Introduction
Knowledge about the carbon (C) cycle is essential for supporting climate change mitigation strategies. However, data on belowground carbon stocks are scarce. Estimates are often made from aerial biomass data, calculating underground biomass as 28% of aboveground biomass. Thus, the global fraction of total C has been calculated to be 113 Gt·C (Ma et al. Reference Ma, Mo, Crowther, Maynard, van den Hoogen, Stocker, Terrer and Zohner2021).
Forests play an essential role as a global carbon sink, particularly the tropical ones are important for their capacity to mitigate atmospheric carbon (Case et al. Reference Case, Johnson, Bartowitz and Hudiburg2021). However, tropical forest carbon may be underestimated due to a lack of data (Houghton Reference Houghton, Yoy, Saugier and Mooney2001). For example, the tropical mountain cloud forest (TMCF) is one of the least studied.
Carbon stocks in TMCF appear similar on average to those in lowland forests but vary over an order of magnitude between sampling sites (Spracklen & Righelato Reference Spracklen and Righelato2014). Environmental heterogeneity and disturbances from human activities may explain differences in biomass and C content in this forest type (Hamilton Reference Hamilton1995).
Variation in belowground carbon stocks is especially uncertain. Belowground carbon is deposited mainly in biomass and as soil organic carbon (SOC). SOC depends on organic matter, decomposition processes in the soil solution, and organic compounds released by living roots (Pausch & Kuzyakov Reference Pausch and Kuzyakov2018). In terrestrial ecosystems, SOC estimates are made relatively frequently but do not correspond to carbon in root biomass. Root biomass may vary with aboveground forest properties. In this sense, Dhyani & Tripathi (Reference Dhyani and Tripathi2000) reported more root biomass near the trees than farther from them. Furthermore, root biomass closely correlates with the diameter of tree trunks (Finér et al. Reference Finér, Ohashi, Noguchi and Hirano2011). Therefore, it would be expected that underground carbon pools scaling with aboveground biomass is greater in better-preserved forests with a higher density of large trees.
In Mexico, TMCF has a restricted distribution in the mountain ranges across the country. Mexican records of underground biomass range from 8.56 to 36 Mg·ha−1 (De Jong et al. Reference De Jong, Cairns, Haggerty, Ramírez-Marcial, Ochoa-Gaona, Mendoza-Vega, González-Espinosa and March-Mifsut1999, Avilés-Hernández et al. Reference Avilés-Hernández, Velázquez-Martínez, Angeles-Pérez, Santos-Posadas and Llanderal2009). The main objective of this work was to estimate underground carbon, considering the role of root biomass and SOC in TMCF fragments in Sierra Madre Oriental, Mexico. The relationship between root biomass with canopy coverage and tree diameter was also analysed. The hypothesis that the concentration of roots in the forest is concentrated near the trunks of trees was evaluated. The results of our research will contribute to improved management of forest systems and maintenance of carbon capture and storage ecosystem services in the TMCF.
Materials and methods
Study area
The study area was located in east-central Mexico in the Sierra Madre Oriental (Figure 1). This is a long mountain range that maintains fragments of TMCF (730–2,500 m asl) with moderate perturbation (Ruiz-Jiménez et al. Reference Ruiz-Jiménez, Téllez-Valdés and Luna-Vega2012). The climate is humid (1,048–2,385 mm) and temperate (mean ∼17°C) with frequent fog (Ruiz-Jiménez et al. Reference Ruiz-Jiménez, Téllez-Valdés and Luna-Vega2012).
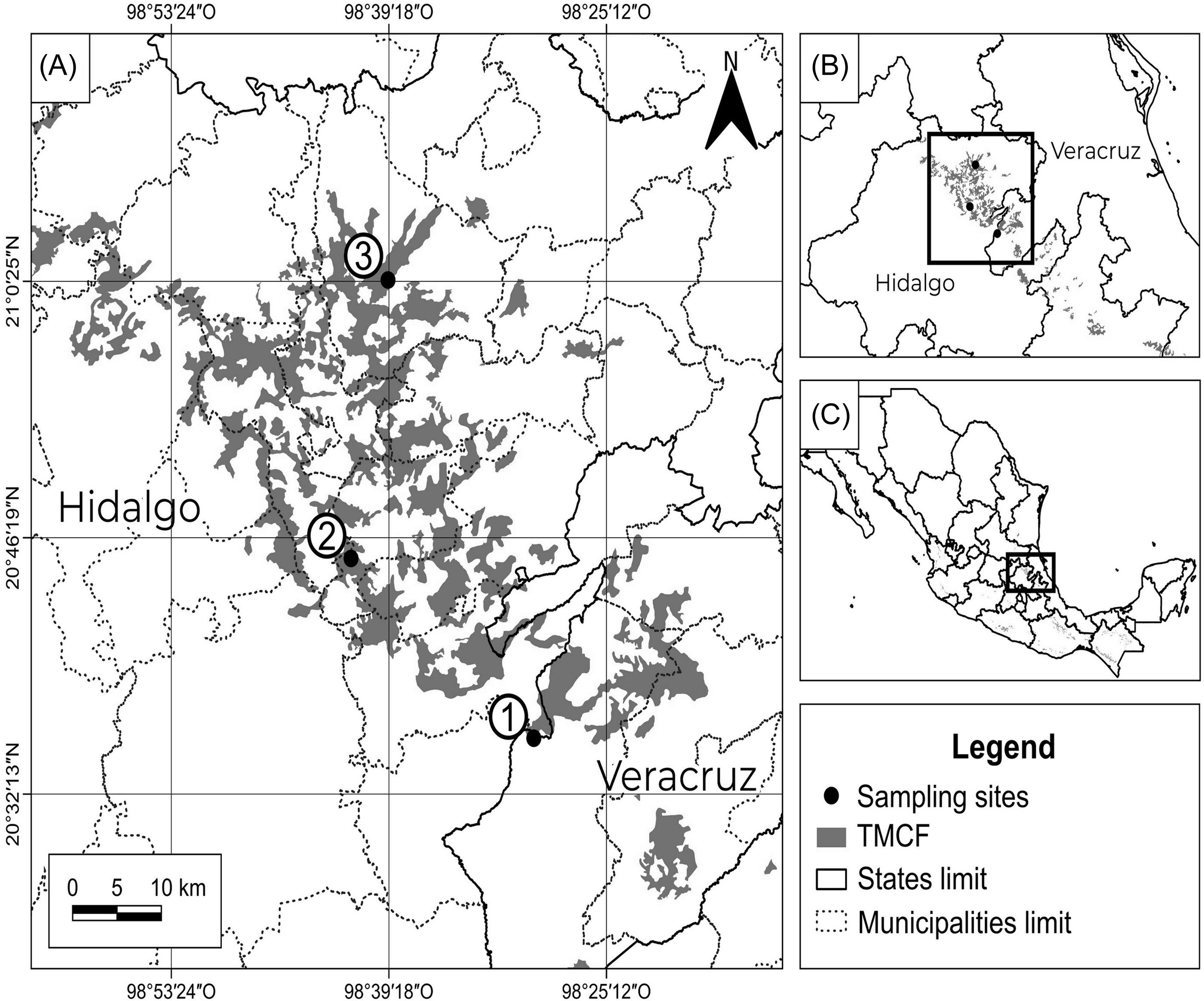
Figure 1. Localization of sampling sites into the TMCF in the central Sierra Madre Oriental in central-eastern Mexico.
The TMCF has holartic and tropical tree elements (González-Espinosa et al. Reference González-Espinosa, Meave, Ramírez-Marcial, Toledo-Aceves, Lorea-Hernández and Ibarra-Manríquez2012). Some of the representative tree genera in the study area are Arbutus, Clethra, Carpinus, Ilex, Liquidambar, Ostrya, and Quercus (Luna-Vega et al. Reference Luna-Vega, Ocegueda and Alcántara1994).
Fieldwork was carried out within three suitable conservation fragments of TMCF, with similar tree cover (87.0–91.5%) and diameter at breast height (DBH) (40–55 cm), located in Huayacocotla, Xochicoatlán, and Tlanchinol municipalities. Tree cover was measured with a spherical densitometer and DBH using a diameter tape.
Sampling method
Sampling was carried out from February to May 2022. Within each fragment, 30 sampling points were located 12 m apart along a 360 m transect, oriented in a northeast–southwest direction. At each sample point, four soil sampling points were established each at the trunk of a focal tree (DBH > 40 cm). Two samples were located 1.5 m from the tree trunk (close) and two others were at least 3 m from any large tree (far). Soil samples 30 cm deep were collected using a corer 8 cm in diameter.
Laboratory work
The soil samples were sieved and roots were then extracted manually and washed and dried in an oven at 75°C for 24 h. The dried roots were classified by their diameter into very fine (<1 mm), fine (1–3 mm), and coarse (>3 mm) roots. The roots of each category were weighed to the nearest 0.01 g.
Subsequently, the soil from the samples with roots removed was mixed. Then, four soil samples were prepared per transect: two samples close to the trunk of the trees and two samples separated from the trunk of any tree. The physical and chemical properties of the soil samples were determined.
SOC (Mg·C·ha−1) at each sampling site was estimated using the following equation (González-Molina et al. Reference González-Molina, Etchevers-Barra and Hidalgo-Moreno2008):
where BD is the bulk density (g·cm−3), OC is the SOC, considering that the organic matter (OM) contains 58% C, and D is the sampled depth of the soil (30 cm). D was 1.1637 for the Huayacocotla site, 1.1197 for Xochicoatlán, and 0.8312 for Tlanchinol.
To estimate the carbon content in the root biomass, the conversion factor used was 0.5 (Carrillo-Anzúres et al. Reference Carrillo-Anzúres, Acosta-Mireles, Flores-Ayala, Juárez-Bravo and Bonilla-Padilla2018).
Statistical analysis
A general linear model was fitted with root biomass as a response variable, and sampling site (Huayacocotla, Tlanchinol, and Xochihuatlán), root type (very fine, fine, and coarse), and location (close and far) as explanatory variables. Jackknife resampling was performed and Tukey’s multiple comparison was used as a post-hoc test. Spearman correlations related to root biomass, DBH, and canopy cover were calculated.
Statistical analyses were calculated using SYSTAT v. 12 and Past v. 4.09
Results
The three TMCF fragments had soil conditions similar to those of loamy and acid soils (pH 4.11–4.46) and electric conductivity values between 0.10 and 1.14 dS m−1. Concerning organic matter, Huayacocotla and Tlanchinol fragments had similar values (4.64–5.51%), while the Xochicoatlán fragment had a significantly higher percentage (10.15%). Regarding forest conditions, the canopy cover was similar in the three fragments, with values higher than 87%. However, the highest average DBH was found in Tlanchinol (55–58 cm) compared to the other two sites (42–52 cm).
Total root biomass was 7.08 ± 1.5 Mg·ha−1, which stores ∼3.54 ± 0.75 Mg·C·ha−1. Coarse roots contributed 36.8%, fine roots 35.5%, and very fine roots 27.7% of the total biomass. The fragment of Tlanchinol contained the highest root biomass at 41.5% (Table 1).
Table 1. Root biomass (g·m−2) for each sampling site and root diameter category. Carbon content per site (g·C·m−2). Mean ± SD is shown
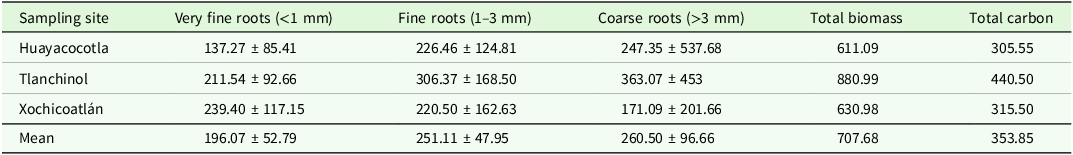
Sampling site (F = 6.43, p = 0.002, df = 2) and root type (F = 3.131, p = 0.044, df = 2) were significant factors. Tlanchinol had significantly more root biomass than Huayacocotla (diff. = −90.611, p = 0.004) and Xochicoatlán (diff. = 83.335, p = 0.008) (Table 1). The biomass of coarse roots was significantly higher than that of very fine roots (diff. = −65.08, p = 0.05) (Table 1). Of all the possible interactions between the factors, only the one between the sampling site and the type of root was significant (F = 2.936, p = 0.02, df = 2).
No significant correlations were recorded between total root biomass and canopy cover in Huayacocotla (r s = 0.002, p = 0.98), Tlanchinol (r s = 0.201, p = 0.237), or in Xochicuatlán (r s = 0.151, p = 0.337). The correlation between total root biomass and DBH was significant (r s = 0.406, p = 0.039) in Huayacocotla but not in Tlanchinol (r s = 2.78, p = 0.234) and Xochicoatlán (r s = 0.072, p = 0.749).
The highest calculated SOC was for Xochicoatlán (146.30 ± 7.62 Mg·C·ha−1), followed by Tlanchinol (93.25 ± 45.19 Mg·C·ha−1), the lowest was in Huayacocotla (85.16 ± 42.21 Mg·C·ha−1).
Total C stored in the soil (C in roots + SOC) by site was 88.21 Mg·C·ha−1 for Huayacocotla, 97.65 Mg·C·ha−1 for Tlanchinol, and 149.46 Mg·C·ha−1 for Xochicoatlán. On average, the underground carbon stored in the TMCF was 111.77 ± 32.97 Mg·C·ha−1.
Discussion
In the study area, SOC contributed 98% of the carbon stock belowground. However, the value obtained in this work was lower than the global average SOC to TMCF (158.65 Mg·C·ha−1, SD = 50) (Llerena et al. Reference Llerena, Kurbatova and Grigorets2021) but similar to the average reported for Mexico (99.6 Mg·C·ha−1, SD = 88.8) (Paz-Pellat et al. Reference Paz-Pellat, Espinoza, Gaistardo, Etchevers and de Jong2016). Roots’ contribution to belowground C in TMCF is low compared with SOC. Values reported, using allometric equations, fluctuate between 3.8 and 19.6 Mg·C·ha−1 (Llerena et al. Reference Llerena, Kurbatova and Grigorets2021) and up to 48.1 Mg·C·ha−1 to Mexico (Masuhara et al. Reference Masuhara, Velarde, Pérez, Gutiérrez, Vázquez, Salcedor, Juárez and Merino2015). While estimates from root sampling, up to 30 cm deep, fluctuate between 2.23 and 2.81 Mg·C·ha−1 (Avilés-Hernández et al. Reference Avilés-Hernández, Velázquez-Martínez, Angeles-Pérez, Santos-Posadas and Llanderal2009, Moser et al. Reference Moser, Leuschner, Hertel, Graefe, Soethe and Lost2011). These values are slightly lower than those reported in this work. The mean root biomass obtained in this study was 707.60 g·m−2, with 353.85 g·C·m−2; these results show the high potential that TMCF has to store underground carbon in roots. In this regard, TMCF had more root biomass and carbon than reported in other tropical forest types (Yuan & Chen Reference Yuan and Chen2010, Liu et al. Reference Liu, Liu, Wan, Wang, Wang and Liu2017, Quintero-Gradilla et al. Reference Quintero-Gradilla, Muñoz and Castillo-Parra2022).
Among other environmental aspects, the complex floristic composition of the vegetation generates a high variation in root biomass and stored carbon, even between nearby locations (Finér et al. Reference Finér, Ohashi, Noguchi and Hirano2011, Rosado et al. Reference Rosado, Martins, Colomeu, Oliveira, Joly and Aidar2011, Quintero-Gradilla et al. Reference Quintero-Gradilla, Muñoz and Castillo-Parra2022). It is expected that the dominant species will make the greatest contribution to biomass. For example, fine root biomass in a forest dominated by Liquidámbar styraciflua was ∼3.8 Mg·ha−1 representing 27% of the total (Coyle et al. Reference Coyle, Coleman and Aubrey2008). In the three forest fragments studied, L. styraciflua was abundant, which undoubtedly influenced the results obtained.
The physical and chemical characteristics of the soil are related to the vertical and horizontal distribution of fine roots (Fujimaki et al. Reference Fujimaki, Tateno, Hirobe, Tokuchi and Takeda2004). In general, the influence of the transfer of carbon from the vegetation to the soil due to the contribution of leaf litter generates that the highest concentration of nutrients occurs in the top few centimeters of the soil, which is why the most significant SOC values and biomass of fine roots occur there. The sampling sites had loamy soils with high organic matter content. This soil type is common in montane forests (González-Espinosa et al. Reference González-Espinosa, Meave, Ramírez-Marcial, Toledo-Aceves, Lorea-Hernández and Ibarra-Manríquez2012). We report acid soils at the three sampling sites, noting that soils with a low pH can potentially favor root growth (Yuan & Chen Reference Yuan and Chen2010).
Regarding the horizontal distribution of roots, several studies reported higher root biomass near the trunks of trees, particularly coarse roots (Macinnis-Ng et al. Reference Macinnis-Ng, Fuentes, O’Grady, Palmer, Taylor, Whitley, Yunusa, Zeppel and Eamus2010, Zhang et al. Reference Zhang, Chen and Jiang2014). While fine roots are more evenly distributed (Macinnis-Ng et al. Reference Macinnis-Ng, Fuentes, O’Grady, Palmer, Taylor, Whitley, Yunusa, Zeppel and Eamus2010), due to their absorptive functionality, they can be expected to search for nutrients and water throughout the forest floor (Vitousek & Sanford Reference Vitousek and Sanford1986). Under dry conditions, trees may extend their roots much further than trees growing in wetter sites, which concentrate their roots below the shade of their crowns (Belsky Reference Belsky1994). In this work, we evaluated the hypothesis that root biomass would be higher near the trunk of the trees; however, it was not possible to support the hypothesis, and it was rejected. This result may be because the sampling sites were preserved with a homogeneous tree cover. In this sense, it has been reported that horizontal soil conditions remain homogeneous in undisturbed forests, so root biomass at shallow depths was similar between stands (Vahedi et al. Reference Vahedi, Bijani-Nejad and Djomo2016). While in disturbed forests, due to selective logging, canopy gaps are generated that have effects on the accumulation of leaf litter in the soil and on the decomposition of organic matter, which results in a decrease in the biomass of fine roots, what has been called belowground gap (Barbhuiya et al. Reference Barbhuiya, Arunachalam, Pandey, Khan and Arunachalam2012, Han et al. Han et al. Reference Han, Tang, Shi and Jin2020). In this sense, a helpful approach in cloud forests would be to relate the correlation between canopy cover and root biomass with the conservation status of the forest.
The biomass of the roots has been correlated with the DBH of the trees (Macinnis-Ng et al. Reference Macinnis-Ng, Fuentes, O’Grady, Palmer, Taylor, Whitley, Yunusa, Zeppel and Eamus2010). This makes sense, considering that larger trees need more structural support from coarse roots (Zhang et al. Reference Zhang, Chen and Jiang2014). However, only in Huayacocotla was the correlation significant. Therefore, the relationship between DBH and root biomass does not occur if only fine roots are considered.
The results of this work are an approximation to the carbon cycle considering the underground C stocks in the TMCF of the Sierra Madre Oriental, Mexico. Without a doubt, it is necessary to evaluate more fragments and increase the sampling effort to increase the precision of the C stock. Additionally, research on belowground productivity and root turnover is needed to understand carbon dynamics in TMCF better. TMCF stores large amounts of carbon belowground, so its conservation is a fundamental strategy for mitigating atmospheric CO2.





