Introduction
It is estimated that there are around 9,000 species of fish distributed throughout the vast bodies of water on the South American continent, however, only 6,200 species of fish have been described (Birindelli and Sidilauskas Reference Birindelli and Sidlauskas2018). Considering the high specificity of fish host–parasite relationships, especially involving monogenean species (Class Monogenea Van Beneden, 1858), it is expected that with the significant increase observed in the last two decades in the description of new fish species (Seidlová et al. Reference Seidlová, Benovics and Šimková2022), there is a growing contribution to the process of knowledge and description of new species of parasites.
Among the monogeneans, the Dactylogyridae family is the most studied, and most of its representatives are ectoparasites of characiform fishes (Luque et al. Reference Luque, Pereira, Alves, Oliva and Timi2017). Urocleidoides Mizelle & Price (Reference Mizelle and Price1964), after the emendation of Kritsky et al. (Reference Kritsky, Thatcher and Boeger1986) is characterized by species that have overlapping or tandem gonads, a male copulatory organ (MCO) coiled with counterclockwise rings, a sinistral vaginal sclerite, unmodified anchors, similar hooks with dilated shanks, and pairs of hooks 1 and 5 with reduced sizes. Fifty-two species of Urocleidoides are considered valid to date and occur especially in Neotropical Characiform fishes (Ferreira et al. Reference Ferreira, Rodrigues, Cunha and Domingues2018; Oliveira et al. Reference Oliveira, Santos-Neto, Tavares-Dias and Domingues2020; Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020; De Oliveira et al. Reference De Oliveira, Da Silva, Vieira and Acosta2021; Santos Neto and Domingues Reference Santos Neto and Domingues2023), especially in hosts from the Anostomidae and Erythrinidae (Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020). Almeida et al. (Reference Almeida, Oliveira and Tavares-Dias2021) confirmed the first record of Urocleidoides for the gills of Hemiodus unimaculatus (Bloch 1794) in the Jari River, a tributary of the Amazon River; however, there has been no description of a species of Urocleidoides for this family of fish.
Molecular tools jointly with morphological analyses have been used to improve the understanding of the taxonomic status of Urocleidoides, as well as to delimit the diagnosis of the genus, based especially on 28S rDNA and cytochrome c oxidase, subunit 1 (COI) sequences (Gasques et al. Reference Gasques, Graça, Prioli, Takemoto and Prioli2016; Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020; De Oliveira et al. Reference De Oliveira, Da Silva, Vieira and Acosta2021, Santos Neto and Domingues Reference Santos Neto and Domingues2023). Although the number of descriptions by integrative taxonomy of new species of monogeneans has increased, more information is still needed to understand the relationships between species in this group (Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020), especially concerning species that have already been described without molecular data.
Therefore, this study aimed to describe a new species of Urocleidoides parasitic on the gills of H. orthonops Eigenmann, Kennedy, 1903 through the analysis of sclerotized structures and internal organs as well to verify the phylogenetic position of the monogenean specimens of the new species through partial sequences of the COI mitochondrial region and 28S rDNA.
Material and methods
Study area and host collection
Specimens of H. orthonops (n=20) were collected by the PELD site-6 project in the years 2020 to 2021, in the floodplain of the Upper Paraná River, Brazil (-22°,761’,100" S -53°,252’,067" W), SISBIO collection authorization under n° 52596-5. This region is the last relatively well-preserved natural area of this river, where there is a great diversity of habitats (Thomaz et al. Reference Thomaz, Bini and Bozelli2007), which are of great importance for the conservation of the diversity of organisms.
The fish were caught using gill nets of different mesh sizes. The captured fish were anesthetized with benzocaine and killed under the Practical Guidelines for Euthanasia of the National Council for the Control of Animal Experimentation (CONCEA), with permission from the Ethics Committee for the Use of Animals of the Universidade Estadual de Maringá (CEUA- nº 1420221018). The fish host was identified according to Ota et al. (Reference Ota, GdeC, WJda and Pavanelli2018).
Parasitological processing
In the Ichthyoparasitology laboratory of the ‘Núcleo de Pesquisa em Limnologia Ictiologia and Aquicultura’ (Nupélia), the host fish were separated and triaged using two different methodologies. First (morphological characterization), the hosts had their gills removed and fixed in a 5% formalin solution, to be subsequently separated and transferred to 70% ethanol. Each specimen was mounted between a slide and a coverslip containing Hoyer’s medium to observe the sclerotized structures, and other specimens were stained with Gomori’s Trichrome to observe the internal organs according to Eiras et al. (Reference Eiras, Takemoto and Pavanelli2006). Secondly (molecular characterization), the hosts were triaged according to the methodology of Da Graça et al. (Reference Da Graça, Fabrin, Gasques, Prioli, Balbuena, Prioli and Takemoto2018) in which the monogeneans are kept intact for molecular analysis. To confirm the morphotype, each specimen was photographed showing the main taxonomic characteristics such as the presence of the vaginal sclerite and the morphology of the male copulatory complex. After confirming the morphotypes, all the materials were transferred to 1,5 ml microtubes each containing 20μl of ultrapure water for subsequent DNA extraction.
The illustrations of the description were prepared with a Nikon Eclipse e200 microscope equipped with a design tube and light phase contrast. All measurements were expressed in micrometers (μm) followed by the mean and amplitude. The ecological descriptors were made according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997), and the specimens were deposited in the ‘Coleção Helmintológica do Instituto Oswaldo Cruz’ – Fiocruz.
Molecular identification and characterization
The DNeasy® Blood and Tissue Kit (QIAGEN®) was used to extract the parasites’ DNA, following the protocol suggested by the manufacturer. A ProFlex™ 3×32-well thermal cycler was used to carry out the PCR. The DNA amplification reaction consisted of 1U of Taq DNA polymerase (5 U/μL, Invitrogen), Tris-KCL (20 mM Tris-HCl pH 8.4; 50 mM KCl), 1.87 mM MgCl2, 0.1 mM of each dNTP, 4μM of each primer, template DNA (10ng), and Milli-Q water for a final volume of 23μL.
Specific primers were used for the mitochondrial region of cytochrome c oxidase, subunit 1 (COI), and the 28S rDNA region to amplify the parasites’ DNA. The primers used for the COI region were COI_Mono_5: 5’TAATWGGTGGKTTTGGTAA-3’; COI_-Mono_3: 5’AATGCATMGGAAAAAAACA-3’; and COI_Mono_int3: 5’ACATAATGAAARTGAGC-3’ using a protocol adapted from Plaisance et al. (Reference Plaisance, Rousset, Morand and Littlewood2008). The partial region of the 28S gene was amplified with primers U178: 5′-GCACCCGCTGAAYTTAAG-3′ and L1642: 5′-CCAGCGCCATCCATTTTCA-3′ (Lockyer et al. Reference Lockyer, Olson and Littlewood2003), and the internal primer 1500R: 5′-GCTATCCTGAGGGAAACTTCG-3′ (Olson et al. Reference Olson, Cribb, Tkach, Bray and Littlewood2003) was used for sequencing. Amplification conditions consisted of an initial denaturation at 94°C for 5 min; 30 cycles at 94°C for 30 s, 54°C for 1 min, 72°C for 1 min; and a final extension at 72°C for 5 min.
The PCR products were checked on a 1% agarose gel, and the size of the fragments obtained was estimated using a marker of known molecular weight (100 bp DNA Ladder, Invitrogen 0.5 μg/μL). The products obtained from DNA amplification were purified following the protocol described by Rosenthal et al. (Reference Rosenthal, Coutelle and Craxton1993). Sequencing was carried out by a private company on an Applied Biosystems® AB 3500 Genetic Analyzer and using the BigDye® Terminator kit. Access to genetic heritage was authorized by the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (registration nº AF3A0E7).
The sequences obtained were visualized and manually edited using the BioEdit v7 program (Hall Reference Hall1999), and the alignment of the sequences was carried out in the MEGA7 software (Kumar et al. Reference Kumar, Stecher and Tamura2016) by the Clustal W (Thompson et al. Reference Thompson, Higgins and Gibson1994). Sequences obtained from GenBank and BOLD Systems were added to the analyses for comparison with the sequences obtained in the present study (COI mtDNA: 3 and 28S rDNA: 1), totaling 30 sequences for COI mtDNA and 17 sequences for 28S rDNA (Table 2).
The JModelTest 2.1.1 software (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012) was used to select the most suitable evolutionary model for Maximum Likelihood (ML). Based on the Bayesian Information Criterion (BIC), the evolutionary model selected was HKY+G+I for 28S rDNA and HKY+G for COI mtDNA. Phylogenetic reconstructions were carried out using the maximum likelihood method with 1000 bootstrap resampling in MEGA7 software and then generated in FigTree v.1.4.3 (Rambaut Reference Rambaut2012) and edited in Open Source Inkscape. Acanthocotyle gurgesiella Ñacari; Sepulveda; Escribano & Oliva, Reference Ñacari, Sepulveda, Escribano and Oliva2018 (Acanthocotylidae) was used as an outgroup for COI (KY379331) and Pseudorhabdosynochus epinepheli (Yamaguti, Reference Yamaguti1938) (Diplectanidae) for 28S (AY553622) according to Zago et al. (Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020).
The genetic distance p was calculated between the species obtained in this study and the sequences available in GenBank using the MEGA7 software. Groups were formed according to the species identified in the database. The sequences obtained in this study have been deposited in GenBank: OR106152 - OR106154 (COI); and OR351225 (28S) (Table 1).
Table 1. List of Urocleidoides spp. included in the molecular analyses, with details of the host, locality, and GenBank accession numbers of sequences from the partial 28S rDNA and COI mtDNA genes. New sequences obtained for the present study are in bold

Results
Taxonomic summary
Class Monogenea Bychoswky, 1937
Order Dactylogyridea Bychoswky, 1937
Family Dactylogyridae Bychowsky, 1933
Genus Urocleidoides Mizelle & Price, Reference Mizelle and Price1964
Species: Urocleidoides luquei n. sp. (Figure 1)

Figure 1. A) Urocleidoides luquei n. sp. (ventral view); B) Copulatory complex; C) Vaginal sclerite; D) Dorsal bar; E) Ventral bar; F) Dorsal anchors; G) Ventral anchors; H) Hooks I: pairs 1 and 5, II: pairs 2, 3, 4, III: pairs 6 and 7.
Type of host: Hemiodus orthonops Eigenmann, Kennedy, 1903
Location type: Upper Paraná River floodplain (-22°,761’,100" S -53°, 252’,067" W)
Infestation site: gill filaments.
Infestation rate: 50% prevalence, 20 hosts analyzed, 10 parasitized; total parasites found: 29; mean abundance: 1.45 ± 2.38 (1-9); mean intensity of infection: 2.9 ± 2.64.
Specimens deposited (Holotype CHIOC 40270 a), (Paratypes CHIOC 40270 b-j).
Etymology: The specific name refers to a tribute to Dr. José Luis Fernando Luque Alejos for his great contributions to fish parasitology studies in Brazil.
Morphological descriptions of new species
Description: (composite drawing). Based on 7 specimens mounted unstained in Hoyer’s medium and 2 stained with Gomori´s trichrome: body elongated, smooth and thin tegument, divided into cephalic region, trunk, peduncle, and haptor. Fusiform body 296.2 (240–84) long and 66.8 (48–96) wide from a central region of the trunk. The cephalic lobes comprises three bilateral pairs of head organs, eyespots, and accessory chromatic granules absent. Mid-ventral subspherical muscular pharynx. Vitellaria dense are distributed throughout the trunk (except in the cephalic region, copulatory complex, peduncle, and haptor). The male copulatory complex is composed of male copulatory organ (MCO) connected to the accessory piece by laminar ligament attached to the base of the MCO; MCO 28 (24.5–34.3) long 1½ rings counterclockwise, base of MCO with sclerotized cap; accessory piece 30.3 (21.5–32.2) long, robust, comprising 2 subunits; anterior subunit serving as guide for de MCO, posterior subunit clamp-shaped; ornamented distal part. Vaginal sclerite 33 (29.4–35.2) long, sigmoidal in shape with single groove striated longitudinally, thumb short, point long. Semi-ovoidal prostatic gland, long and narrow. Testicle partially in tandem with the ovary, Mehlis’ glands and seminal vesicle not observed. Vaginal pore dextral ventral; vaginal vestibule slightly sclerotized, vaginal canal comprising slim delicate tube. Sub-hexagonal haptor 76.4 (60–88.8) wide and 57.9 (45.6–74.5) long equipped with seven pairs of hooks according to the distribution of dactylogyrid (Mizelle Reference Mizelle1936), similar in shape; Pairs 1 and 5 reduced size; Pair 1 10.2 (7.8–12.7) long, Pair 5 14.6 (12.7–15.6) long; Pairs 2-3-4-6 and 7 similar sizes 34 (29.4–39.2) long; Dorsal anchor 20.5 (17.6–22.5) long, base 8.1 (6.8–8.8) wide, well-developed superficial root and deep root, straight shaft and recurved point. Dorsal bar ‘V’ shaped 28.4 (24.5–29.4) length. Ventral anchors 22.8 (19.6–24.5) high and 8.6 (4.9–9.8) wide the base, well-developed superficial root and deep root, straight shaft, blade and recurved tip longer than the superficial root. Ventral bar 32 (29.4–35.2) long, with expanded ends wider than long. Ventral bar 32 (29.4–35.2) long, with expanded ends wider than long.
Remarks: Urocleidoides luquei n. sp. resembles U. paradoxus Kritsky, Thatcher & Boeger, Reference Kritsky, Thatcher and Boeger1986 and U. surianoae Rossin & Timi Reference Rossin and Timi2016. Both species have a laminar ligament connecting the base of the MCO to the accessory piece, with the main significant difference from the other Urocleidoides spp. described as valid. However, it can be easily distinguished from U. paradoxus by the shape of the very ornate accessory piece distally. When compared to U. surianoae, both species are similar in terms of the morphology of the accessory piece; however, it can be easily distinguished by the morphology of the bars. U. surianoae has a large medial anteroposterior development on the ventral bar, a feature not found in the new species.
Molecular data
The partial sequence of the 28S rDNA, with 951 base pairs (bp) after editing and alignment, was obtained for the new species, showing p-distance values ranging 23% to 29.8% compared to other sequences in the database, being closer to U. brasiliensis Rosim, Mendoza-Franco & Luque, Reference Rosim, Mendoza-Franco and Luque2011 and more distant from U. indianensis De Oliveira et al. Reference De Oliveira, Da Silva, Vieira and Acosta2021. The genetic distance values involving the new species were slightly higher when compared to genetic distance values between other species of the genus (Table 2). Based on the 28S rDNA gene tree (Figure 2), U. luquei n. sp. was positioned in a distinct clade, even separated from U. paradoxus, a morphologically closer species due to the linkage structure between the base of the MCO and the accessory piece.
Table 2. Genetic distance p obtained from sequences of the 28S rDNA region for species of the genus Urocleidoides. The sequence obtained in this study is in bold

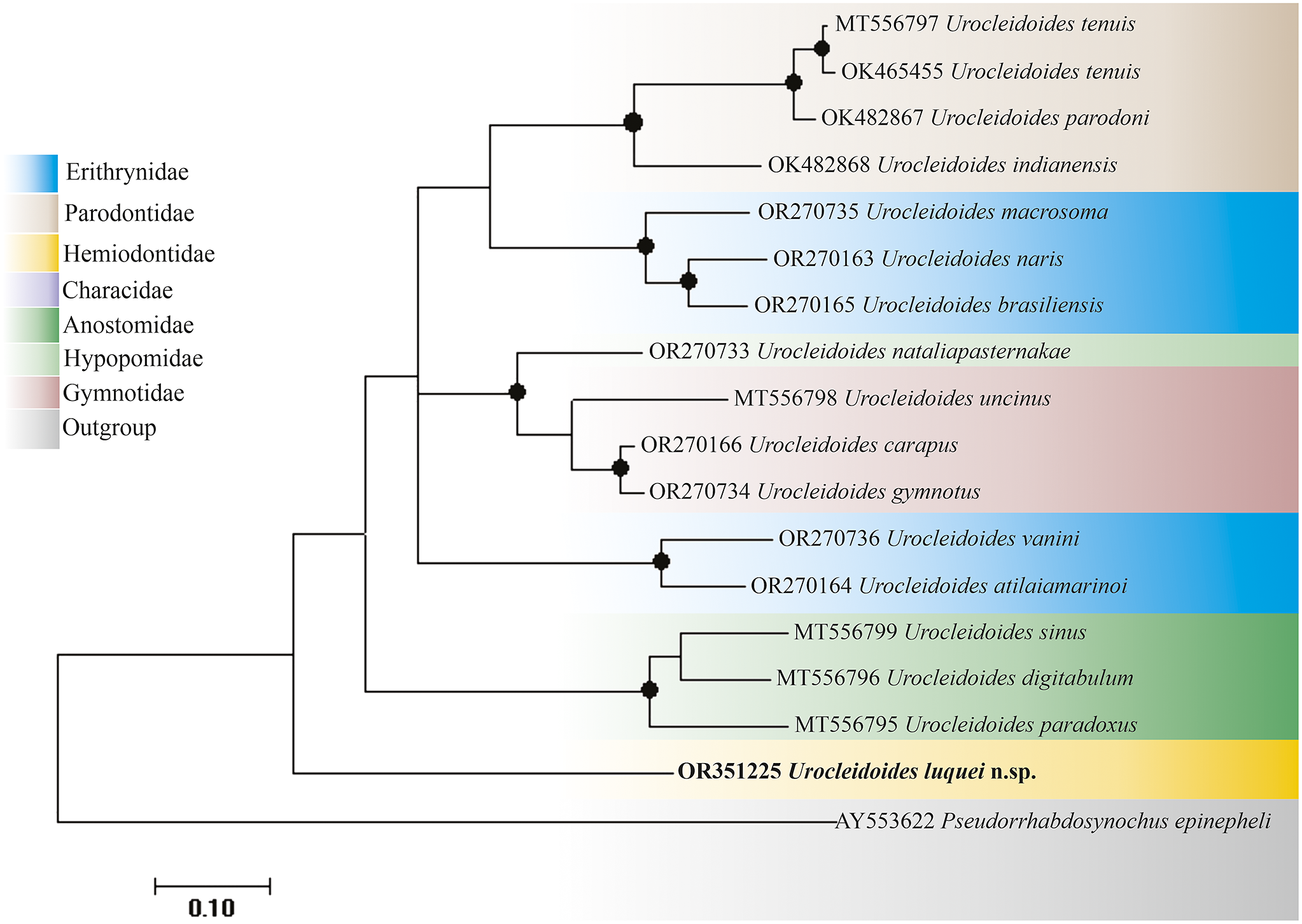
Figure 2. Gene tree constructed using the maximum likelihood method with 1000 bootstrap resamplings for the 28S molecular marker, where Pseudorhabdosynochus epinepheli (Yamaguti, Reference Yamaguti1938) was used as the outgroup and the nucleotide substitution model used was HKY+G+I. The sequence highlighted in bold is the one obtained in this study. Support values above 85 are highlighted with circles, and the colors refer to the host fish’s family.
Furthermore, it is possible to verify a phylogenetic relationship between the species U. sinus Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020, U. digitabulum Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020, and U. paradoxus, all of which parasitize Anostomidae’s fishes. This relationship between parasites that share the same host family can also be seen for U. tenuis De Oliveira et al. Reference De Oliveira, Da Silva, Vieira and Acosta2021, U. parodoni De Oliveira et al. Reference De Oliveira, Da Silva, Vieira and Acosta2021, and U. indianensis De Oliveira et al. Reference De Oliveira, Da Silva, Vieira and Acosta2021, fish parasites from the Parodontidae. Parasites of the Gymnotidae (U. uncinus Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020, U. carapus Mizelle, Kritsky & Crane, Reference Mizelle, Kritsky and Crane1968, and U. gymnotus Mizelle, Kritsky & Crane, Reference Mizelle, Kritsky and Crane1968) also formed a distinct clade, closer to U. nataliapasternakae Santos Neto & Domingues, Reference Santos Neto and Domingues2023, the only representative of Hypopomidae for this marker.
Parasites of the Erythrinidae were the only ones that did not group, forming two distinct clades, one including the species U. macrossoma Santos Neto & Domingues, Reference Santos Neto and Domingues2023, U. naris Rosim, Mendoza-Franco & Luque, Reference Rosim, Mendoza-Franco and Luque2011, and U. brasiliensis and the other with U. vanini Santos Neto & Domingues, Reference Santos Neto and Domingues2023 and U. atilaiamarinoi Santos Neto & Domingues, Reference Santos Neto and Domingues2023.
Partial sequences of the COI region, with 671 bp after editing and alignment, were obtained for the new species, which were identical to each other and had distances ranging from 58.5% to 65.9% to the other Urocleidoides specimens obtained from the database, being closer to U. vanini and more distant from U. cuiabai Rosim, Mendoza-Franco & Luque, Reference Rosim, Mendoza-Franco and Luque2011 (Table 3). Considering the high genetic distance values obtained for U. luquei n. sp. When compared to the other species of the genus, the mitochondrial marker shows that the new species is differentiated genetically from the others, which is also reflected in the gene tree (Figure 3).
Table 3. Genetic distance p obtained from COI sequences for species of the genus Urocleidoides. The sequences obtained in this study are in bold
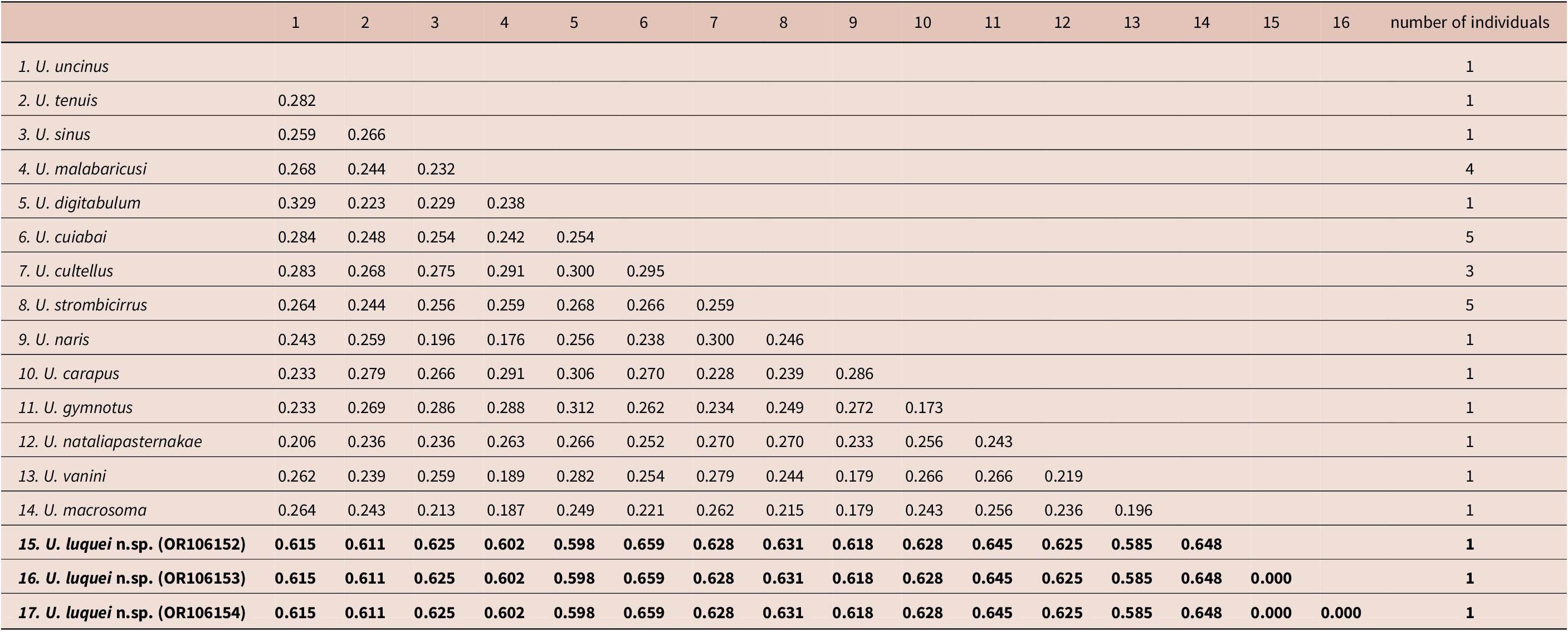

Figure 3. Gene tree constructed using the maximum likelihood method with 1000 bootstrap resamplings for the COI molecular marker. Acanthocotyle gurgesiella Ñacari et al., 2017 was used as an outgroup, and the nucleotide substitution model used was HKY+G. The sequences highlighted in bold are those obtained in this study. Support values above 85 are highlighted with circles, and the colors refer to the host fish’s family.
The gene tree for COI shows that the new species of Urocleidoides (highlighted in bold) constituted a clade distinct from the others. All the sequences analyzed from U. strombicirrus (Price & Bussing Reference Price and Bussing1967), the only parasite representing the Characidae, were grouped. Parasites of the Gymnotidae (U. uncinus, U. carapus, and U. gymnotus) also formed a distinct clade, closer to Hypopomidae representatives.
Just like the ribosomal marker, parasites of the Erythrinidae did not group together and formed two distinct clades, one including the species U. macrossoma, U. naris, U. vanini, and U. malabaricusi Rosim, Mendoza-Franco & Luque, Reference Rosim, Mendoza-Franco and Luque2011 and the other with U. cuibai Rosim, Mendoza-Franco & Luque, Reference Rosim, Mendoza-Franco and Luque2011. Finally, representatives of Anostomidae (U. digitabulum and U. sinus) were also not placed in the same clade.
Discussion
The current study presents a description of a new species of Urocleidoides Mizelle & Price (Reference Mizelle and Price1964), rising to 53 the number of valid species parasitizing various types of hosts recorded in other localities on the Neotropical continent (Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020; Santos Neto and Domingues, Reference Santos Neto and Domingues2023). In molecular aspects, U. luquei n. sp. is genetically differentiated from the species that have sequences available in databases, mainly concerning the mitochondrial marker.
Morphology has undergone changes about the genus. Kritsky et al. (Reference Kritsky, Thatcher and Boeger1986) based on the genus review proposed a new diagnostic criterion (the presence of a vaginal sclerite); although this characteristic is adopted as a definitive criterion, it has generated doubts because some species are described as representatives of Urocleidoides and do not have vaginal sclerite as a diagnostic feature (Kritsky et al. Reference Kritsky, Thatcher and Boeger1986; Mendoza-Franco and Reina Reference Mendoza-Franco and Reina2008; Cohen et al. Reference Cohen, Justo and Kohn2013; Santos Neto and Domingues, Reference Santos Neto and Domingues2023). However, it was suggested by Santos Neto and Domingues (Reference Santos Neto and Domingues2023) that the isolated presence or absence of the vaginal sclerite is insufficient for the diagnosis of the species of the genus. Concerning that, in our analysis, there is no molecular separation, regarding 28S rDNA, between U. vanini Santos Neto & Domingues, Reference Santos Neto and Domingues2023 (absence of vaginal sclerite) and U. atilaiamarinoi Santos Neto & Domingues, Reference Santos Neto and Domingues2023 (presence of vaginal sclerite), both grouping in the same clade.
Considering molecular and morphological aspects, there are differences between species. Morphologically, U. luquei n. sp. is closer to U. paradoxus and U. surianoae due to a laminar ligament that connects the base of the MCO to the accessory piece. However, concerning genetic similarity, U. luquei was closer to U. brasiliensis and U. vanini, considering 28S and COI markers, respectively. Currently, for COI mtDNA, there are 27 sequences available in the database representing 26.92% of the total species described so far for the genus Urocleidoides spp. (14 of 52 species) and 16 sequences of 28s rDNA, representing only 28.84% of the species described ( of 52 species). So this study used all the sequences available for the genus in the databases. However, the unavailability of sequences to the same species, both for COI and 28S marker, did not allow us to infer, with information from both regions of the DNA, which species is phylogenetically closest to U. luquei n. sp.
Based on 28S sequences, the phylogenetic analyses with the marker show close molecular relationships between the species U. digitabulum, U. sinus, and U. paradoxus, all of which parasitize Anostomidae, as well as the similarity between U. sinus and U. digitabulum has also been observed in previous studies (Zago et al. Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020; Santos Neto and Domingues Reference Santos Neto and Domingues2023). Furthermore, it is possible to note the relationship between U. tenuis, U. parodoni, and U. indianensis, the three parasites of Parodontidae, as observed by Santos Neto and Domingues (Reference Santos Neto and Domingues2023). Finally, U. nataliapasternakae is the only representative of Hypopomidae, so it is not possible to verify the relationship of parasites belonging to this family.
The species of Urocleidoides reported for Erythrinid fish, as observed by Zago et al. (Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020) and Santos Neto and Domingues (Reference Santos Neto and Domingues2023), do not represent a monophyletic group, being divided into two clades in the analysis carried out (Figure 2 and 3, blue color) for both markers. This separation may be associated with host exchange events as well as their geographical distribution, which can contribute to shaping the sharing of these parasites (Braga et al. Reference Braga, Razzolini and Boeger2015; Santos Neto and Domingues Reference Santos Neto and Domingues2023), and this pattern can be observed for both studied markers.
Urocleidoides tenuis, being the only representative of Parodontidae for this marker, does not group with the other species; this pattern was also observed by Zago et al. (Reference Zago, Yamada, de Oliveira Fadel Yamada, Franceschini, Bongiovani and da Silva2020) and Santos Neto and Domingues (Reference Santos Neto and Domingues2023). Moreover, Gymnotidae and Hypopomidae were mixed according to Santos Neto and Domingues (Reference Santos Neto and Domingues2023); however, in our analysis, although there was also this mixture, for Hypopomidae there was no clumping pattern since one of the sequences was separated from the rest; the same happened with the representatives of Anostomidae.
Finally, this study contributes to the knowledge of the diversity of the parasitic fauna present in the floodplain of the upper Paraná River, Brazil, especially concerning the hosts of the genus Urocleidoides. In addition, because U. luquei n. sp. is the first species described parasitizing H. orthonops, which belongs to the Hemiodontidae family, more studies are needed to monitor the occurrence of this monogenean in the family and elucidate unresolved phylogenetic issues. Just as Santos Neto and Domingues (Reference Santos Neto and Domingues2023) pointed out, we believe that the phylogenetic relationships in Urocleidoides could be elucidated in future studies, with the possible inclusion of DNA sequences of all the species described in the analyses.
Acknowledgements
The authors are grateful to Capes and CNPq for funding the study, to the Núcleo de Pesquisa em Limnologia Ictiologia e Aquicultura - NUPÉLIA-UEM for providing the infrastructure, and to PELD site 6 for the samples obtained in this work.
Financial support
PELD site 6 and Conselho Nacional do Desenvolvimento Científico e Tecnológico supported this work.
Data availability
The datasets generated and analyzed during the study are available in the GenBank repository under the following accession numbers: OR106152, OR106153, OR106154 (COI) and OR351225 (28S).
Competing interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable guidelines for the care and use of animals were followed.








