Significant outcome
PTSD and non-PTSD patients showed persistently different scores of mental distress 1 year after psychiatric treatment. In contrast, their cytokine levels did not differ 1 year after discharge from a psychiatric treatment inpatient stay.
Limitations
The patients with higher scores of mental distress were those who attended the follow-up stay, introducing a selection bias to the study. Further, it was not assessed whether the patients were fasting or smoking cigarettes prior to blood collection, or their sleeping or physical exercise status.
Introduction
The notion of inflammation as a cause, consequence, or correlate in psychiatric diseases has been extensively investigated in recent decades (Reus et al., Reference Reus, Fries, Stertz, Badawy, Passos, Barichello, Kapczinski and Quevedo2015). Studies of inflammatory markers have provided evidence of elevated levels of interleukin-6 (IL-6), interleukin 1β (IL-1β), and interferon-γ (IFN-γ) in people diagnosed with post-traumatic stress disorder (PTSD) (Gill et al., Reference Gill, Saligan, Woods and Page2009; Passos et al., Reference Passos, Vasconcelos-Moreno, Costa, Kunz, Brietzke, Quevedo, Salum, Magalhaes, Kapczinski and Kauer-Sant’anna2015). However, PTSD has largely been investigated in cross-sectional studies (Sumner et al., Reference Sumner, Nishimi, Koenen, Roberts and Kubzansky2020), not providing insight into the development of inflammatory markers over time. Furthermore, the body of evidence is still mixed (Michopoulos et al., Reference Michopoulos, Powers, Gillespie, Ressler and Jovanovic2017; Toft et al., Reference Toft, Bramness, Lien, Abebe, Wampold, Tilden, Hestad and Neupane2018a; Lima et al., Reference Lima, Hammadah, Wilmot, Pearce, Shah, Levantsevych, Kaseer, Obideen, Gafeer, Kim, Sullivan, Lewis, Weng, Elon, Li, Bremner, Raggi, Quyyumi and Vaccarino2019; Sumner et al., Reference Sumner, Nishimi, Koenen, Roberts and Kubzansky2020).
Trauma is a prerequisite of PTSD, but other parts of its aetiology remain largely unknown (Hori et al., Reference Hori, Yoshida, Itoh, Lin, Niwa, Ino, Imai, Ogawa, Matsui, Kamo, Kunugi and Kim2019). The investigation of certain inflammatory biomarkers commonly related to PTSD may enhance understanding of the role that biological factors play in PTSD (Hussein et al., Reference Hussein, Dalton, Willmund, Ibrahim and Himmerich2017). It has been suggested that an altered inflammatory marker profile is a risk factor for developing PTSD (Michopoulos et al., Reference Michopoulos, Beurel, Gould, Dhabhar, Schultebraucks, Galatzer-Levy, Rothbaum, Ressler and Nemeroff2019; Sumner et al., Reference Sumner, Nishimi, Koenen, Roberts and Kubzansky2020). Also, higher levels of IL-6 and IL-8 have been associated with higher symptoms of acute stress and post-traumatic stress symptoms (Cohen et al., Reference Cohen, Meir, Klein, Volpin, Assaf and Pollack2011).
Inflammation in PTSD has previously been investigated in adult individuals who experienced childhood maltreatment (Wieck et al., Reference Wieck, Grassi-Oliveira, Hartmann Do Prado, Teixeira and Bauer2014; Koenig et al., Reference Koenig, Karabatsiakis, Stoll, Wilker, Hennessy, Hill and Kolassa2018), in patients with war-related trauma (Lindqvist et al., Reference Lindqvist, Dhabhar, Mellon, Yehuda, Grenon, Flory, Bierer, Abu-Amara, Coy, Makotkine, Reus, Bersani, Marmar and Wolkowitz2017), and in patients admitted to emergency wards immediately after trauma exposure (Michopoulos et al., Reference Michopoulos, Beurel, Gould, Dhabhar, Schultebraucks, Galatzer-Levy, Rothbaum, Ressler and Nemeroff2019). There is still a gap in the literature on longitudinal studies of cytokines in psychiatric patients. This study recruited psychiatric patients who participated in an inpatient, intensive treatment programme lasting 12 weeks. Their suffering had persisted over time, and previous treatment efforts were insufficient to achieve recovery. The current study included patients who attended a 5-day follow-up stay 1 year after treatment termination. Thus, this study design that includes measurement points at admission, at mid-treatment, at discharge, and at 1-year follow-up enables us to contribute to the long-running debate on whether cytokines are trait or state markers in patients and whether there are cross-diagnostic differences. We have previously found the cytokines IL-1β, TNF-α, IL-1RA, and the chemokine MCP-1 to be elevated in inpatients with PTSD when compared to patients without PTSD (Toft et al., Reference Toft, Bramness, Lien, Abebe, Wampold, Tilden, Hestad and Neupane2018a). This was, however, observed over a period of only 12 weeks. In the present study, we aimed to explore the cytokine levels and psychometric scores in patients with and without PTSD at 1-year follow-up. Accordingly, our research question was whether cytokine levels and mental distress in patients with and without PTSD would be similar or different 1 year later.
Material and methods
Study participants and recruitment procedure
Patients were recruited from the Modum Bad Psychiatric Center, a specialised psychiatric treatment centre for adults in Norway, offering treatment to patients with long-standing or treatment-resistant trauma, anxiety, or eating and depressive disorders. Patients who are currently using illegal substances or are alcohol dependent are not eligible for treatment at the centre and are referred elsewhere. The facility offers group and individual therapies in a 12-week inpatient treatment programme. The patients were admitted in groups of eight at a time. The patients were given a 15-minute presentation of the study during group therapy by the first author during the first days of their stay. Written information was handed out, explaining the aim of the study and the procedures involved. A consent form was also distributed to each potential participant. Data from the mainstay were collected from March 2015 to April 2016, while the follow-up measurements took place approximately 1 year after discharge from the mainstay. There were some minor differences in the precise number of months from discharge to 1-year follow-up [mean 14.5 months, standard deviation (SD) 1.5]. In total, 90 (61.2%) of the 147 patients who consented to participate in the study during their mainstay participated at the follow-up measurement. Of these 90 patients, there were 24 men (mean age 48, SD 8.4), while 66 were women (mean age 38, SD 10.3). The genders differed significantly in age (t = 2.11, p = < 0.001). Of the 57 patients who did not attend the follow-up stay, there were 18 men (mean age 48, SD 10.9) and 39 women (mean age 41, SD 13.3). Again, there was a significant age difference between genders (t = 4.34, p = 0.04). When comparing those who attended follow-up with those who did not, the 90 attendees had a mean age of 41 years (SD 10.8), while the 57 non-attendees had a mean age of 43 years (SD 13.0). There was thus no significant age difference between attendees and non-attendees at the follow-up stay (p = 0.326). There were nine patients who had missing data on diagnosis, and these were excluded from the statistical analyses.
As part of clinical interviewing, the patient’s trauma history was assessed by the therapists at the clinic. The clinic used five questions developed on-site to assess the degree of trauma exposure. The first three questions assess childhood trauma, while the last two assess adulthood trauma. In the current paper, having experienced any of these kinds of trauma at all was recorded as having trauma experience. The questions were as follows: (1) Has the patient been exposed to sexual assaults in childhood? (2) Has the patient been exposed to physical abuse in childhood? (3) Has the patient experienced other traumatic childhood events, which led to serious problems later? (4) Has the patient been exposed to sexual assaults or abuse in adulthood (after 18 years of age)? (5) Has the patient experienced other traumatic events in adulthood, which led to serious problems later?
All patients were interviewed by trained psychologists or psychiatrists using the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998). The MINI interview provides information to be used when assessing diagnoses in the 10th revision of the International Classification of Diseases and Related Health Problems (ICD-10). Other helpful information for staff assessment of patients’ disorders included a combination of several psychometric questionnaires, interviews, and clinical judgement. All disorders were in the areas of depression, anxiety, and eating disorders. The ICD-10 diagnosis F43.1 PTSD is a chronic condition that develops in response to extreme stressors like military combat, sexual, or physical abuse in childhood or adulthood, natural disasters, or similar catastrophic events (Foa & Yadin, Reference Foa and Yadin2011). In order to diagnose PTSD, the symptoms must have been present for more than 1 month (Grinage, Reference Grinage2003).
The Hopkins Symptom Checklist-90 Revised (HSCL-90R) questionnaire was administered to assess the patients’ mental distress (Derogatis et al., Reference Derogatis, Lipman and Covi1973). Patients completed the HSCL-90R self-report questionnaire either on a computer or on a digital tablet. The HSCL-90R is a 90-item questionnaire measuring the levels of psychological distress during the past 7 days. A mean score, the Global Severity Index (GSI), is calculated from all items, each ranging from 0 to 4. The questionnaire assesses symptoms of somatisation, obsession, and compulsion, depression, anxiety, and hostility. The GSI is an overall scale taking various symptoms into account, thus tapping into symptoms common to a heterogeneous psychiatric patient sample (Rytila-Manninen et al., Reference Rytila-Manninen, Frojd, Haravuori, Lindberg, Marttunen, Kettunen and Therman2016). The HSCL-90R has been shown to provide a psychometrically valid evaluation of psychiatric patients regarding the severity of depression, specific anxiety, and interpersonal sensitivity (Bech et al., Reference Bech, Bille, Moller, Hellstrom and Ostergaard2014). A cut-off for caseness was set at a GSI score of 0.85 (Pedersen & Karterud, Reference Pedersen and Karterud2004). The HSCL-90R questionnaires were submitted within 1 week of entering treatment (T0), at the halfway stage (T1), and a few days before discharge (T2). Patients were also followed up approximately 1 year after discharge from their mainstay (T3).
Blood collection and serum preparation
The blood samples were drawn at T0, T1, T2, and T3. They were collected between 08 : 00 am and 09 : 00 am, except for 16 patients from the depression ward, who had their blood drawn between 12 : 00 am and 03 : 00 pm. Patients sat in an upright position when blood was collected. Vacuette 8ml serum containers were used for blood collection. These were turned upside-down 8–10 times immediately after the blood was collected, and allowed to clot for 30–60 min. The samples were then spun at 1917 g for 10 min at room temperature. Separated serum samples were put to freeze at −80°C until assay.
Cytokine and chemokine measurements
We analysed seven cytokines and one chemokine based on the available literature on the neuroimmune correlates of psychiatric disorders: IL-1β, IL-1RA, IL-6, IL-10, IL-17A, IFN-y, MCP-1, and TNF-α. The unit of measurement was picograms per millilitre (pg/ml). Four cytokines had too many values under the limit of detection (LOD) (> 55%) and were excluded from the study. The following cytokines were taken into consideration: IL-1β, IL-IRA, MCP-1, and TNF-α. Serum samples were thawed on ice, vortexed, and then spun down a tube with 250 μl serum at 14 000 g for 10 min at 4°C, before dilution (1 : 5) and further processing. The cytokine measurements were performed using Bio-Plex xMAP Technology (Bio-Rad, Austin, TX, USA) with a Luminex IS 100 instrument (Bio-Rad, Hercules, CA, USA), powered using Bio-Plex Manager (version 6.0.1) software. The assay was performed according to the manufacturer’s instructions, but an additional standard point was included. To achieve a more reliable result, individual sets of samples from patients were run in the same assay, all samples were assayed in duplicate, and a magnetic plate washer was used during assay set up. The StatLIA software package (version 3.2; Brendan Scientific, Carlsbad, CA, USA) incorporates a weighted, five-parameter logistic curve-fitting method and was used to calculate sample cytokine concentrations. Longitudinal controls were used in order to validate inter-assay variation: IL-1β (18.1%), IL-1RA (10.2%), MCP-1 (6.7%), and TNF-α (7.4%). The inter-assay percentage of variability (CV) in parentheses is a measure of variation between plates, where a lower figure is better. Any figure below 21% is considered acceptable. The mean inter-assay percentage CV for all blood sample plates was 10.4%. An inter-assay percentage CV of 10–12% is common. The blood samples from the follow-up stay (T3) were analysed in a separate batch by the same laboratory as the first three measurements. These samples were analysed in 2018. The same procedures as described for the first batch were applied. The inter-assay percentage CVs were IL-1β (12.3%), IL-1RA (8.6%), MCP-1 (7.8%), TNF-α (8.1%). The mean inter-assay percentage CV for all blood sample plates was 11.4%.
Limit of detection and imputation
The values below the LOD were replaced with the LOD values (values in pg/ml). The amounts of zero values and the LOD varied across each of the four measurements. For IL-1β at T0 and T1, there were 44 zero values at each measurement point (48.9% and 49.4% imputations, respectively) and the LOD was 0.01. At T2, there were 42 zero values (47.7%) and the LOD was 0.02. At T3, there were 62 zero values (69.7%) and the LOD was 0.01. For IL-1RA at T0, there was 1 zero value (0.01%) and the LOD was 7.87. There were no zero values for IL-1RA at T1 and T2. For IL-1RA at T3, there were 8 zero values (9.9%) and the LOD was 20.01. For MCP-1 at T0, there were 5 zero values (5.7%) and the LOD was 0.76. At T1, there were 4 zero values (4.44 %) and the LOD was 1.72. At T2, there were 5 zero values (5.6%) and the LOD was 1.55. At T3, there were no zero values. For TNF-α at T0, there were 37 zero values (41.1%) and the LOD was 0.02. At T1, there were 29 zero values (32.6%) and the LOD was 0.03. At T2, there were 31 zero values (35.2%) and the LOD was 0.02. All patients showed up for blood sampling at T0. There was 1 patient who did not show up for blood sampling at T1, 2 patients who did not show up at T2, and 1 patient who did not show up for the blood sampling at T3. These patients still participated by submitting their HSCL-90R GSI scores. At T0, all patients successfully submitted their GSI scores, but at T1, data were missing for 6 patients. At T2 and T3, data were missing for 13 patients. The study was approved by the Norwegian Regional Ethics Committee prior to data collection (reference number 2014/2189).
Statistical analyses
The Shapiro–Wilk test was used to test for normal distribution in all variables. The HSCL-90R GSI scores were normally distributed at T0 (p = 0.213) and T1 (p = 0.104). Age was also normally distributed (p = 0.287). All other variables were not normally distributed. To maintain parsimony, the non-parametric Mann–Whitney U-test was employed to assess differences between PTSD and non-PTSD patients for all variables. Pearson’s chi-square test was used to assess differences between categorical variables. Fisher’s exact test was used for analysing categorical variables with fewer than five observations. Logistic multilevel regression models were used to assess possible interaction effects between time-varying cytokines/chemokine and GSI scores on PTSD as a binary-dependent variable. The advantage of multilevel models is that they handle the dependency of measurements within each patient, thus constituting a two-level structure (Sommet & Morselli, Reference Sommet and Morselli2017). All statistical analyses were conducted with Stata (StataCorp. 2019, Stata Statistical Software: Release 16, College Station, TX, USA: StataCorp LLC).
Results
Table 1 presents means and frequencies of gender, age, trauma history, mental distress, as well as mood, anxiety, and eating disorders stratified by PTSD diagnosis. Figure 1 ( A–E) shows the mean levels at T0–T3 for cytokines/chemokine and GSI, thus graphically presenting the levels and development across time. The PTSD patients had significantly higher GSI levels than non-PTSD patients at the follow-up stay (T3) (p = 0.048). This difference was also seen at T0 (p = 0.014) and T2 (p = 0.050). The cytokines/chemokine did not differ significantly between the PTSD and non-PTSD group at T3. In contrast, the groups exhibited different levels (trend to significance) of IL-1β (p = 0.053), IL-1RA (p = 0.042), and TNF-α at T2 (p = 0.037), with the PTSD patients having the higher levels. In addition, longitudinal multilevel logistic regression models were employed to test the possible interaction effects between cytokines/chemokine and GSI scores on the association with having PTSD compared with not having PTSD. These models were run separately for each cytokine/chemokine and adjusted for age and sex. There were no significant interactions (data are not shown).
Table 1. Patient characteristics at 1-year follow-up
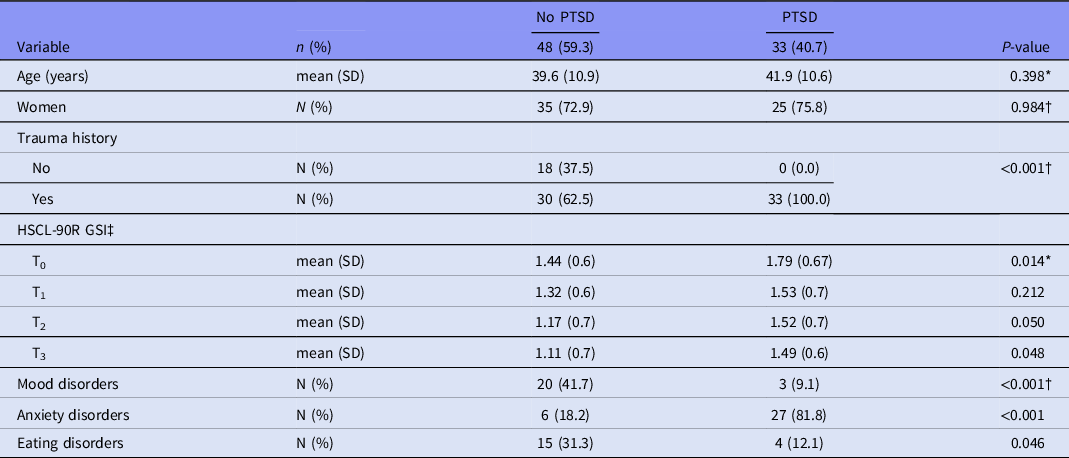
*Mann–Whitney U-test. †Pearson’s chi-square. Fisher’s exact test when fewer than five observations. ‡HSCL-90R GSI = Hopkins Symptom Checklist-90 Revised Global Severity Index.
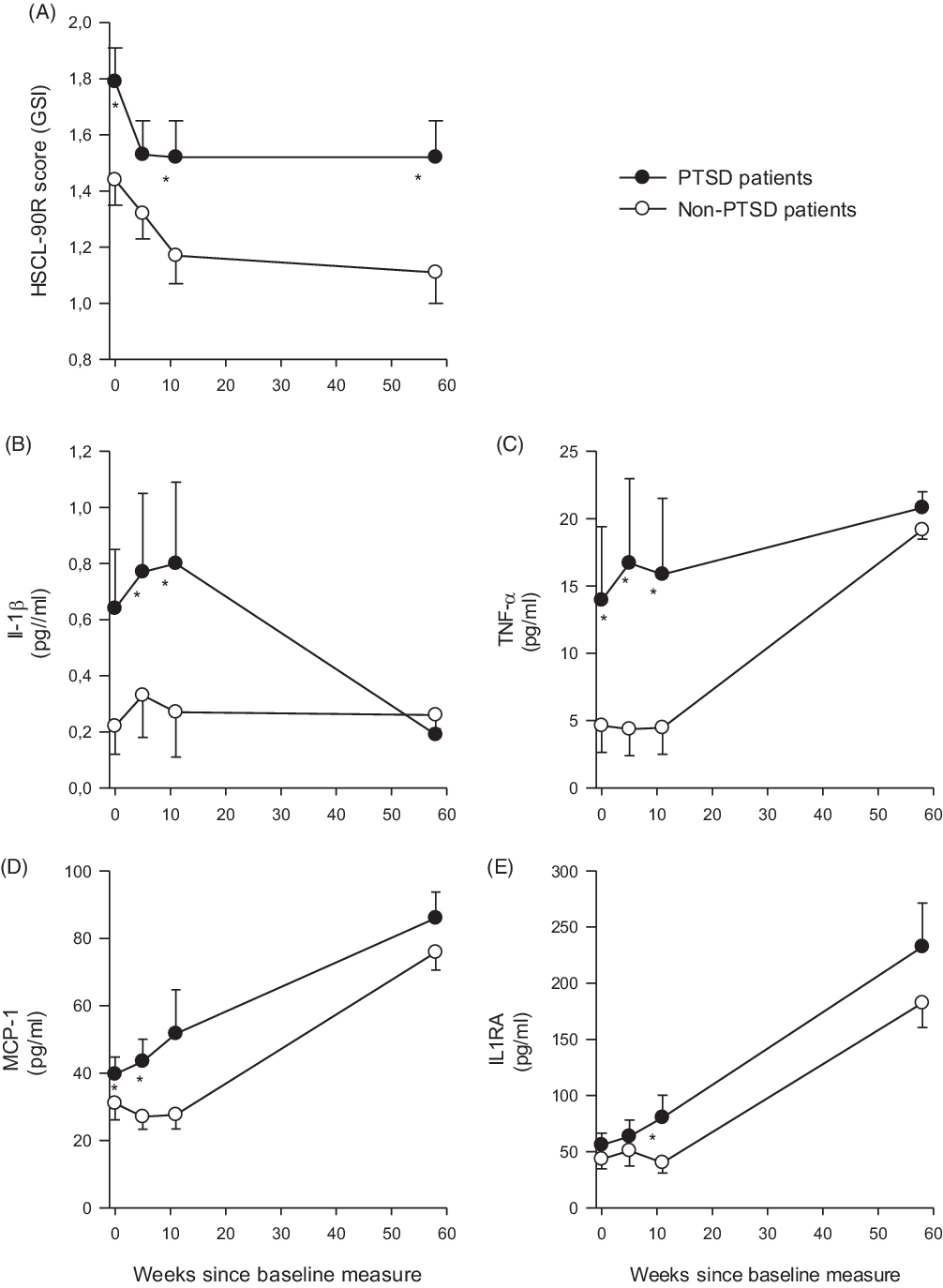
Fig. 1. Development of symptoms of mental distress (A) and inflammatory markers (B–E) at psychiatric inpatient treatment and at 1-year follow-up stay. (A) Depicts the measurements (means, SD) of mental distress measured by the Hopkins Symptom Checklist-90 Revised Global Severity Index (HSCL-90R GSI) questionnaire. The inflammatory markers (B–E) were analysed in serum blood samples, which were collected during the first week of the treatment, at weeks 5 and 11, and lastly at the 1-year follow-up stay.
Discussion
In this 1-year post-discharge follow-up study, PTSD patients continued to report significantly higher levels of mental distress than non-PTSD patients. This difference was not reflected in the levels of some commonly measured cytokines, where we observed more similar levels at the 1-year follow-up. The GSI scores in both patient groups remained high, indicating that a severe but unchanging psychiatric state could be associated with escalating levels of IL-1RA, MCP-1, and TNF-α, regardless of diagnosis. Put differently, the levels of the cytokine/chemokines are not necessarily reflected in the psychiatric symptom scores. This is somewhat in line with our previous study that found decreasing GSI scores, but increasing cytokine levels in PTSD patients (Toft et al., Reference Toft, Neupane, Bramness, Tilden, Wampold and Lien2018b). The unchanged mental distress since discharge from the mainstay may indicate that the effect of the treatment is beyond the treatment period, at least in this particular patient sample. Moreover, the declining levels of IL-1β in PTSD patients suggest that these patients have an inflammatory response that does not entirely follow the pattern of patients without PTSD, again underscoring the distinct neuroimmune features of PTSD patients, at least for cytokine IL-1β. This finding is not in line with the expectation that levels of IL-1β and IL-1RA would generally follow each other, as IL-1RA acts as an antagonist and is secreted in response to increasing levels of IL-1β (Gabay et al., Reference Gabay, Lamacchia and Palmer2010).
Hypoactivity of the hypothalamic–pituitary–adrenal axis, along with the hyperactive sympathetic nervous system, is considered a trademark of PTSD (Hori & Kim, Reference Hori and Kim2019). PTSD patients usually exhibit decreased secretion of cortisol, as well as having glucocorticoid receptors (GRs) more sensitive to cortisol, altogether rendering the individual vulnerable to suffering from an abnormal immune response (Hori & Kim, Reference Hori and Kim2019). This could reflect their high scores in mental distress, which might be explained by a blunted cortisol feedback system in response to stressors, or possibly a habituation to repeated exposures to stressors in individuals diagnosed with PTSD (Michopoulos et al., Reference Michopoulos, Beurel, Gould, Dhabhar, Schultebraucks, Galatzer-Levy, Rothbaum, Ressler and Nemeroff2019). The similar cytokine levels of PTSD and non-PTSD patients at the follow-up stay could be explained by enhanced GR sensitivity and reduced cortisol levels in both patient groups (Hori & Kim, Reference Hori and Kim2019). These are features of PTSD patients that distinguish them from patients with major depression disorder (MDD), as MDD patients are reported to exhibit hypercortisolism in addition to reduced GR sensitivity. Both groups shared a history of trauma, and thus did not differ greatly in this regard. The trauma experience might be the necessary prerequisite for suppressed cortisol levels and increasing cytokines as a consequence (Hori & Kim, Reference Hori and Kim2019), suggesting that the type of diagnosis is not as relevant as the trauma experience in itself. This provides a rationale for why we see increased IL-1RA, MCP-1, and TNF-α in both PTSD and non-PTSD groups in the current study.
In our previous papers (Toft et al., Reference Toft, Bramness, Lien, Abebe, Wampold, Tilden, Hestad and Neupane2018a; Toft et al., Reference Toft, Neupane, Bramness, Tilden, Wampold and Lien2018b; Toft et al., Reference Toft, Lien, Neupane, Abebe, Tilden, Wampold and Bramness2020), PTSD patients exhibited escalating cytokine responses in comparison to patients without PTSD who were diagnosed with MDD, anxiety disorders, and eating disorders. The lack of differences at follow-up could indicate that at 1 year, the patients’ immune system had either adapted to a more normalised state, or that both groups exhibited abnormal levels regardless of diagnosis. This finding is in line with a prospective study on PTSD patients which found levels of TNF-α to differ from those of healthy controls at the first measurement, but did not find any difference at the second measurement 5 years later (Vidovic et al., Reference Vidovic, Vilibic, Sabioncello, Gotovac, Rabatic, Folnegovic-Smalc and Dekaris2009). Despite differences in follow-up time and different control groups, the results are in line with the results from other studies (Vidovic et al., Reference Vidovic, Vilibic, Sabioncello, Gotovac, Rabatic, Folnegovic-Smalc and Dekaris2009).
When considering the findings of no differences in IL-1β in the current paper together with previous findings of higher IL-1β levels in PTSD patients during the mainstay (Toft et al., Reference Toft, Bramness, Lien, Abebe, Wampold, Tilden, Hestad and Neupane2018a), the results appear to point in different directions. Finding conflicting results is in line with results reported in a 2018 systematic review on IL-1β in PTSD patients (Waheed et al., Reference Waheed, Dalton, Wesemann, Ibrahim and Himmerich2018). The authors suggested that it is premature to consider IL-1β as a biomarker of PTSD, as the results when comparing levels of IL-1β in PTSD patients and controls were mixed. Moreover, the review assessed two longitudinal studies on IL-1β in PTSD, both of which lent some support to findings in the current study. IL-1β was not detectable in a proportion of participants at both time points (Jergovic et al., Reference Jergovic, Bendelja, Savic Mlakar, Vojvoda, Aberle, Jovanovic, Rabatic, Sabioncello and Vidovic2015), not unlike the current study, which had approximately 50% imputation of IL-1β. Furthermore, IL-1β increased during the first 3 months (Jergovic et al., Reference Jergovic, Bendelja, Savic Mlakar, Vojvoda, Aberle, Jovanovic, Rabatic, Sabioncello and Vidovic2015), which was similar to our findings from the mainstay. The other longitudinal study found the levels of IL-1β to decrease in both PTSD and controls in a double-blind, randomised controlled trial (Tucker et al., Reference Tucker, Ruwe, Masters, Parker, Hossain, Trautman and Wyatt2004). This partly agrees with the findings in the current study, where PTSD patients had lower levels than the year before.
This study has limitations and strengths. It is a strength that we had four measurements, as the cytokines exhibited a non-linear pattern from the mainstay to the follow-up stay. This indicates the necessity of analysing cytokines across a long period of time, as the associations follow somewhat unexpected patterns.
It is a limitation that the patients who chose to come back 1 year later and complete the follow-up treatment had considerably (but not significantly) higher distress measured by GSI than those who only participated at the mainstay. This was found in preliminary analyses of GSI scores at T2 between those who did and did not participate at follow-up (data are not shown), although the difference was not significant. This represents a selection bias that could explain why all cytokines, apart from IL-1β, were higher at the follow-up stay. Having been away from treatment for 1 year could have resulted in a more excessive inflammatory state at follow-up, although not reflected in GSI scores, than if all 147 patients had been included.
Furthermore, the follow-up measurement was analysed 2 years later than the first three measurements, although by the same laboratory personnel and in the same laboratory. Different batches are prone to give different results, leaving the results between the two blood sample batches susceptible to measurement differences.
Further, we did not record whether patients were fasting prior to blood collection. We did not assess body mass index, whether they were smokers, or if the patients had been physically active prior to blood collection. These are all factors known to influence cytokine production (Rubin et al., Reference Rubin, Mcmurray, Harrell, Thorpe and Hackney2008; Belchamber et al., Reference Belchamber, Hall and Hourani2014; Rivera et al., Reference Rivera, Locke, Corre, Czamara, Wolf, Ching-Lopez, Milaneschi, Kloiber, Cohen-Woods, Rucker, Aitchison, Bergmann, Boomsma, Craddock, Gill, Holsboer, Hottenga, Korszun, Kutalik, Lucae, Maier, Mors, Muller-Myhsok, Owen, Penninx, Preisig, Rice, Rietschel, Tozzi, Uher, Vollenweider, Waeber, Willemsen, Craig, Farmer, Lewis, Breen and Mcguffin2017; Sieske et al., Reference Sieske, Janssen, Babel, Westhoff, Wirth and Pourhassan2019). In addition, there was a rather high amount of zero values in cytokine IL-1β, and no firm conclusions should be made about this cytokine against this background.
In conclusion, this study suggests that having a PTSD diagnosis or not is unrelated to inflammatory levels 1 year after psychiatric treatment. Despite exhibiting different cytokine levels and trajectories at the mainstay, these differences between PTSD and non-PTSD patients were eliminated 1 year later.
Acknowledgements
The authors would like to thank the staff at the Modum Bad Psychiatric Center for their contributions and support in all phases of this study.
Author contributions
HT collected the data, conducted the statistical analyses, and drafted and revised the manuscript. JG was involved in the planning and design of the study, interpretation of data and revision of the manuscript. TT was involved in the planning and design of the study, and revision of the manuscript. IB was involved in the interpretation of the data and revision of the manuscript. LL was involved in the planning and design of the study, interpretation of data and revision of the manuscript.
Financial support
The study was funded by the Innlandet Hospital Trust.
Conflict of interest
The authors state that they have no conflicts of interest in the current study.
Ethical standards
The authors state that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 (Williams, 2008).





