INTRODUCTION
Basking sharks (Cetorhinus maximus), the second largest fish species, occur in temperate coastal seas in both northern and southern hemispheres, and in the Atlantic range as far north as northern Norway and the south-west coast of Iceland (Sims et al., Reference Sims, Fowler, Clò, Jung, Soldo and Bariche2015) and as far south as the Falkland Islands (Bigelow & Schroeder, Reference Bigelow and Schroeder1948). In the NE Atlantic they are primarily observed during mid- to late-summer surface feeding at sites along the west coasts of Britain and Ireland (Nicholson et al., Reference Nicholson, Harris and Pollard2000; Southall et al., Reference Southall, Sims, Metcalfe, Doyle, Fanshawe, Lacey, Shrimpton, Solandt and Speedie2005; Bloomfield & Solandt, Reference Bloomfield and Solandt2008; Speedie et al., Reference Speedie, Johnson and Witt2009). Evidence suggests that most individuals make local seasonal movements (Sims, Reference Sims2008) while some larger individuals also undertake transoceanic migrations (Gore et al., Reference Gore, Rowat, Hall, Gell and Ormond2008; Skomal et al., Reference Skomal, Zeeman, Chisholm, Summers, Walsh, McMahon and Thorrold2009). In summer amongst the highest concentrations of surface-feeding sharks occur around the islands of Coll, Gunna and Tiree in the Inner Hebrides, Scotland (Speedie et al., Reference Speedie, Johnson and Witt2009; Gore et al., Reference Gore, Frey, Ormond, Allan and Gilkes2016), where on favourable days up to 200 individuals have been observed, and up to 800 individuals may occur over a 7–10 day period (Gore et al., Reference Gore, Frey, Ormond, Allan and Gilkes2016).
While normally in such areas most sightings are of solitary sharks, up to a quarter or more may be of sharks in loose aggregations of up to nine or more sharks feeding within a few hundred metres of each other (Gore et al., in prep.). The occurrence of such distinct groups was noted from the Minch off the Outer Hebrides Island of Barra as far back as 1947 by Matthews (Reference Matthews1950) who suggested that social behaviour might be involved. Likewise Hallacher (Reference Hallacher1977) observed that in Carmel Bay, California, groups of two or three sharks were common, and described a group of 10 basking sharks (5–8 m in length) feeding in a straight line or circling before continuing ahead. Similarly, Harvey-Clark et al. (Reference Harvey-Clarke, Stobo, Helle and Mattson1999) described behaviour within an aggregation of 13 basking sharks (6–8 m length) during a 5 min aerial observation period off Nova Scotia, Canada. They observed groups of two or three individuals swimming in slow tight circles, nose-to-tail following, parallel swimming, and echelon swimming (with one individual to the side and behind another). They also described individuals making a flank approach, approaches leading to rostral contact with the tail, pectoral fin, vent or back, and also possible pectoral biting and nudging. They noted that some sharks had discrete white patches on their dorsum and fins, which they suggested could be related to mating behaviour. As with Wilson's (Reference Wilson2004) observations, Harvey-Clark and colleagues concluded that the aggregation involved group reproductive activity rather than feeding behaviour.
Sims et al. (Reference Sims, Southall, Quayle and Fox2000) also described possible social behaviour in basking sharks observed over 5 years between May and July during 25 separate events in coastal front areas in SW England. They described groups of two to four sharks participating in nose-to-tail following, echelon swimming and close flank approaches. They noted that while larger sharks of 5–8 m length were involved, those of 3–4 m were not, and that on three occasions they were able to identify the lead shark as female. They suggested that while the individual sharks may initially be attracted to aggregation areas to feed, it is feasible that they may then use the opportunity to find mates. The size at maturity for basking sharks is uncertain, but generally the females are thought to mature at a larger size (~7.24 m: Matthews, Reference Matthews1950) than males (>6.22 m: Matthews, Reference Matthews1950).
Sims et al. (Reference Sims, Southall, Quayle and Fox2000) also recorded full breaches by basking shark taking place in the area where close-following was observed and suggested there may be a link. Breaching behaviour not related to fishing has been observed in several other shark species, including thresher (Alopias vulpinus), spinner (Carcharhinus brevipinna), blacktip (C. limbatus) and white shark (C. carcharias). A wide variety of functions for the behaviour have been proposed, including feeding, predator avoidance, mating, parasite removal and signalling (Curtis & Macesic, Reference Curtis and Macesic2011).
Despite the above observations and other detailed studies of basking sharks over 70 years, remarkably little is known of the species’ reproductive cycle. Neither mating nor natural parturition has ever been observed, although one female has been reported to have delivered premature young after being harpooned by fishers in Norway (Sund, Reference Sund1943), and a pregnant female was found to have 34 egg cases at the beginning of gestation (Ali et al., Reference Ali, Saad, Reynaud and Capapé2012). Published descriptions of mating in any shark species are scarce with most observations having been made in captivity. In a number of shark species biting of the female by the male leading to intromission was reported to result in scarring of the female in gill, pectoral fin and tail areas (Bres, Reference Bres1993). In the wild mating has been observed in whitetip reef sharks (Triaenodon obesus) (Tricas & Le Feuvre, Reference Tricas and Le Feuvre1985; Whitney et al., Reference Whitney, Pratt and Carrier2004) and silky shark (Clarke et al., Reference Clarke, Lea and Ormond2013). Presumed courtship behaviours involving close-following and sometimes parallel swimming and fin-biting have been described in bonnethead sharks, Sphyrna tiburo (Myrberg & Gruber, Reference Myrberg and Gruber1974), grey reef sharks, C. amblyrhynchos (Bres, Reference Bres1993), nurse sharks, Ginglymostoma cirratum (Klimley, Reference Klimley1980; Pratt & Carrier, Reference Pratt and Carrier2001), blacktip reef sharks, C. melanopterus (Johnson & Nelson, Reference Johnson and Nelson1978) and lemon sharks, Negaprion brevirostris (Pratt & Carrier, Reference Pratt and Carrier2001).
While these comparisons support the interpretation that close-following and related behaviours in basking shark may be related to courtship, that this is the prime function has not been established. We took the opportunity of frequent encounters with basking sharks around the islands of Coll, Gunna and Tiree to investigate the hypotheses that (a) close-following and echelon swimming are a form of pre-courtship behaviour, (b) that abrasions on females are associated with males approaching females and (c) breaching by basking sharks is also related to courtship.
MATERIALS AND METHODS
Observations described here were made between 22 June and 14 August 2016 in the waters around the islands of Coll, Gunna and Tiree in the Inner Hebrides off the west coast of Scotland (Figure 1). Coll and Tiree are both inhabited islands ~20 km long and 2–8 km wide, with their long axes orientated north-east south-west. Gunna is a much smaller (1.75 km length) uninhabited island lying in the channel, Gunna Sound, that runs between the adjacent ends of the two larger islands. Strong tidal currents run through Gunna Sound and appear to result in surface concentrations of zooplankton on which the sharks feed. For recording purposes the study area has been divided into seven subareas (Figure 2), although during the main recording period survey work was confined to only five of these. Areas were primarily demarcated to reflect differences in coastal geomorphology and hence typical sea conditions and extent of use by basking sharks. Larger areas are ones where fewer observations were made per unit area, data from possible smaller sub-areas being combined so as to permit comparison of encounter rates per unit time.
Fig. 1. Map of Scotland and western isles with inset of Inner Hebrides, showing the Isles of Coll, Gunna, Tiree, Treshnish and Mull (OS Open Data 2016).
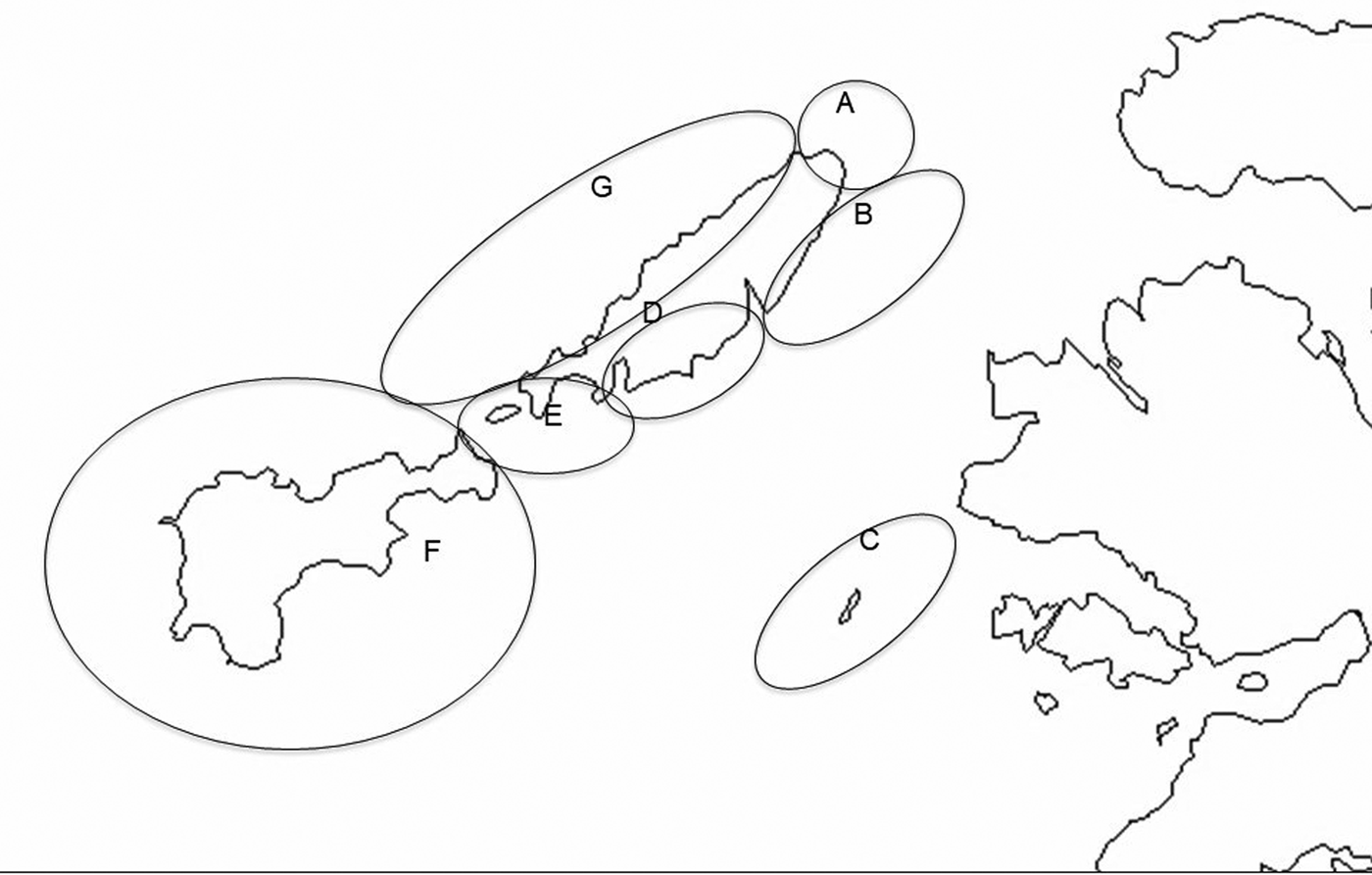
Fig. 2. Map of the Isles of Coll and Tiree with seven sub-areas distinguished in survey work. (A) Cairns of Coll; (B) North-East Coll; (C) Treshnish Isles; (D) South-East Coll; (E) Gunna Sound; (F) Tiree; (G) West Coll (OS Open Data 2016).
Observations of basking sharks were made from a 9.8 m long RIB which, conditions permitting, made daily excursions to whichever areas, given prevailing weather, seemed most likely to reveal surface-feeding sharks. For each shark the total length was estimated and the following data recorded: location, date, time, sea surface temperature (SST, measured at −0.5 m using a Raymarine Dragonfly sensor), sea state (using the World Meteorological Organisation sea state scale), wind speed, wind direction, cloud cover and state of the tide (UKHO, 2016, for Gott Bay, Isle of Tiree). Measurements given for basking sharks are total lengths estimated for nearby sharks in relation to markings on the boat and as double the linear distance between the tip of the first dorsal fin and the tip of the tail, when at a distance only these features were visible. When possible, a record was made of how many other sharks were present within a distance of 100 m and whether the focal shark appeared to be solitary or a member of a pair or larger group. Under most conditions other individuals surface feeding within 100 m were readily detectable by eye and usually would also be plotted beforehand or afterwards as the observation boat moved along the coast. Any instances where a basking shark was observed to be within 25 m of a conspecific(s) so that it could sense and initiate or react to the behaviour of another shark were designated as putative ‘shark interaction’.
Weather and sea state (related to wave size) affect the ease with which the dorsal fin of surface-feeding basking sharks can be detected from a distance by boat-based observations. Above sea state 3 sharks cannot reliably be spotted at distances of more than 25 m, whereas in sea state 0 sharks can be regularly spotted from 2 or 3 km away.
Seventy-nine hours of observation were completed within the 54 day study period. It should be noted however that because of the extended time spent observing many individual sharks, the overall encounter rate per unit time is considerably lower than it would have been had the survey boat been motoring forward throughout the survey periods. Survey work usually began in the two or three subareas most likely to yield sharks, with the survey route not being designed to obtain an overall estimate of shark density.
Three methods were used to observe and record the features and behaviour of a proportion of the sharks:
1. Above-water observations were made and recorded by a boat-based researcher who whenever practicable also noted the distinctive features of each individual shark so as to permit individual identification (see Gore et al., Reference Gore, Frey, Ormond, Allan and Gilkes2016). The behaviours shown by the sharks were recorded using a pre-defined set of categories, as characterized in Table 1.
2. Underwater observations were made by snorkellers who were either paying guests or the crew of the basking shark tour operator Basking Shark Scotland (BSS). Most snorkellers took underwater still or video images of the sharks with a variety of cameras. Many snorkellers made their imagery available to the research team when requested. BSS crew were asked if they could determine from observation whether each shark appeared to be male (with visible claspers) or female. It is possible however that some small immature sharks with no visible claspers (NVC) were counted as female. Snorkellers were also asked to note the position on the body of fresh abrasions (skin patch colour from red to pink for recent occurrence or white when healing has begun). Snorkellers reported their observations to the boat-based researcher on return to the boat. Where possible photographs taken by crew and guests were also used to facilitate individual identification of the sharks.
3. Where practicable aerial video imagery of examples of close-following and similar behaviour were obtained by co-author LS using a Phantom 3 drone.
In all cases the boat, observer and snorkellers respected the responsible wildlife watching guide published by Scottish National Heritage (see http://www.snh.gov.uk/): boats did not normally approach sharks directly to within closer than 100 m and snorkellers in a group to within 4 m, but both positioned themselves where it was anticipated a shark might approach them, which they frequently did, sometimes to within a distance of a metre or two. When using drones, in conformity with SNH's 2017 ‘A Guide to Best Practice for Watching Marine wildlife’ advice that care should be taken not to disturb marine wildlife by flying too close to them, we avoided positioning a drone lower than 10 m above a swimming shark and more usually kept at more than twice this altitude. In no case was there any indication that the sharks even noticed the presence of the drone.
Table 1. Description of behavioural categories used during the study. A body length was ~5 m.

RESULTS
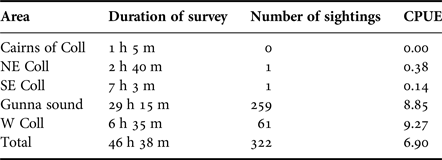
Table 2 shows the hours surveyed and the number of sharks encountered during the peak period (19 July–14 August 2016) within the main subareas. During this period a total of 322 sharks were encountered at a mean overall rate of 6.90 sharks h−1, the highest rates of sighting being in the west Coll (9.27 h−1) and Gunna Sound (8.85 h−1) subareas.
Table 2. The duration of basking shark surveys, the numbers of basking sharks sighted and the number of basking sharks encountered per hour (catch per unit effort, CPUE) during the peak observation period 19 July–14 August 2016 of the main subareas in the study.
On 234 occasions it was possible to determine the number of sharks visible within a 100 m radius of the focal shark. On over half of these occasions (54.7%) there were a total of 2–5 sharks present within this distance, on 20.1% of occasions 6–9 sharks and on 10.7% of occasions more than 10 sharks. On only 14.5% of occasions were solitary sharks the only individuals present within 100 m (Table 3).
Table 3. The number of focal basking sharks observed in groups of different sizes during the study overall.

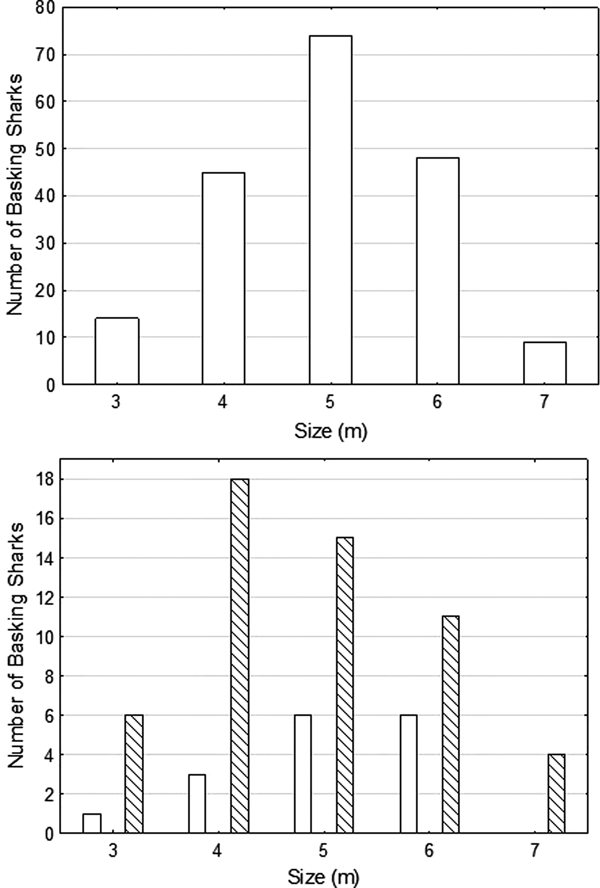
The mean size of sharks whose lengths could be estimated during the focal period was 4.96 m (±0.98 SD; range 3–7 m, N = 190) (Figure 3A), with the result of a Shapiro–Wilk-test (W = 0.9234, P = 0.5521) suggesting that size range of sharks was normally distributed. The sex of the shark could be determined in 70 cases; 54 were female or immature, 16 were male. The mean size of confirmed male sharks (mean = 5.06 ± 0.90 SD, range: 3–6 m) was greater than that of female sharks (or possibly NVC sharks) (mean = 4.79 ± 1.11 SD, range: 3–7 m) (Figure 3B), but not the range of size.

Fig. 3. The size distributions of basking sharks encountered during the main observation period of 19 July–14 August 2016, where size could be estimated, (Top) all sharks, (Bottom) males (empty bars) and females (or males with no visible claspers, NVC) (cross hatched bars).
During the study period the mean sea state was 3.6 (range 1–5), mean wind strength was Force 3.6 (range 1–7) with mean gust strength at 5.4 (range 3–9), while SST ranged from 13.4–15.0°C. No strong trends in relation to basking shark aggregating behaviour were revealed on analysis, other than that the sightings rate was much reduced as sea-state increased. This finding may be solely due to the increasing difficulty of spotting shark fins amid larger waves or reflect in part reduced surface feeding when plankton swarms are dispersed by wave action. However, basking sharks were sometimes encountered surface feeding intensely amid waves as high as 1.5 m.
Shark interactions
There were 48 occasions during the main study period when basking sharks were recorded interacting within 100 m of each other. Twenty-three of the 48 were observed from the boat, 18 by snorkelling and seven by drone. The sizes of the groups of sharks were a pair in 29 events, a trio in 11 events, four sharks feeding in four events, and one event each with five and with six sharks.
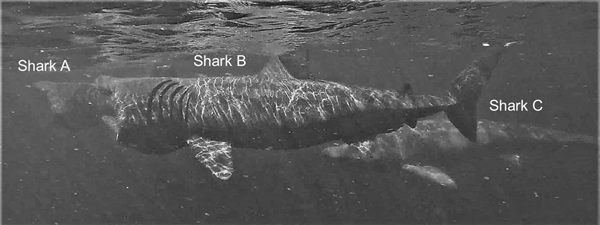
Basking sharks mostly fed independently (Figure 4) but were also on occasion approached directly by another shark, often as a prelude to close-following, as illustrated in the image (Figure 4) captured by drone. Various forms of close-following were observed on 41 occasions. Of these occasions, in 11 the lead shark was larger than the follower, in nine they were of equal size, and in 17 the lead shark was smaller than the follower, while in four cases the sizes could not be determined satisfactorily. An analysis of these data shows that there was overall no significant difference in the relative sizes of the lead and close-following shark (χ2 = 2.81, df = 2, P = 0.3).
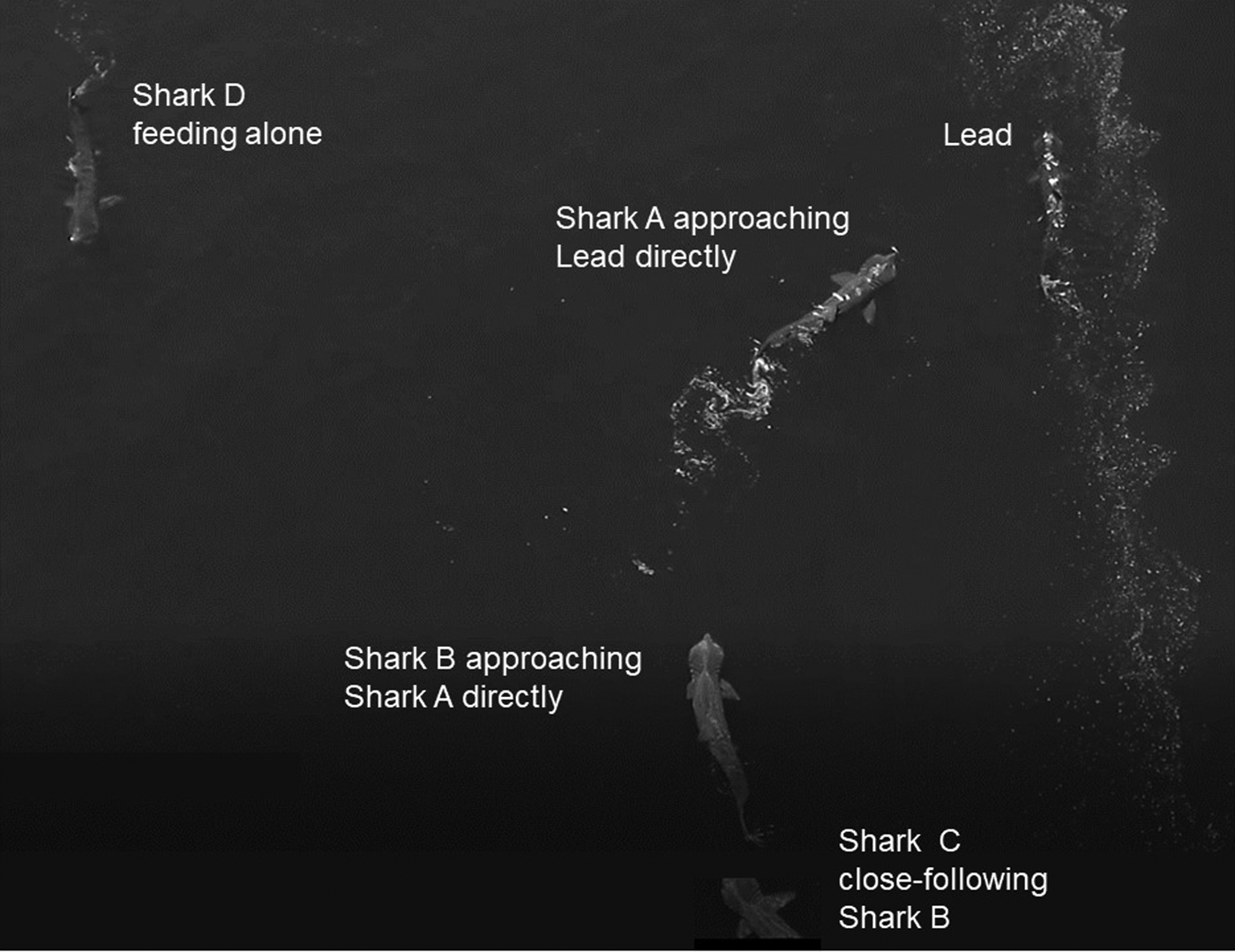
Fig. 4. Two examples of sharks approaching another directly captured by drone: Shark A approaching the lead shark and shark B approaching Shark A. Shark C is close-following shark B, while shark D is feeding alone.
Parallel swimming occurred in various group sizes, but tended to become echelon swimming, with one shark falling behind rather than remaining parallel (for example see Figure 5). In all 40 sharks were observed swimming in echelon. Two stacking events were recorded, one involving four female sharks and one of six sharks with five females and a male at the rear; in both events all the sharks were feeding. Cruising (that is, swimming without feeding) was observed both in solitary sharks and on occasions within groups.
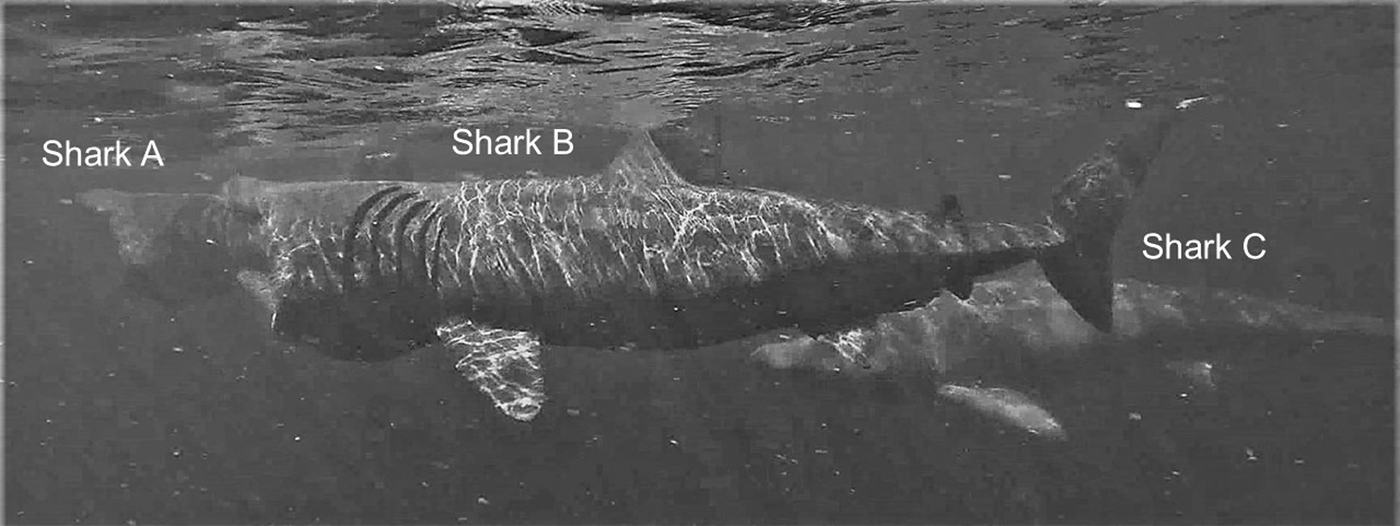
Fig. 5. Three basking sharks swimming in echelon, as seen underwater.
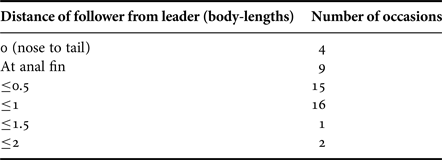
The sustained distance of the following shark from the lead shark varied markedly from the follower being at the anal fin of, or nose-to-tail with, the leader, to the follower being up to two body lengths away (Table 4). There was a significant difference in the number of sharks following at different distances behind the leader (χ2 = 27.4, df = 5, P < 0.001), with the majority being <0.5 body length from the leading shark. The position of the following shark was to the left on 16 (72.7%) occasions and to the right on five events. The additional video recordings (see Appendix 1(a–f)) revealed four occasions with the follower to the left and one with the follow to the right. The results show significantly more occasions where the following shark was on the left side of the lead shark (χ2 = 7.5, df = 1, P < 0.001).
Table 4. The distances between lead and follower sharks estimated as body lengths (~5 m).
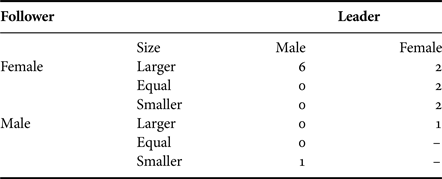
Where the sex of individuals could be determined during a close-following event (Table 5), a significant difference was found between the frequencies of different types of pairing (χ2 = 8.8, df = 3, P = 0.04). The most frequent occurrence (66.7%) was that both sharks appeared to be female (or NVC). Only on one (4.2%) of these occasions was a male (5.5 m) confirmed to be close-following a female (4 m). Inspection of additional underwater video material (Appendix 1(b)) provided seven occasions on which the sex of both the lead shark and one close-following behind could be determined (Table 5): on four occasions a male was recorded following a female, but on the other three occasions one female was observed to be following another. Together, the data show that males were following females (and possibly on occasion NVC sharks) in only 12.9% of the events.
Table 5. The sex of basking sharks observed participating in close-following events when the sex of both sharks could be determined. Note that Female may include small male sharks with no visible claspers.

When the sex and size of both sharks could be clearly determined and are taken together (Table 6), there was again a significant difference in the frequency of different types of pairing (χ2 = 21.7, df = 9, P = 0.01). The predominant result was that females following a male were more often larger than the male, while in only one instance was a male (5.5 m) following a female (4 m).
Table 6. Relationship between position, sex and size in close-following events, when both sex and size of both individuals could be estimated. ‘–’ represents redundant category.
Abrasions on individuals
During the study the natural markings and fin shape of 38 sharks were documented to allow individual identification (see Gore et al., Reference Gore, Frey, Ormond, Allan and Gilkes2016); of these sharks 23 were female (or NVC sharks) and 10 were male. Abrasions on pectoral fins of the type suspected of resulting from being held by a male seeking to mate were recorded on 43.5% of females (for example, see Figure 6) and 10.0% of males (Table 7). Abrasions on the nose, of the type suspected of being the result of a male pursuing and pushing at a female with his nose, were observed on 20.0% of males and 21.7% of females (Table 7). Most of the abrasions seen on the dorsum could be attributed to lampreys (for example, see Figure 7). Two males, 4 and 5 m, had abrasions on their gill slits, a location where scarring might be expected on females as a result of mating attempts by males.

Fig. 6. The right pectoral fin of a female basking shark showing white abrasions.

Fig. 7. A female basking shark (close-following a mature male out of image) with lamprey scars (inside white ring) on dorsum between dorsal fins. A live lamprey (Petromyzon marinus) is attached to her dorsum towards the rear of this area.
Table 7. The frequency of abrasions on one or both pectoral fins or the nose of individually identified male and female basking sharks.

Breaching events
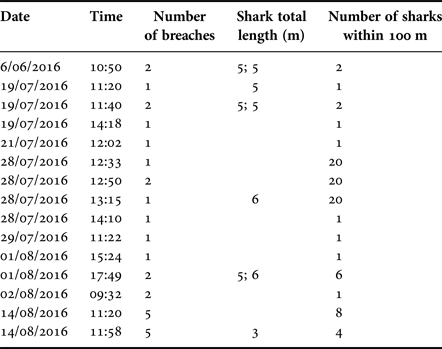
Twenty-eight breaches were recorded during 15 events through the whole study period 22 June–14 August 2016, with the number of breaches per event ranging between one and five (Table 8). The number of sharks present within 100 m of a breaching individual varied between zero and 20. Seven of the 15 events involved solitary individuals, one of which breached five times, while eight events occurred within 100 m of 1–20 other sharks. Most breaches were performed by sharks with an estimated length of 5 m, with 6 m being the largest estimated length and 3 m the smallest (Table 8). Conditions during breaching varied in respect to sea state (range 0–3), SST (range 12.5–15.0°C), weather (sunny, cloudy and rain) and time of day (09:32–17:49). However, breaching events (whether single or multiple) were recorded on only eight separate days through the survey period.
Table 8. Breaching events recorded between 22 June–14 August 2016.
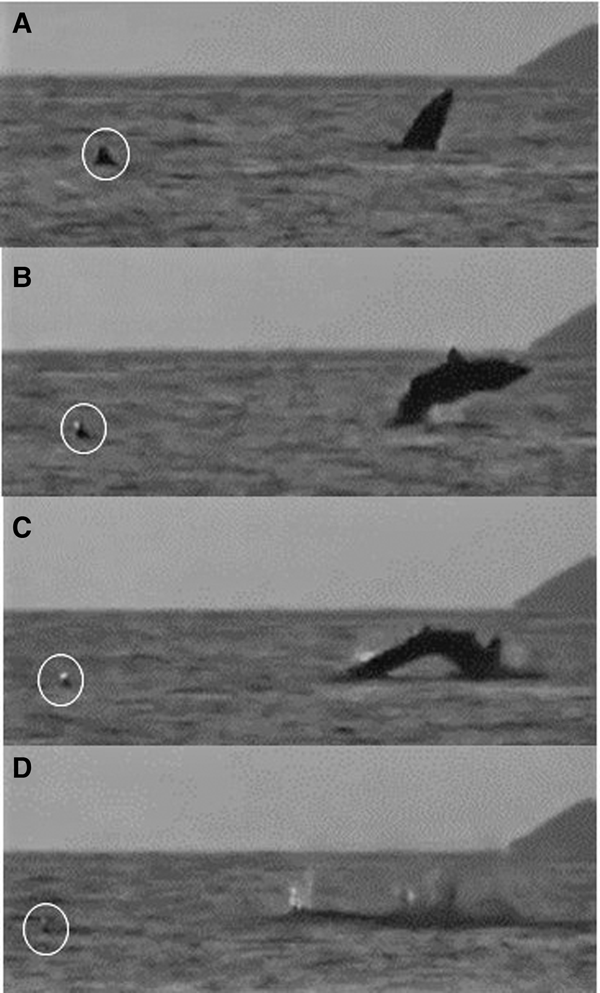
One breaching incident within an aggregation was recorded on a drone video (Figure 8; Appendix 1) and involved a 6 m lead shark and a 5 m (female) follower that maintained her position behind and to the left of the lead; both sharks swam directly forwards feeding for 3.5 min. The lead shark then closed its mouth and accelerated while diving. It happened by then to be about 3 body lengths (~16 m) from a group of waiting snorkel observers. After 7 s, the pressure wave from the lead shark's dive footprint reached the nose of the follower, which then accelerated and also dived (but could still be seen clearly by the drone). The follower then breached, 14 s after it had submerged, while the lead shark then breached 13 s later. In Figure 9, the sequence of a basking shark breaching close to and away from a conspecific can be seen off the Cairns of Coll at around 21:00 on 11 August 2015. The shark breached three times consecutively.

Fig. 8. Sequence of two basking sharks breaching captured by drone. (Top) a 6 m lead and a 5 m close-following female, both feeding. (Middle) the lead shark diving and the pressure wave from the dive footprint about to reach the follower's nose. (Bottom) the footprint of the two breaches (solid white arrow) and the position of a group of nearby snorkellers (arrow outline) and the accompanying RIB (inflatable boat) in the top left of the image.
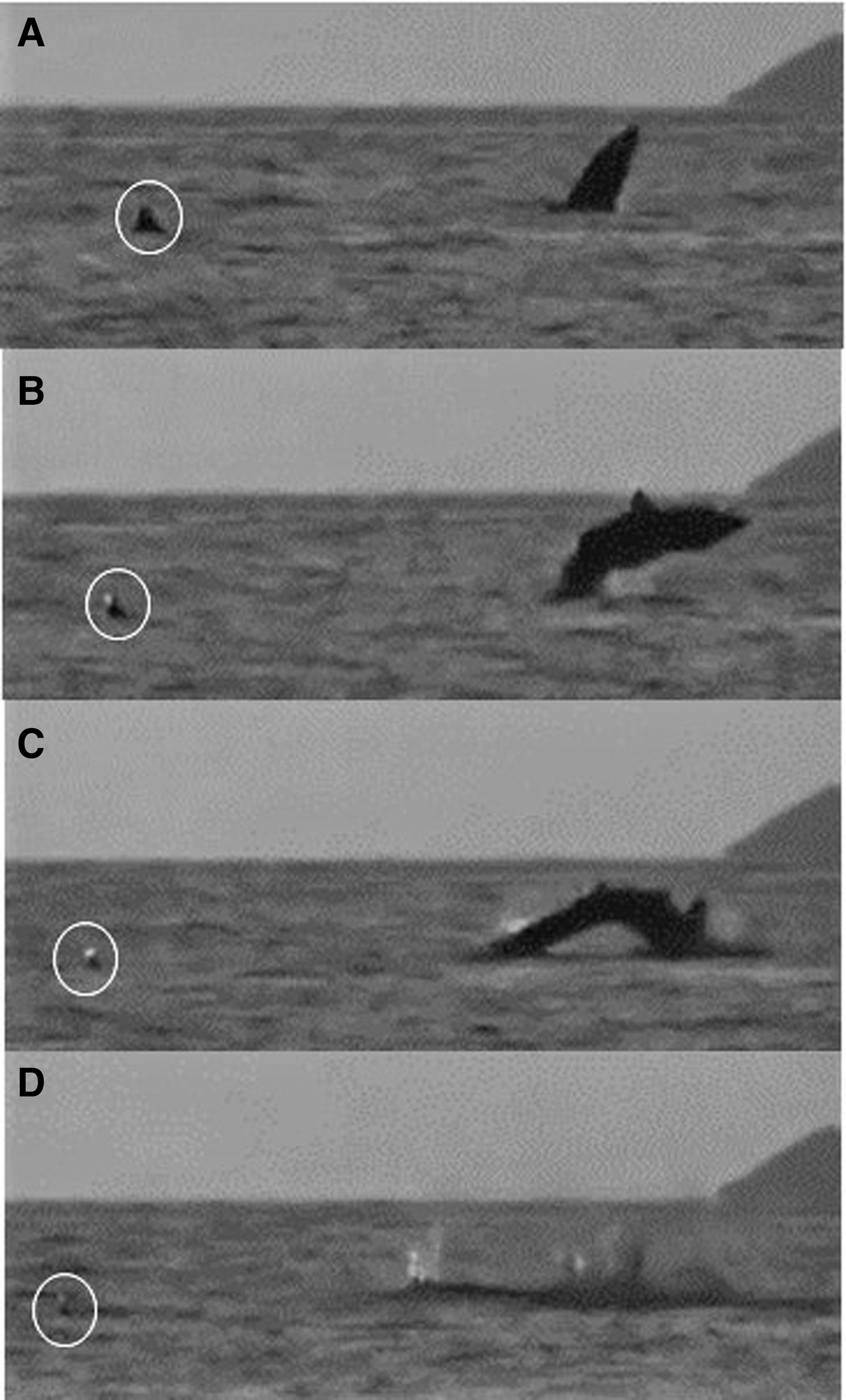
Fig. 9. A basking shark breaching at dusk 21:00 on 22 July 2015 close to and away from a conspecific (circled) (Appendix 1(h)) off the Cairns of Coll, Scotland. Breaching begins at (A) and ends with (D).
DISCUSSION
Methodological issues
During visual observation of basking sharks from above water aspects of their behaviour cannot be discerned with clarity and it is only occasionally that sharks can be sexed by an observer looking down through the water column. We therefore involved volunteer snorkellers to make underwater observations of the sharks and where possible obtain still or video imagery of them. We also used a radio-controlled aerial drone to record video imagery from overhead. While both close approach by boat and underwater observation during snorkelling might interfere with the basking sharks’ natural behaviour, drones provided a method of precisely recording the behaviour and horizontal movements of surface swimming sharks with reduced risk of influencing their activity. A limitation was that the drone could only be deployed when weather conditions were suitable and the sharks present within range of a stable homing base. There may have been instances of sharks below the surface not seen using the boat or drone. Employing the three techniques in concert helped overcome the limitations of each single method.
Even with experienced snorkel observers it can be difficult to be certain whether a shark is male or female. Immature males have only very small claspers that typically do protrude from the anal fin area, and so may not be readily visible. Thus some small males may have been reported as female or NVC individuals. At the same time a significant proportion of basking sharks (a third or more in this study area) carry lampreys, Petromyzon marinus, attached to their body. They are often attached to the base of dorsal or pectoral fins or the adjacent dorsum, or close to the area of the cloaca. In the latter location lampreys may be mistaken for claspers, hence conversely in a few cases individuals reported as male may actually have been female.
Shark interactions
While in many cases where basking sharks were close to one another there appeared to be no behavioural response by either shark, in other instances there clearly was a reaction. Bres (Reference Bres1993) noted that social behaviour in the form of dominance hierarchy based on size and sex has been observed in bull (C. leucas), bonnethead (S. tiburo), lemon (N. brevirostris) and Galapagos (C. galapogensis) sharks. Also, in their review, Jacoby et al. (Reference Jacoby, Croft and Sims2012) noted grouping behaviour in juvenile and adult sharks that could be distinguished as either aggregations or social groups. They suggested that social groups could be very short term and sporadic and may function for finding a partner and mating. In species that form schools, it has been proposed that population density may be needed to trigger mating behaviour (Demski, Reference Demski1990).
Are abrasions related to mating?
We hypothesized, following Bres (Reference Bres1993) and Harvey-Clark et al. (Reference Harvey-Clarke, Stobo, Helle and Mattson1999), that the abrasions and scarring often noticeable on the dorsum, gills and pectoral fins of many individuals were injuries to the female caused by the attention of males looking to mate. The abrasions evident could be injuries to males caused by them repeatedly nudging and pushing against females prior to attempts at mating. We therefore expected abrasions in these areas to occur almost exclusively on females, and nose abrasions on males.
Abrasions on the dorsum of both sexes mostly appeared a result of recent lamprey attachment, and not a consequence of mating. Close inspection of images (for example, see Figure 7) show that the scars are mostly round in shape rather than elongated or unformed, and that they approximate to the size of lamprey mouths. Older lamprey scars appear as black rings, which could be the result of healing of the characteristic near-circular white scars being logged in this study. Abrasions on the nose seem not to be related to the sex of the shark, being evident on almost the same proportions of individuals of each sex (20.0% of males and 21.7% of females). However, abrasions on the pectoral fins were observed in 43.5% of females but only 10.0% of males, suggesting that some or most of these abrasions may be a consequence of recent attempted mating out of view of the observers. The only abrasions on gills recorded were on two males.
Breaching behaviour
While breaching clear of the water has been reported in some shark species (see Introduction), comparable breaching behaviour is more frequently associated with marine mammals, notably some large whale species. The factors prompting breaching in whales are also uncertain, but in humpback whales (Megaptera novaeanglia) these include increased frequency during windy conditions and the presence of multiple calves and of conspecific groups 1–4 km away (Kavanagh et al., Reference Kavanagh, Owen, Williamson, Blomberg, Noad, Goldizen, Kniest and Cato2016). In sperm whales (Physeter macrocephalus) breaching bouts can last several hours (Waters & Whitehead, Reference Waters and Whitehead1990). Aerial behaviour including breaching in humpback whales has been shown to occur prior to a change in behaviour and Frankel et al. (Reference Frankel, Clark, Herman and Gabriele1995) suggest that intentionally or not it may convey information to other whales, such as an animal's location or behavioural state. These authors also commented that aerial behaviour is presumably much more energy consuming than vocalization and so is unlikely to be used exclusively for acoustic signalling.
The observations described here confirm that in contrast to some earlier opinions breaching in basking sharks is not infrequent and suggest that it may have more than one trigger. Breaching was observed to occur both in sharks that appeared to be alone and by others that were in aggregations. Six of the seven occasions involving two to five repeated breaches occurred when the breaching shark was close to one or more conspecifics, while the fact that breaching was only observed on certain days suggested that breaching by one shark may trigger breaching by another (for example, see Figure 8). The shark launches itself partially or wholly clear of the water, typically landing close by at a near-horizontal angle with a considerable and audible splash (for an example see Appendix 1(g)). The impact on the water on re-entry (APPEA, 2013; Kavanagh et al., Reference Kavanagh, Owen, Williamson, Blomberg, Noad, Goldizen, Kniest and Cato2016) could communicate both its presence and size. Both trends suggest that breaching may have a social function, possibly providing a mechanism by which an individual can draw the attention of others to its presence. Alternatively breaching might possibly be used to deter or escape a potential predator such as an orca (Orcinus orca), small numbers of which occasionally occur in the study area, although none were noted during the present study. Conversely, white sharks breach when hunting their seal prey, but will launch suboptimal strikes in areas of intraspecific competition, leading to a lower predation success (Martin et al., Reference Martin, Hammerschlag, Collier and Fallows2005). There is the possibility in the present study that some breaching may have been partly triggered by the shark sensing the presence of snorkellers (Figure 8). However a breaching event outside the present study was observed by MG from close-by (sitting 10 m above overlooking the cove) without any possible disturbance as follows. An immature 3 m basking shark swam alone into a small deserted sandy cove and suddenly breached, before unhurriedly swimming back out of the cove again. No other shark was in or near the cove.
Another explanation sometimes advanced is that breaching may serve to rid sharks of parasites or commensals. Jumping out of the water in an apparent attempt to dislodge sharksuckers, Echeneis naucrates, attached to their body has been described for both blacktip and white sharks (Ritter & Brunnschweiler, Reference Ritter and Brunnschweiler2003; Brunnschweiler, Reference Brunnschweiler2006). As noted above, in the present study some basking sharks had lampreys attached to their body. Among the few cases where breaching sharks could be checked for the presence of lampreys, one shark did appear to have a lamprey attached to the first dorsal fin, but this appeared to be still attached as the shark re-entered the water.
Basking shark breaches also occur frequently off Malin Head, Co. Donegal, Ireland, where about 600 have been noted by Bren Whelan (personal communication). Matching our own data, he has observed a very large number of multiple breaches including one occasion when apparently the same shark breached three times within 90 s. He also recorded an occasion when two sharks breached in near unison within 50 m of each other. Both Whelan's experience and our own records noted that breaching was much more frequent in 2016 than in 2015, and in general seems to be more frequent in some years than others. Whelan has noted that SST at Malin Head varies significantly from year to year and suggests breaching may be more common when the sea is warmer. Along similar lines Schwartz (Reference Schwartz2013) has suggested that an abrupt change in water temperature may trigger jumping and spinning out of the water in both blacktip and spinner sharks (C. brevipinna).
Is close-swimming related to courtship?
The sizes of basking sharks recorded during the study showed a normal distribution (Figure 2) with a mean size of 4.96 m. Where individuals could be sexed the size range of males was 3–6 m and of females 3–7 m. Size at maturity for basking sharks is uncertain. In Scotland basking sharks have been suggested to mature at >6.22 m total length (N = 1) with a mean of 8.12 ± 0.16 m (N = 3) for males and 7.24 m (N = 1) with a mean of 8.24 ± 0.12 m (N = 5) for females (Matthews, Reference Matthews1950). By contrast Bigelow & Schroeder (Reference Bigelow and Schroeder1948) found in the western North Atlantic that males mature at 4.57–6.10 m. Ali et al. (Reference Ali, Saad, Reynaud and Capapé2012) recorded a 6.9 m female basking shark off Syria that was pregnant. These results suggest that the larger females and males in the present study could have been mature and so pre-courtship and mating behaviour might be expected.
Nonetheless, no indication was observed throughout the study of a following shark attempting to grab the pectoral fin of the shark it was following. Further, as the leader and follower shark were equally likely to be of either sex, and there was no observed mean difference in size between leader and follower, this suggests that the following behaviour observed is not primarily related to courtship. The difficulty in being sure about the sex of a proportion of individuals might explain occasional records of females apparently following males, even if in fact it is exclusively males that follow females. However it is unlikely that occasional errors of this type could explain the complete lack of any correlation between gender and leader or follower position. Similarly, given that a significant difference was observed between mean size of males and females, a difference between mean size of leader and follower might have been expected if it is normally one sex that follows the other, whereas in fact none was observed.
Swimming patterns of basking sharks in aggregations have been described to include milling, flank approaching from one side, parallel swimming, swimming in echelon formation, following, nose-to-tail following and cart-wheeling (Hallacher, Reference Hallacher1977; Sims et al., Reference Sims, Southall, Quayle and Fox2000; Wilson, Reference Wilson2004), although these terms have not always been clearly defined. Sims et al. (Reference Sims, Southall, Quayle and Fox2000) described how a leading shark may be followed by up to three sharks within a distance of less than half a body length. We documented these behaviours, although it must be emphasized that intentional swimming by one shark towards another only happened on a minority 25.4% of occasions when two or more sharks were in proximity (within 100 m) of each other; on the greater number of occasions individuals appeared to be feeding independently albeit on the same concentrations of zooplankton.
Reports of mating behaviour in some other shark species have also described close-following, for example of a female blacktip reef shark by a male, with a second male following 5 m behind the pair (Johnson & Nelson, Reference Johnson and Nelson1978). In this case the female swam with a distinct tail-up posture, which however was not observed in basking sharks in the present study. Other features of the behaviour observed in the present study also fail to point to close-swimming behaviour being related primarily to courtship. First, in most cases of close following, both sharks were feeding (83.7%) and continued to feed despite the presence of the other shark. Secondly, where the sex of both sharks could be determined, there was no tendency for a male shark to be following a female as might have been anticipated on the basis of comparison with observations of mating in other shark species. On occasions where sharks of the opposite sex were involved, in only 12.9% was the male following the female, while in the other cases it was the female that was following a male. Thirdly, in a majority (64.5%) of all cases where we could identify the sex of individuals, the following shark belonged to the same sex as the lead shark. Thus in the cases we observed the sex of both lead and follower sharks appeared to be determined randomly. Close-following has also been reported in non-copulatory situations in bonnethead and grey reef sharks (Bres, Reference Bres1993).
The species known so far to show behaviour and prey preference most similar to that of basking sharks at aggregation sites are manta rays, Manta birostris and M. alfredi. A series of locations have now been described, notably in Japan (Homma et al., Reference Homma, Maruyama, Itoh, Ishihara, Uchida, Seret and Sire1999; Yano et al., Reference Yano, Sato and Takahashi1999), the Maldives (Stevens & Rubin, Reference Stevens and Rubin2008; Anderson et al., Reference Anderson, Adam and Goes2011), Mozambique (Marshall & Bennett, Reference Marshall and Bennett2010) and Hawai'i (Deakos, Reference Deakos2012), where seasonal aggregations of mantas form to take advantage of dense swarms of zooplankton concentrated by the local topography. Here feeding mantas often show echelon swimming, with sometimes up to 10 or more animals swimming in extended formation, presumably to benefit hydrodynamically from the swimming movements of the individuals forward of them. However pre-copulatory behaviour has also been observed at these sites, with one or more males forming a ‘mating train’ swimming in fast pursuit of a single female (Yano et al., Reference Yano, Sato and Takahashi1999; Stevens & Rubin, Reference Stevens and Rubin2008; Marshall & Bennett, Reference Marshall and Bennett2010). The fast swimming is interrupted by quick turns and somersaults initiated by the female and often mimicked by the pursuing males (Marshall & Bennett, Reference Marshall and Bennett2010). Mating itself has also on occasion been observed; the leading male, typically after several attempts, bites the female's pectoral fin before twisting to insert a clasper into the female's cloaca (Yano et al., Reference Yano, Sato and Takahashi1999). Thus, in these species, there is clearer circumstantial evidence to indicate that while formation swimming is primarily related to feeding, males also take the opportunity to pursue available females. To date, there are no published records of close-following behaviour in the two other plankton-feeding elasmobranchs, whale (Rhincodon typus) and megamouth (Megachasma pelagios) sharks.
Alternative explanations for close swimming
A number of alternative explanations for close-following and echelon swimming suggest themselves. First, it may provide an energy saving hydrodynamic advantage to the following shark. A basking shark positioned near to the pectoral fin of another may benefit from the echelon formation, as geese and some other large flocking birds are believed to do (Lebar Bajec & Heppner, Reference Lebar Bajec and Heppner2009), while a shark directly behind another may be in its slipstream. There would be an even greater hydrodynamic advantage by swimming with the mouth closed. Saving energy through swimming in schools is known for other fish, for instance for the golden grey mullet, Liza aurata (Marras et al., Reference Marras, Killen, Lindström, McKenzie, Steffensen and Domenici2015). In addition, Reale (Reference Reale2012) designed a ‘Strait Power’ turbine based on filter feeding in basking sharks. His calculations suggest that the shape of the shark's head is such that as it swims along a pressure differential is created between the mouth and the body below the gills. This draws water passively through the gills, in contrast to the increased pressure required to push water through the gills such as is believed to occur in ram filtering in whale sharks and megamouth sharks (Motta & Wilga, Reference Motta and Wilga2001; Nakaya et al., Reference Nakaya, Matsumoto and Suda2008). It is possible that this same hydrodynamic effect may also benefit an individual feeding alongside or behind another, or it may result in the plankton missed by the first individual being funnelled towards the second within the turbulence created by the leader.
The behaviour of zooplankton themselves may also benefit an echelon-swimming or close-following basking shark. The copepod Calanus finmarchicus forms an important part of basking shark diet (Blumer et al., Reference Blumer, Mullin and Thomas1964; Sims et al., Reference Sims, Southall, Tarling and Metcalfe2005). Fields et al. (Reference Fields, Shema, Browman, Browne and Skiftesvik2012) studied the escape response of this species and found that later life stages are able to use mechano-sensing to detect pressure changes associated with the approach of a predator. They are also able to make large jumps relative to their body size, involving 400 times their normal energy expenditure, as opposed to the breast stroke movements mainly used while feeding (Wadhwa et al., Reference Wadhwa, Andersen, Kiørboe and Bohr2015). This suggests that while some of the calanoid prey immediately ahead of an approaching basking shark may be able to jump out of its way, a second shark may benefit by positioning itself behind and to one side, so that it can catch the copepods after they have jumped. The ability of the copepod to make a second jump is much reduced as a result of the loss of energy reserves after the first jump (Wadhwa et al., Reference Wadhwa, Andersen, Kiørboe and Bohr2015), leaving it in the path of a following shark.
Our data suggest that even if on at least some occasions close following and echelon swimming in basking sharks were to lead to mating, it is not the only or primary reason why these sharks may interact with each other. In the great majority of instances they focused strongly on feeding even when close following or echelon swimming, and there are probably one or more mechanisms by which a following shark can gain some energy advantage, either by using less energy or gaining more prey. In our dataset there was no tendency for sharks swimming close to each other to be of opposite sexes and no tendency for either the lead shark or the follower to be of a particular sex. It is also possible that basking sharks, particularly when younger, cannot tell the sex of another individual except by its response to a sexual advance. This is known to be the case in a number of bird species, most famously the European robin (Lack, Reference Lack1943). It also remains possible, as proposed by Sims et al. (Reference Sims, Southall, Quayle and Fox2000) that, while basking sharks aggregate in certain areas primarily in order to feed, it may benefit males to take advantage of the close proximity of other individuals to test whether or not females may be receptive. Such a system is common among herbivorous mammals, such as Uganda kob (Leuthold, Reference Leuthold1966), with mature males competing for dominance of the prime areas where females concentrate to feed.
CONCLUDING REMARKS
The present study has confirmed that basking sharks will, at locations where feeding aggregations tend to form, show following and close-following behaviour. We have been able to provide clear documentation of this behaviour on video sequences captured by an overhead drone. These actions have been interpreted in the literature as courtship or pre-mating behaviour. However, we observed no sign of actual mating and contrary to expectation, we found no relationship between the sex of a shark or its size and close-following. This suggests that following behaviour in these circumstances is not primarily courtship. Further, abrasions on the nose suspected to be related to mating were found to occur on both sexes. Breaching by basking sharks has also been proposed as a means of attracting the opposite sex. We observed breaching to be not infrequent and captured examples of the behaviour on video, but again there was no clear evidence to indicate that breaching is strongly associated with mating. It seems more likely that individuals show close-following primarily for feeding-related hydrodynamic advantage. It remains plausible that mature sharks use feeding aggregations to make initial contact with receptive females. Thus for the time being it seems safest to retain the terminology proposed by Sims et al. (Reference Sims, Southall, Quayle and Fox2000) and refer to such behaviour as pre-courtship (rather than courtship) activity. Further research is needed to resolve these issues, ideally at locations where a higher proportion of individuals are mature.
ACKNOWLEDGEMENTS
We would like to thank Dan Beecham, Bren Whelan and Laurent Cocherel for allowing us to analyse video footage of basking sharks that they had taken and Bren Whelan for very helpful discussions on breaching behaviour. We also thank Anthony Reale for his insight and discussion of modelling basking shark feeding mechanisms. Basking Shark Scotland is a member of the WiSE scheme.
APPENDIX 1
List of additional video recordings.
(a) Basking Shark Scotland, Oban, Scotland, UK.
(b) Beecham, D. 2007. Sennen Cove.
(c) Carthy, J., Caliso Bay, County Waterford, Ireland. 12.5.2016, https://www.youtube.com/watch?v=CsF50PM3hDQ
(d) Creamer, R., Caliso Bay, County Waterford, Ireland. 13.5.2016, www.youtube.com/watch?v=q2VmWgyzl0w
(e) Ring, E., Youghal Bay, County Cork, Ireland. 6.6.2016, https://www.youtube.com/watch?v=fnHQ0mD-knE&t=159
(f) Ring, K., Youghal Bay, County Cork, Ireland. 1.6.2014, https://www.youtube.com/watch?v=CsF50PM3hDQ
(g) Whelan, B., Malin Head, County Donegal, Ireland. 22.9.2015, https://www.youtube.com/watch?v=0jwX_gb_CHQ
(h) Cocherel, L. Gunna Sound, Scotland, https://vimeo.com/135502913