding factor when assessing associations between obesity and length of breast-feeding.
Further, in a study by Olson et al. among women in the USA, an interaction between income and gestational weight gain in relation to their effects on PPWR at 1 year postpartum was found( Reference Olson, Strawderman and Hinton 20 ). Women with low income had higher weight retention 1 year postpartum than the high-income women with the same weight gain during pregnancy. This indicates that modifying effects of socio-economic factors could play a role also in the relationship between breast-feeding and PPWR. None of the previous studies of breast-feeding and PPWR have evaluated socio-economic factors as effect modifiers in this association.
The focus of the current study was to evaluate the complex associations between breast-feeding and PPWR in the large, population-based, Norwegian Mother and Child Cohort Study (MoBa). Here, we had the opportunity to evaluate socio-economic factors not only as confounders but also as effect modifiers. In addition, we were able to assess PPWR up to 36 months. Longer follow-up time has to our knowledge been available in only one study, and then with considerably fewer participants( Reference Wiklund, Xu and Lyytikainen 21 ). Our aim was to investigate the association between full and partial breast-feeding duration and PPWR at 6, 18 and 36 months after delivery. An additional objective was to evaluate socio-economic status as an effect modifier in this relationship.
Materials and methods
The Norwegian Mother and Child Cohort Study
MoBa is a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health( Reference Magnus, Irgens and Haug 22 , Reference Nilsen, Vollset and Gjessing 23 ).
The participants were recruited to the study through a postal invitation in connection with a routine ultrasound examination offered to all pregnant women in Norway. No exclusion criteria were applied( Reference Brantsaeter, Birgisdottir and Meltzer 24 ). Participants were recruited from all over Norway between 1999 and December 2008, and 38·5 % of invited women consented to participate. The cohort now includes 108 000 children, 90 700 mothers and 71 500 fathers. Several sub-studies are conducting additional collections of data and biological materials.
The current study is based on version 5 of the quality-assured data files released for research in 2010. Version 5 represented the latest data set available at the time of the present analysis. Informed consent was obtained from each MoBa participant upon recruitment. The study was approved by The Regional Committee for Medical Research Ethics in South-Eastern Norway.
Follow-up is conducted by questionnaires at regular intervals and linkage to national health registries( Reference Brantsaeter, Haugen and Samuelsen 25 , Reference Haggkvist, Brantsaeter and Grjibovski 26 ).
Questionnaires
The participants received three self-administered questionnaires by mail during the pregnancy. After birth, the questionnaires were sent to the women when the child was 6, 18 and 36 months old. A woman could participate in MoBa with more than one pregnancy. Since the pregnancy is the unit of observation, each pregnancy is given an identification number and all data from the mother, father and child are linked to this number( Reference Magnus, Irgens and Haug 22 ). Pregnancy and birth records from the Medical Birth Registry of Norway (MBRN) are linked to the MoBa database.
For the present study, the questionnaires Q1, Q4, Q5 and Q6 were used. The first, which was sent to the women in pregnancy weeks 13–17, consisted of questions regarding medical history, medications, occupation, income and lifestyle habits. Q4 was sent to the women when the child was 6 months old, and included questions about the child's health, mother's health and lifestyle. Also in this questionnaire, questions regarding infant feeding in the child's first 6 months were asked. Q5 was sent to the women when the child was 18 months old, with the main focus being the child's developmental status; however it also contained questions on food consumed by the child and breast-feeding continuing after 6 months( Reference Magnus, Irgens and Haug 22 ). Finally Q6 focused on the child's developmental status and was sent to the women when the child was 3 years old.
Variable definitions
Outcome variable
The outcome measure in the present study was PPWR. PPWR at 6 months was calculated as the weight difference between the body weight reported by the women in Q4 and pre-pregnant weight from Q1. For calculation of weight retention at 18 months maternal weight reported in Q5 was used and at 36 months weight reported in Q6 was used. All weights used for generating the outcome measure in the present study were self-reported.
Breast-feeding variables
Retrospective recall of breast-feeding and infant feeding was self-reported at 6 months postpartum for generating the breast-feeding variables up to and including 6 months (Q4), and partial breast-feeding beyond 6 months was reported retrospectively at 18 months (Q5).
The question in Q4 was ‘What has your child been given to drink during the first 6 months of his/her life?’ For these questions more than one liquid could be ticked and the answers were specified for 0, 1, 2, 3, 4, 5 and 6 months. The food question was ‘How often does your child eat the following food at the moment, and how old was your child when you started giving him/her this food?’ and also had several possibilities to tick for semi-solid and solid food. In Q5 the question was ‘What kind of milk has your baby been given since he/she was 6 months old?’ More than one liquid could be ticked and the alternatives stated in the questionnaire were 6–8 months, 9–11 months, 12–14 months and 15–18 months.
In the present study, breast-feeding was evaluated as the monthly duration of both full and partial breast-feeding from 0 up to 6 months as independent variables in the same models. ‘Full breast-feeding’ was defined as answering breast-feeding in the specified months, but no infant formula, other milk, semi-solid or solid food had been given. ‘Partial breast-feeding’ was defined as breast-feeding in combination with infant formula, other milk and/or semi-solid or solid food. ‘Any breast-feeding’ denotes both full and partial breast-feeding up to 6 months. The breast-feeding variables and their distribution in the MoBa sample are described in more detail in a previous publication( Reference Haggkvist, Brantsaeter and Grjibovski 26 ).
Other variables
In the present paper we have made an effort to estimate relative socio-economic status. One variable represents the family unit, i.e. family income. The other variable represents the mother and her educational attainment.
From Q1 the covariates representing socio-economic status, including maternal education and household income, were retrieved. These variables were operationalized as follows. Maternal education level was divided into four groups: <12 years, 12 years, 13–16 years and ≥17 years. The two lower groups were combined and denoted ‘low education’ and the two upper groups (college and university) were combined and denoted ‘high education’ in the stratified analyses. Household income was categorized on the basis of both the participant's and her partner's income (both incomes <300 000 NOK, one income ≥300 000 NOK or both incomes ≥300 000 NOK; 300 000 NOK (Norwegian krone) are equivalent to about 39 000 € (Euros)). In the stratified analyses, the lowest group was denoted ‘low income’ and the two other groups were combined and denoted ‘high income’.
Self-reported pre-pregnant weight and height from Q1 were used to calculate pre-pregnant BMI. BMI was modelled as a continuous variable, but for descriptive statistics it was categorized according to the WHO classification as normal (18·5–24·9 kg/m2), underweight (≤18·5 kg/m2), overweight (25·0–29·9 kg/m2), obese grade 1 (30·0–34·9 kg/m2) or obese grade 2 (≥35·0 kg/m2).
Marital status was defined as married, cohabitants, single or widow or not answered. Maternal age at time of delivery was also used as a continuous variable except for descriptive statistics. Smoking at 6 months postpartum was divided into three categories (daily smokers, occasional smokers and non-smokers). Exercise during pregnancy was based on respondents’ reports of their participation in thirteen different types of recreational exercise and divided into four categories (no exercise, less than once weekly, 1–2 times weekly and 3+ times weekly). Parity was estimated based on combined information from MoBa Q1 and MBRN. Gestational weight gain was calculated as the difference between weight at the time of delivery reported in Q4 and pre-pregnant weight reported in Q1. This variable was used as a continuous variable except for in descriptive statistics.
Sample selection
We identified 67 023 women who were eligible for the present study based on delivering singleton live infants and having data available from MBRN and from MoBa questionnaires Q1 and Q4 (Fig. 1). We then excluded women who had participated with more than one pregnancy, women who had not answered the questions regarding breast-feeding and women who had not reported their body weight at the relevant time points (pre-pregnancy, end of pregnancy, 6, 18 and 36 months postpartum). Furthermore, we also excluded women who reported being pregnant again in Q5 (18 months) or Q6 (36 months). The final sample for analysis at 6 months comprised 49 675 women. Of these, 27 187 (55 %) were available for analysis at 18 months and 17 343 (35 %) at 36 months. The main reason for much lower sample sizes at 18 and 36 months was that the index child had not yet reached the corresponding age.
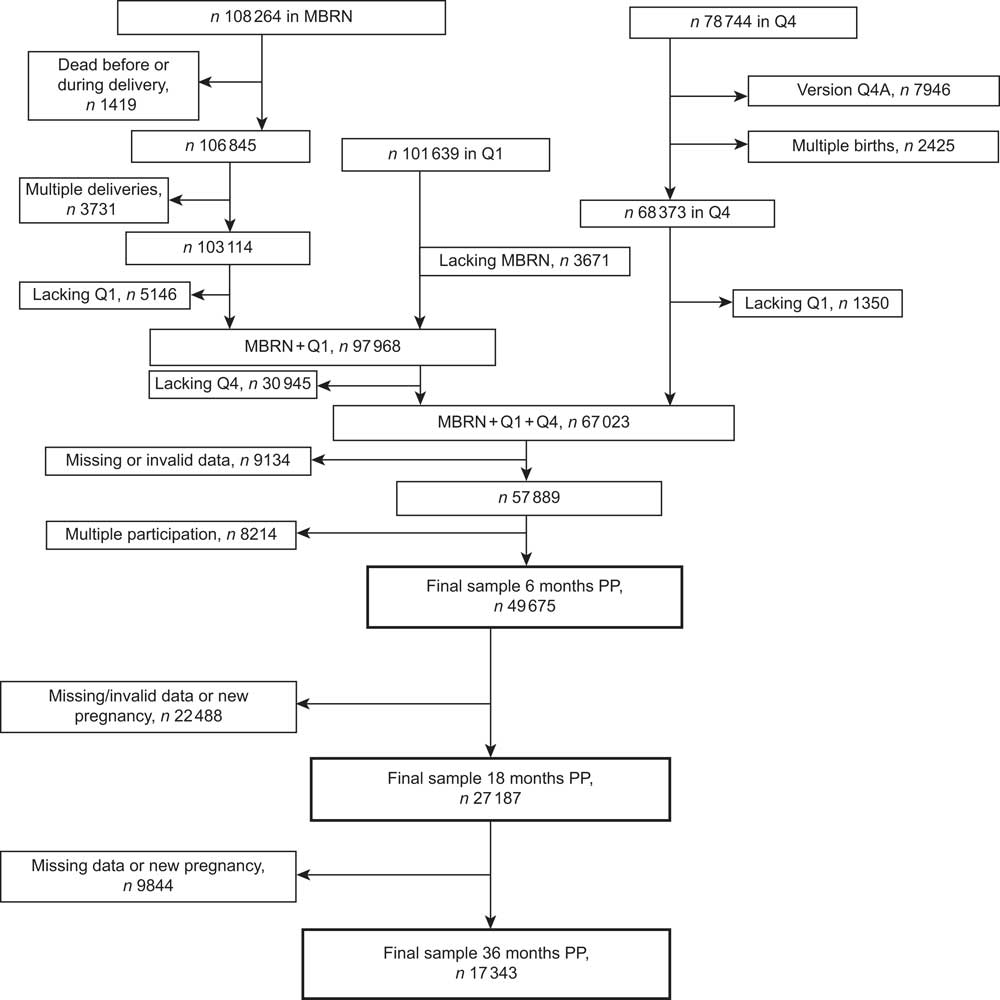
Fig. 1 Flow diagram showing the selection of participants for the present study from the Norwegian Mother and Child Cohort (MoBa). MBRN, Medical Birth Registry of Norway; PP, postpartum
Statistical analysis
Student's t test and ANOVA were used to study differences between subgroups for the normally distributed variables, length of full and partial breast-feeding. Bivariate and multivariate regression analyses were performed to study the association between duration of breast-feeding and PPWR. First, associations between length of full and partial breast-feeding up to 6 months and PPWR at 6 months postpartum were evaluated. Second, associations between length of full and partial breast-feeding up to 6 months as well as length of partial breast-feeding beyond 6 months and PPWR at 18 and 36 months postpartum were evaluated. Here, the intervals used were 6–11 months and 12–18 months and were modelled as dichotomous variables. The β values in the models for full breast-feeding denote weight change in kilograms per month of full breast-feeding. The β values for partial breast-feeding denote weight change in kilograms for partial breast-feeding relative to no breast-feeding in the given interval.
Possible confounding factors were initially evaluated in bivariate regression analyses. The variables tested were maternal education, household income, maternal age, parity, Caesarean section, pre-pregnant BMI, pregnancy weight gain, marital status, alcohol intake, smoking and physical activity. Among these, pre-pregnant BMI, pregnancy weight gain, maternal age and parity were found to be confounders and adjusted for in the basic multivariate regression models. Thus, maternal education and household income did not exert confounding effects on the relationship between breast-feeding and PPWR in our analyses. In addition, fully adjusted multivariate regression analyses were performed including also covariates reported by many other studies, i.e. maternal age, parity, Caesarean section, pre-pregnant BMI, pregnancy weight gain, marital status, alcohol intake and physical activity.
Tests were performed to evaluate possible interactions between variables indicating socio-economic status and breast-feeding duration, in their effect on PPWR. In analyses of interaction, a more generous significance level <0·1 was used to make sure that important interactions were detected. A significant interaction between income and full breast-feeding was detected; thus stratified analyses were performed for groups with high/low income (coded 0/1). All statistical analyses were performed using the statistical software package PASW Statistics version 18·0·1 and significance level α = 0·05 was used for all but the analyses of interaction.
Results
Table 1 shows the length of full and any breast-feeding in the 49 675 women included in the 6 months PPWR analyses in relation to lifestyle factors and demographic and health characteristics. Length of both full and any breast-feeding differed significantly in relation to sub-categories of a number of the background variables.
Table 1 Breast-feeding in relation to characteristics of participants at 6 months postpartum analyses, Norwegian Mother and Child Cohort Study (MoBa)
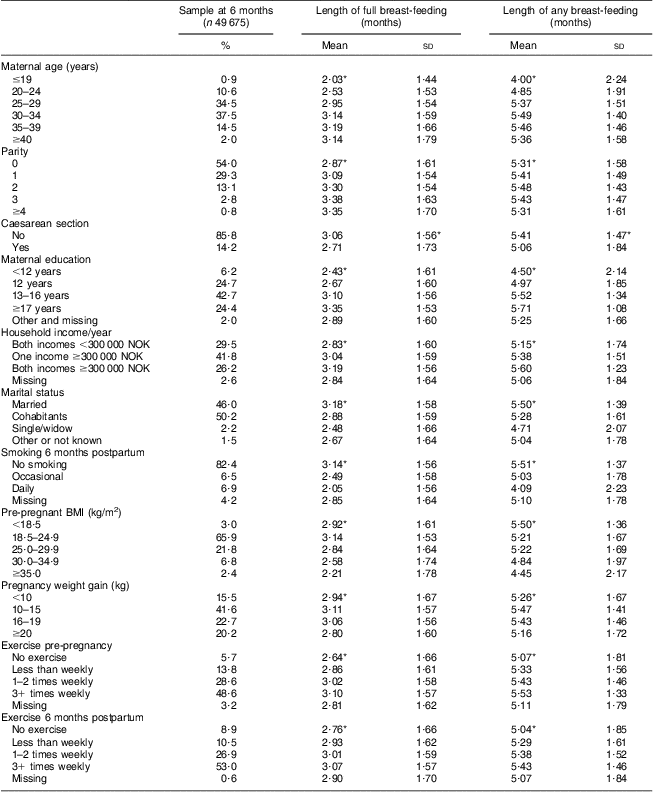
*Length of breast-feeding differed significantly according to category, P < 0·05 (ANOVA).
Maternal PPWR at 6, 18 and 36 months postpartum, in relation to months of full and any breast-feeding, is shown in Table 2. Longer duration of full breast-feeding as well as any breast-feeding was related to lower PPWR at 6 and 18 months, respectively. At 36 months postpartum, only full breast-feeding was related to PPWR.
Table 2 Breast-feeding duration in relation to weight retention at 6, 18 and 36 months postpartum, Norwegian Mother and Child Cohort Study (MoBa)
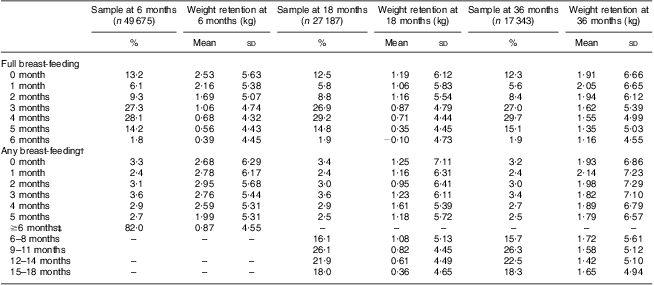
†Any breast-feeding denotes both full and partial breast-feeding up to 6 months and partial breast-feeding beyond 6 months.
‡In total, 40 237 (81 %) women breast-fed for at least 6 months. Weight retention at ≥6 months postpartum is here shown for these women jointly.
In Table 3 results from multivariate regression analysis of PPWR at 6 months postpartum in relation to breast-feeding patterns are shown. In the model including only covariates that had an impact on the association between breast-feeding and weight retention in bivariate regression analysis, full and partial breast-feeding were significantly inversely related to PPWR (P < 0·001). Fully adjusted analyses (including also covariates reported by other studies, i.e. maternal age, parity, Caesarean section, pre-pregnant BMI, pregnancy weight gain, maternal education, household income, marital status, alcohol intake, smoking and physical activity) also revealed a significant relationship between longer duration of breast-feeding and lower PPWR at 6 months (P < 0·001, data not shown).
Table 3 Weight retention at 6 months postpartum in relation to full and partial breast-feeding duration (n 49 675), Norwegian Mother and Child Cohort Study (MoBa)
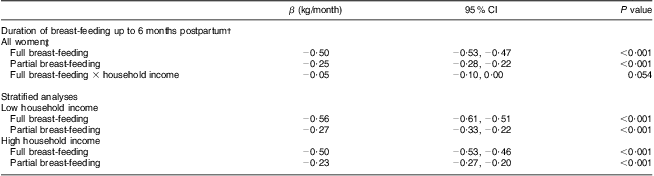
†Results from multivariate regression models. Both full and partial breast-feeding in the same model; all models adjusted for pre-pregnant BMI, pregnancy weight gain, maternal age and parity.
‡Interactions between income and education (respectively, 1 for lower levels/0 for higher levels) and full and partial breast-feeding were all tested. The only significant interaction was for full breast-feeding and household income.
An interaction between household income and full breast-feeding, in their effects on PPWR, was found (P < 0·1). However, no significant interaction between education and full or partial breast-feeding in relation to PPWR was found. In the stratified analyses, with subgroups on high and low household income, the differences in regression β coefficients between the groups were small.
Longer duration of full breast-feeding was significantly associated with lower PPWR at 18 months postpartum (P < 0·01) in the basic model (Table 4). Partial breast-feeding during 0–6 months was not significantly associated with PPWR at 18 months. Likewise, partial breast-feeding during 6–11 months was not associated with PPWR at 18 months, whereas partial breast-feeding during 12–18 months was significantly inversely associated with PPWR at 18 months (P < 0·01). There was a statistically significant interaction between household income and full breast-feeding in their effects on PPWR at 18 months (P < 0·01), with lower weight retention per month of full breast-feeding in the low-income household. No significant interactions were found between partial breast-feeding and household income. Also, no interaction between breast-feeding patterns and maternal education on PPWR at 18 months postpartum was found.
Table 4 Weight retention at 18 months postpartum in relation to breast-feeding duration (n 27 187), Norwegian Mother and Child Cohort Study (MoBa)

†Results from multivariate regression models. Both full and partial breast-feeding in the same model; all models adjusted for pre-pregnant BMI, pregnancy weight gain, maternal age and parity.
‡Interactions between income and education (respectively, 1 for lower levels/0 for higher levels) and full and partial breast-feeding were all tested. The only significant interaction was for full breast-feeding and household income.
At 36 months postpartum, length of full breast-feeding was significantly inversely associated with PPWR (P < 0·01; Table 5). Length of partial breast-feeding up to 6 months was not significantly associated with weight retention at 36 months. Likewise, partial breast-feeding during 6–11 months was not associated with PPWR at 36 months, and neither was partial breast-feeding during 12–18 months. There was a statistically significant interaction between household income and full breast-feeding in their effects on PPWR at 36 months (P < 0·1), with lower weight retention per month of full breast-feeding in the low-income household. No significant interactions were found between partial breast-feeding and household income. No interaction between breast-feeding patterns and maternal education on PPWR at 36 months postpartum was found.
Table 5 Weight retention at 36 months postpartum in relation to breast-feeding duration (n 17 343), Norwegian Mother and Child Cohort Study (MoBa)

†Results from multivariate regression models. Both full and partial breast-feeding in the same model; all models adjusted for pre-pregnant BMI, pregnancy weight gain, maternal age and parity.
‡Interactions between income and education (respectively, 1 for lower levels/0 for higher levels) and full and partial breast-feeding were all tested. The only significant interaction was for full breast-feeding and household income.
In addition, we repeated the statistical analyses at 6 months and 18 months postpartum with the smaller 36 months sample size of n 17 343 (data not shown). This yielded results similar to those found with the larger sample sizes.
Discussion
The main finding in the present study was the significant inverse association between duration of full breast-feeding and PPWR up to 36 months. The benefits of breast-feeding are well established regarding infant health outcomes, while long-term health benefits for the mother are less well studied( 27 ). Our results support that longer duration of breast-feeding contributes to lower PPWR and hence might be of importance to obesity risk. The finding that the protective association with full lactation also persisted in lower-income women is a novel finding and suggests that breast-feeding promotion may be an important strategy for weight management in this vulnerable group.
The main strength of the present study is the large study sample and the cohort being population-based. MoBa women thus represent women with a wide range of pre-pregnant weight, BMI, PPWR, breast-feeding patterns and socio-economic factors. However, it has previously been shown that MoBa participants differ from the non-participants( Reference Nilsen, Vollset and Gjessing 23 ). Participating women include fewer young mothers (<25 years) and fewer women living alone, and are thus not truly representative of all Norwegian women. Nevertheless, evaluation of a potential bias due to self-selection in MoBa showed that despite differences in prevalence estimates between the cohort participants and the total population of pregnant women, no statistically relevant differences in association measures were found previously regarding eight exposure–outcome associations evaluated previously( Reference Nilsen, Vollset and Gjessing 23 ).
An important limitation in the present study is the lack of measured body weight at pre-pregnancy, during pregnancy and postpartum. To our knowledge there are no studies investigating the reliability in reported pre-pregnancy weight while in early pregnancy. In our study, the question regarding pre-pregnant weight was asked in the first questionnaire, sent to the women around pregnancy week 18, when current weight also was asked for. In a review article, Engström et al. ( Reference Engstrom, Paterson and Doherty 28 ) examined the accuracy of self-reported weight among non-pregnant women. Although mean variations between self-reported and measured values were small, all included studies showed that women underestimated body weight. A significant percentage of women in study groups also had very large errors. A similar pattern was observed by Rowland( Reference Rowland 29 ) who also found that weight reporting error was associated with degree of overweight, with the largest under-reporting found in severely overweight non-pregnant women, defined as BMI > 32·3 kg/m2. In the MoBa study only a small proportion of the women reported BMI values this high. None of these studies, however, addressed the additional challenges of recalling pre-pregnancy weight while in pregnancy. In sum, likely the women in our study underestimated their actual weight pre-pregnancy, during pregnancy and postpartum. This bias may be larger the heavier the women. However, if the degree of underestimation remains fairly constant per woman, our estimates of changes in weight may be quite accurate.
Another limitation of our study is the 6-month retrospective recall of infant nutrition. The long recall period may have caused overestimation of the duration of full breast-feeding.
In MoBa, the number of participants at the different time points varied, with n 49 675 at 6 months postpartum, n 27 187 at 18 months and n 17 343 at 36 months. For this reason we also performed a sensitivity analysis using the sample of n 17 343 at all time points. Nevertheless, we believe that there are strong reasons for doing the 6 months analyses in the full sample, the 18 months analyses in the second sample, and the 36 months analyses in the smallest sample. This is in order to avoid self-selection that comes with dropout at 18 and 36 months of follow-up, i.e. to prevent ending up with an even less representative sample than the one we started with. In addition, we are able to catch the later recruits in MoBa who had not reached their later follow-up at the time we created the sample.
Several studies have found that breast-feeding is inversely associated with weight retention after birth( Reference Baker, Gamborg and Heitmann 13 , Reference Dewey, Heinig and Nommsen 14 , Reference Kac, Benicio and Velasquez-Melendez 30 ). In none of these studies, however, did the follow-up time exceed 18 months. Janney et al. found inverse associations between breast-feeding and PPWR, yet the authors concluded that the effect of lactation on PPWR was small and that promoting breast-feeding was not relevant in order to minimize PPWR( Reference Janney, Zhang and Sowers 15 ). Maternal obesity is a complex interplay between a number of factors and Birdsall et al. concluded in a review article on interventions on maternal obesity that there is a deficiency of appropriately designed interventions in this field( Reference Birdsall, Vyas and Khazaezadeh 12 ).
In the present study an inverse association between duration of full and partial breast-feeding and maternal PPWR was found at 6 months. The possibility of confounding was reduced by adjusting for a number of relevant factors. At 18 months postpartum the association between full breast-feeding and PPWR persisted, whereas the association for partial breast-feeding in the first 6 months was no longer significant. At 36 months, full breast-feeding still was significantly inversely associated with PPWR.
When evaluating education and household income as effect modifiers in the relationship between breast-feeding and PPWR, no significant interaction was found for low (≤12 years) v. high (>12 years of school) education. However, a significant effect modification was seen for full breast-feeding and low v. high family income at 6, 18 and 36 months postpartum. We found a larger weight decline for each month of full breast-feeding among women in the low-income group than among women in the high-income group at 6 months (0·56 kg/month v. 0·50 kg/month), at 18 months (0·20 kg/month v. 0·11 kg/month) and at 36 months postpartum (0·31 kg/month v. 0·10 kg/month). This may be in part because lower-income women had higher pregnancy weight gain as previously shown( Reference Olson, Strawderman and Hinton 20 ) and also observed here (data not shown). However, including gestational weight gain in the models’ analyses should have partially controlled for such confounding.
Another possible explanation could be that women with high income are more weight conscious and lose weight independent of their breast-feeding behaviour. Nevertheless, women in MoBa are not a truly representative sample, as included women smoke less often, are less overweight and obese and have higher education than the general population of women giving birth( Reference Magnus, Irgens and Haug 22 , Reference Nilsen, Vollset and Gjessing 23 ). It could therefore be argued that the potential modification effects are attenuated by the population being too homogeneous. Hence, it could be speculated that low-income women in societies with more pronounced economic inequality would benefit even more from breast-feeding in respect to reducing PPWR.
The variables used in the present study to represent socio-economic status were household income and maternal education level. These are two crude indicators of socio-economic status. However, with the maternal education variable we aimed to capture the socio-economic status of the mother herself, while the combined household income variable would capture the socio-economic status of the family as a unit. In our statistical analyses, the two variables both acted as independent predictors, supporting our decision to study both of these indicators. Both maternal education and household income have also been used in previous papers( Reference Haggkvist, Brantsaeter and Grjibovski 26 ).
The overall relationship between breast-feeding and PPWR was attenuated at 18 and 36 months postpartum compared with results from the 6 months analyses. These findings could be due to biological factors or the different sample sizes at the different occasions. Sample size in the analyses of PPWR decreased from 49 675 women at 6 months to 27 187 women at 18 months and 17 343 women at 36 months. However, additional sub-analyses, where we repeated the 6 months and 18 months PPWR analyses on the same sample as the 36 months analyses (i.e. 17 343 women), did not change the significance of these results (data not shown). Similar results, where the impact of breast-feeding on PPWR was attenuated over time, have been obtained by Dewey et al.( Reference Dewey, Heinig and Nommsen 14 ). In the present study, the inverse association between full breast-feeding and weight retention was still significant at 36 months, even if the magnitude of the effect was lower compared with 6 and 18 months.
The overall effect of breast-feeding on maternal weight status, together with the added impact in low-income women, has public health implications, even in a country with relatively high rates of breast-feeding. As previously reported, 98·7 % of MoBa mothers were found to initiate breast-feeding and 80·0 % of the children were breast-fed at 6 months; however only 2·1 % were fully breast-fed at 6 months( Reference Haggkvist, Brantsaeter and Grjibovski 26 ). There was a pronounced decline in full breast-feeding between 3 and 4 months, from 70·9 % to 44·0 %. The prevalence of full breast-feeding is thus not in line with the Norwegian health authorities’ recommendation of exclusive breast-feeding during the first 6 months.
The present results support full breast-feeding for the first 6 months in order to facilitate return to pre-pregnant weight in all women. The interaction found between full breast-feeding and household income in relation to weight retention is interesting and could be useful for midwives and other health-care professionals when giving advice regarding breast-feeding. Low-income women often have a lower prevalence of breast-feeding and the causes are multifactorial( Reference Amir and Donath 19 , Reference Shortt, McGorrian and Kelleher 31 ). Health-care professionals working with women in lower socio-economic circumstances could use the lower weight retention as an incentive when promoting breast-feeding. In the future, it would also be interesting to conduct intervention studies comparing weight retention in breast-feeding and non-breast-feeding women.
Conclusion
The present MoBa study supports the hypothesis that breast-feeding contributes to lower PPWR and showed that the effect is manifest for as long as 3 years postpartum. The study also showed that the relationship between full breast-feeding and weight retention is modified by household income up to 36 months.
Acknowledgements
Sources of funding: The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research; the National Institute of Environmental Health Sciences (NIEHS; contract no. NO-ES-75558) and the National Institute of Neurological Disorders and Stroke (NINDS; grant no. 1 UO1 NS 047537-01) of the US National Institutes of Health (NIH); and the Norwegian Research Council/FUGE (grant no. 151918/S10). The present analyses were supported by the Swedish Council for Working Life and Social Research (Project Grant 2008-0728; Principal Investigator A.W.). The funders (the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS, NIH/NINDS, the Norwegian Research Council/FUGE and the Swedish Council for Working Life and Social Research) had no role in the design, analysis or writing of this article. Conflicts of interest: No author has any conflict of interest. Authors’ contributions: M.B. is the main author and performed statistical analyses; L.L. provided help with statistical methods and language; A.L.B. provided practical help with the (MoBa) data, in the early stages of analysis and all along the way; H.M.M. provided expert knowledge about the MoBa data; A.-P.H. provided expert help with the breast-feeding variables definitions; M.H. provided expert help with the MoBa data; A.W. assisted in writing and statistical analysis strategies on a daily basis, together with L.L. she originally created the research question in this article. All co-authors have contributed to the work in a substantial way.








