Introduction
Parasitism is an important factor influencing population dynamics of wild hosts (Moss et al. Reference Moss, Watson and Ollason1982; Price et al. Reference Price, Westoby, Rice, Atsatt, Fritz, Thompson and Mobley1986). Indeed, parasitism can impact individual fitness through its effects on various components of a host's life such as its reproductive success and survival (Valkiūnas, Reference Valkiūnas2005; Holmstrup et al. Reference Holmstrup, Bindesbøl, Oostingh, Duschl, Scheil, Köhler, Loureiro, Soares, Ferreira, Kienle, Gerhardt, Laskowski, Kramarz, Bayley, Svendsen and Spurgeon2010). Parasite transmission is driven by complex interactions among diverse environmental variables that are generally heterogeneous in the landscape (Lachish et al. Reference Lachish, Knowles, Alves, Wood and Sheldon2011). Therefore, to better understand and predict the dynamics of parasite infection in wild hosts, it is crucial to assess the effects of environmental factors on prevalence at a fine spatial scale (Gonzalez-Quevedo et al. Reference Gonzalez-Quevedo, Davies and Richardson2014). Furthermore, for host species with large-scale migration patterns, documenting the extent of spatial heterogeneity in parasite prevalence at the population level may also provide some key insights about transmission dynamics (Bensch and Åkesson, Reference Bensch and Åkesson2003; Knowles et al. Reference Knowles, Wood, Alves and Sheldon2014). A homogeneous distribution of parasites prevalence on breeding sites generally suggests a transmission occurring after post-reproduction dispersal on wintering grounds. Alternatively, a more heterogeneous distribution should suggest a transmission occurring on breeding sites, which would reflect the influence of local environment variables on vectors (Knowles et al. Reference Knowles, Wood, Alves and Sheldon2014).
In birds, several environmental factors such as climate, landscape composition and avian population density and diversity have been shown to affect the spatial distribution of avian vector-borne parasites (Sérandour et al. Reference Sérandour, Girel, Boyer, Ravanel, Lemperière and Raveton2007; Galardo et al. Reference Galardo, Zimmerman, Lounibos, Young, Galardo, Arruda and D'Almeida Couto2009; Okanga et al. Reference Okanga, Cumming, Hockey and Peters2013). In general, the geographic distribution of parasites will usually reflect vector abundance, which can be modulated by environmental conditions throughout the landscape (Hellgren, Reference Hellgren2005; Loiseau et al. Reference Loiseau, Iezhova, Valkiūnas, Chasar, Hutchinson, Buermann, Smith and Sehgal2010; Grillo et al. Reference Grillo, Fithian, Cross, Wallace, Viverette, Reilly and Mayer2012; Ellis et al. Reference Ellis, Collins, Medeiros, Sari, Coffey, Dickerson, Lugarini, Stratford, Henry, Merrill, Matthews, Hanson, Roberts, Joyce, Kunkel and Ricklefs2015). For instance, higher temperatures may increase (especially for Plasmodium spp.) the rate of parasite development in vectors, allowing them to reach their infectious stage before the mosquitoes’ next blood meals, which should promote parasite transmission (Paaijmans et al. Reference Paaijmans, Read and Thomas2009; Garamszegi, Reference Garamszegi2011). Other studies have shown that high vector–host encounter rates are generally associated with a higher host density (Fourcade et al. Reference Fourcade, Keišs, Richardson and Secondi2014; Ellis et al. Reference Ellis, Medeiros, Collins, Sari, Coffey, Dickerson, Lugarini, Stratford, Henry, Merrill, Matthews, Hanson, Roberts, Joyce, Kunkel and Ricklefs2017).Characteristics of landscape composition, such as proximity to water bodies, also can be important factors affecting the development of some vectors because of habitat-specific phases of their lifecycles (Wood et al. Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007; Lachish et al. Reference Lachish, Knowles, Alves, Wood and Sheldon2011; Okanga et al. Reference Okanga, Cumming, Hockey and Peters2013).
Negative effects of parasitism can be amplified by interactions with environmental stressors of anthropogenic origin, such as habitat loss or exposure to contaminants (Clinchy et al. Reference Clinchy, Zanette, Boonstra, Wingfield and Smith2004; Coors et al. Reference Coors, Decaestecker, Jansen and De Meester2008; Holmstrup et al. Reference Holmstrup, Bindesbøl, Oostingh, Duschl, Scheil, Köhler, Loureiro, Soares, Ferreira, Kienle, Gerhardt, Laskowski, Kramarz, Bayley, Svendsen and Spurgeon2010; Dunn et al. Reference Dunn, Goodman, Benton and Hamer2013). These potentially negative synergistic interactions can be particularly detrimental for declining wild populations that may already show signs of reduced immunological capacity and/or increased physiological demands (Clinchy et al. Reference Clinchy, Zanette, Boonstra, Wingfield and Smith2004; Sih et al. Reference Sih, Bell and Kerby2004). For example, agricultural intensification, which results in landscape homogenization and increased use of pesticides, appears to be responsible for the decline in quality and quantity of breeding habitats for several farmland birds (Jobin et al. Reference Jobin, DesGranges and Boutin1996; Benton et al. Reference Benton, Bryant, Cole and Crick2002; Chiron et al. Reference Chiron, Chargé, Julliard, Jiguet and Muratet2014). Pesticide applications during the breeding season can indeed play an important role by altering the immune system and reducing the breeding success and survival of farmland birds, particularly for insectivores through both toxicological and trophic effects (Benton et al. Reference Benton, Bryant, Cole and Crick2002; Hart et al. Reference Hart, Milsom, Fisher, Wilkins, Moreby, Murray and Robertson2006; Chiron et al. Reference Chiron, Chargé, Julliard, Jiguet and Muratet2014; Hallmann et al. Reference Hallmann, Foppen, van Turnhout, de Kroon and Jongejans2014; Lopez-Antia et al. Reference Lopez-Antia, Ortiz-Santaliestra, Mougeot and Mateo2015). Given the possible negative interaction with pesticides, parasitism may play a major role in the decline of farmland birds in agricultural landscapes (Dunn et al. Reference Dunn, Goodman, Benton and Hamer2013). Yet, to our knowledge, no studies have assessed the influence of agricultural intensification, as a proxy of pesticide use, on the prevalence of vector-borne parasites in declining populations of insectivorous birds (Dunn et al. Reference Dunn, Goodman, Benton and Hamer2013; see also: Zehtindjiev et al. Reference Zehtindjiev, Križanauskienė, Scebba, Dimitrov, Valkiūnas, Hegemann, Tieleman and Bensch2012a).
Avian haemosporidians are vector-borne blood parasites that cause a malaria-like disease. This group of parasitic protists is ubiquitous and abundant throughout avian species (Valkiūnas, Reference Valkiūnas2005). Parasites of three genera (Haemoproteus spp., Plasmodium spp. and Leucocytozoon spp.) commonly infect birds and differ in some aspects of their life cycle and vectors (e.g. Haemoproteus species main vectors: Hippoboscidae and Ceratopogonidae; Plasmodium: Culicidae; Leucocytozoon: Simuliidae) (Valkiūnas, Reference Valkiūnas2005; Marzal, Reference Marzal and Okawa2012). Previous studies have identified negative effects of haemosporidian parasites on body condition, breeding success and survival of many bird species (Dawson and Bortolotti, Reference Dawson and Bortolotti2000; Hatchwell et al. Reference Hatchwell, Wood, Anwar, Chamberlain and Perrins2001; Navarro et al. Reference Navarro, Marzal, de Lope and Møller2003; Marzal et al. Reference Marzal, Bensch, Reviriego, Balbontin and de Lope2008; Knowles et al. Reference Knowles, Palinauskas and Sheldon2010; Lachish et al. Reference Lachish, Knowles, Alves, Wood and Sheldon2011). For instance, Asghar et al. (Reference Asghar, Hasselquist, Hansson, Zehtindjiev, Westerdahl and Bensch2015) showed long-lasting effects of avian malaria (Plasmodium and Haemoproteus spp.), such as reduced lifespan and lifetime number of offspring, in Great reed warblers (Acrocephalus arundinaceus). Moreover, the prevalence of haemosporidian parasites has been shown to depend upon environmental conditions (Okanga et al. Reference Okanga, Cumming, Hockey and Peters2013; Gudex-Cross et al. Reference Gudex-Cross, Barraclough, Brunton and Derraik2015; Hernández-Lara et al. Reference Hernández-Lara, González-García and Santiago-Alarcon2017). For instance, Okanga et al. (Reference Okanga, Cumming, Hockey and Peters2013) identified higher avian malaria prevalence in wetland habitats related to the presence of rich nutrient sources and suitable breeding site for vector development. However, the effects of human-driven environmental changes on haemosporidian parasite prevalence, particularly for Leucocytozoon species, have never been addressed within an agricultural context.
This study aims to identify the environmental factors affecting haemosporidian parasite prevalence and patterns of transmission in a declining wild population of Tree swallows (Tachycineta bicolor), in southern Québec, Canada. More specifically, our objectives are to: (1) quantify the prevalence and diversity of haemosporidian parasites in this population using a large sample size collected over 4 years; (2) determine if transmission occurs during the breeding season; and (3) identify environmental variables, such as climate, landscape composition, as well as avian population density and diversity, associated with the infection status of individuals within this population. The Tree swallow is a small passerine bird that migrates annually between its wintering grounds in the southern USA and Central America to its breeding sites over much of North America (Winkler et al. Reference Winkler, Hallinger, Ardia, Robertson, Stutchbury and Cohen2010). This aerial insectivore nests in secondary cavities in open areas such as pastures and agricultural fields. The studied population is located within a gradient of agricultural intensification that was previously linked to variation in the density of its main prey (i.e. Diptera; Rioux Paquette et al. Reference Rioux Paquette, Garant, Pelletier and Bélisle2013). Tree swallow populations are declining in northeastern North America (Michel et al. Reference Michel, Smith, Clark, Morrissey and Hobson2016), and our studied population is no exception (Rioux Paquette et al. Reference Rioux Paquette, Pelletier, Garant and Bélisle2014).
METHODS
Study system and bird sampling
This study is the part of a long-term project that monitors a breeding population of Tree swallows in southern Québec (Canada) since 2004. The study system, characterized by an east–west gradient of agricultural intensification, covers an area of 10 200 km2 and consists of 400 nest boxes uniformly distributed among 40 farms [Fig. 1; see Ghilain and Bélisle (Reference Ghilain and Bélisle2008) for details on the study system]. All nest boxes were visited every 2 days during the breeding season (early May to mid-July). Breeding females and males were captured directly in the nest box during incubation and food provisioning, respectively. For this study, 1150 adult Tree swallows were sampled between 2012 and 2015 (females = 687, males = 463). Nestlings were captured directly in the nest at 16 days old in 2015 on six farms (see Fig. 1). These farms were selected because infected adults were detected at those locations between 2012 and 2014. We determined the sex of adult birds in the field by the presence of a brood patch and subsequently confirmed it with molecular techniques [see Lessard et al. (Reference Lessard, Bourret, Bélisle, Pelletier and Garant2014) for details]. At first capture, each bird was identified with a unique US Fish and Wildlife service aluminium band. A blood sample was taken from the brachial vein of each individual on a qualitative P8 grade filter paper (Thermo Fisher Scientific). Blood samples were dried and stored at room temperature until DNA extractions. For individuals found dead in the study system, a piece of tissue (25 mm2) at the base of a leg was taken for DNA extractions and preserved in 70% ethanol. All manipulations were approved by the Université de Sherbrooke Animal Care Committee (protocol number DG 2010-01 and DG 2014-01).
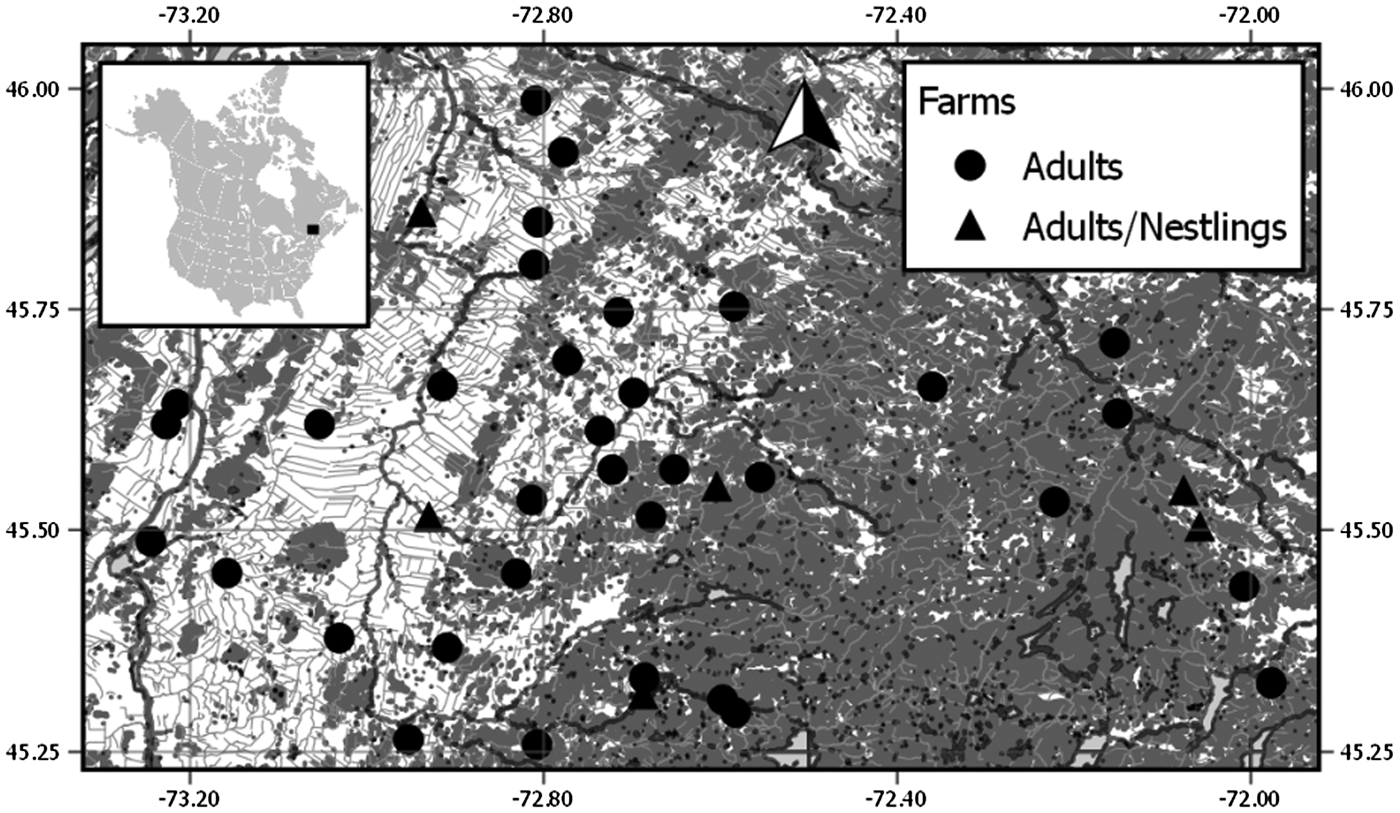
Fig. 1. Map of the 40 farms (black circle and triangle) used to sample Tree swallows in southern Québec, Canada. Land cover types are based on a mosaic of classified Landsat-TM satellite images (Canadian Wildlife Service, 2004) and include forest cover (dark grey), and intensive cultures (white). Water bodies are shown in light grey. Coordinates are in decimal degrees. This map was created with QGIS 2·0 (QGIS Team Development, 2016)
Molecular analyses
DNA was extracted from blood samples with an overnight proteinase K digestion followed by a salt extraction (Aljanabi and Martinez, Reference Aljanabi and Martinez1997). Extracted DNA samples were then checked and quantified on 1% agarose gels. The detection of haemosporidian parasites from bird blood samples was performed with a nested polymerase chain reaction (PCR), which consists of two successive PCR amplifications (Hellgren et al. Reference Hellgren, Waldenström and Bensch2004). The first amplification targets a conserved section of mitochondrial DNA common to parasites of the three genera we studied with the following primers pairs: HaemNF1/HaemNR3 [designed by Hellgren et al. (Reference Hellgren, Waldenström and Bensch2004)]. This first step increases the performance of the second PCR, which has specific primers for the amplification of Plasmodium and Haemoproteus species together: HaemF/HaemR2 and Leucocytozoon species separately: Haem FL/HaemR2L (Hellgren et al. Reference Hellgren, Waldenström and Bensch2004). The first PCR was performed in a total volume of 25 µL containing 40–60 ng of genomic DNA, 10 mm Tris–HCl (pH = 9), 50 mm KCl, 0·1% Triton X-100, 2·5 mm MgCl2, 0·1 mm dNTPs, 0·4 mm of each primer and 1 U µL−1 AmpliTaq Gold (Applied Biosystems). The second PCR was performed with similar conditions, except that we used 5 µL of the first PCR product instead of genomic DNA. The thermal profile for both PCRs consisted of an initial denaturation of 2 min at 94 °C followed by 35 cycles of 30 s at 94 °C, 30 s at 50 °C and 45 s at 72 °C and a final elongation period of 10 min at 72 °C. To determine if the PCR amplification was successful, 5 µL of the second PCR product was migrated on a 2% agarose gel stained with ethidium bromide and visualized under UV light. Each batch of PCR amplification contained a positive control (i.e. DNA of a known infected individual) and a negative control (i.e. H2Odd) to control for possible contamination or problems during manipulations (Szöllősi et al. Reference Szöllősi, Hellgren and Hasselquist2008). The presence of an infection was confirmed by the detection of an amplification at ca. 500-bp (478-bp for Leucocytozoon species and 480-bp for Plasmodium and Haemoproteus species without primers).
Sequencing and identification of lineages
PCR products from all positive amplifications were sent to the Plateforme de séquençage et de génotypage du Centre de Recherche du CHUL (CHUQ, Québec, Canada) for sequencing with an AB 3730xl Data Analyser (Applied Biosystems). Obtained sequences were compared with those present in the MalAvi database of haemosporidian avian parasites, which contains more than 2400 lineages (Bensch et al. Reference Bensch, Hellgren and Pérez-Tris2009). Identified sequences were aligned with their closest sequence matches in MalAvi and analysed using MEGA version 7 (Kumar et al. Reference Kumar, Stecher and Tamura2016). The electropherograms of each sequence were visually checked for the presence of sites with double base calling suggesting a mixed infection. All samples with potential mixed infections were re-amplified and re-sequenced to validate the presence of mixed infections and identify the lineage of the additional infection [see Matthews et al. (Reference Matthews, Ellis, Hanson, Roberts, Ricklefs and Collins2015) for details on this approach].
Landscape characterization
We characterized the proportion of different land covers around each nest box at a large (5-km radius) and a fine (500-m radius) spatial scale. These 5-km and 500-m radii encompass, respectively, the area used by Tree swallows during the food-provisioning period (Ghilain and Bélisle, Reference Ghilain and Bélisle2008) and the local environment of the farm (Lagrange et al. Reference Lagrange, Pradel, Bélisle and Gimenez2014). Land cover at the 5-km scale was estimated using a mosaic of geo-referenced and classified Landsat-7 satellite images taken between August 1999 and May 2003 (Canadian Wildlife Service, 2004) and ArcView GIS spatial analyst 2·0a (ESRI, 2005). At the 500-m scale, land cover was assessed annually in the field by determining visually the crop growing in each field polygon delineated with orthophotos (scale: 1:40 000). Land cover was classified in five categories at both scales: (1) intensive cultures (e.g. corn and soyabean), (2) extensive cultures (e.g. pastures and perennial cultures, such as various grasses, alfalfa and clover), (3) water bodies, (4) forested areas and (5) anthropic areas (e.g. buildings and roads). We also conducted livestock (i.e. number of horses, cattle and sheep) counts at the beginning of each farm visit within a 500-m radius from the central point of the farm. These counts were then averaged across visits for each farm and livestock category.
Weather variables
Temperature and precipitation data were collected on each farm every year. Each weather variable was obtained relative to each bird sampled by using data collected between the start of the field season and the date of the blood sampling. Thus, each weather variable reflected the conditions that may have affected the infection status of each bird prior to blood sampling. Temperature (±0·5 °C) was taken every hour with a temperature data logger (Thermocron iButton DS 1992 L; Dallas Semiconductor, Dallas, Texas, USA) installed under the central nest box on each farm. We used five different temperature variables: (1) mean daily temperature, (2) mean diurnal temperature (6 AM to 8 PM, inclusively), (3) mean minimum daily temperature, (4) mean maximum daily temperature and (5) mean daily temperature variation (i.e. mean maximum daily temperature – mean minimum daily temperature). Precipitation data (±0·5 mm) were obtained on a 48-h basis using pluviometers installed on each farm.
Bird population density and diversity index
We used multiple indices estimating the availability of bird hosts on and surrounding each farm. First, we determined the proportion of nest boxes (e.g. avian density) occupied by Tree swallows and other cavity-nesting species within a 15-km radius around each farm. A nest box was considered occupied when at least one egg was laid during the breeding season. A 15-km radius was used to consider the territory covered by Tree swallows when searching for extra-pair copulations [see Lessard et al. (Reference Lessard, Bourret, Bélisle, Pelletier and Garant2014) for details]. In addition, we estimated the total farmland bird abundance as well as their species richness and diversity (i.e. Shannon's equitability) based on point counts conducted on each farm during the breeding season of 2004. At least six 5-min point counts were conducted from the central point of each farm during which birds from a pre-established species list (see Table A1) were counted within an unlimited radius.
Statistical analyses
We used mixed logistic regression models to quantify the influence of environmental variables on the haemosporidian infection status of Tree swallows (binomial factor: 0 for uninfected and 1 for infected). For adults and nestlings, we assessed infection status patterns for each parasite genus detected separately (Plasmodium or Leucocytozoon species). We also analysed all parasite genera combined for nestlings, given the lower number of individuals tested from a smaller number of farms. All explanatory variables were standardized (zero mean, unit variance) before model selection (see Table A2 and A3). Explanatory variables were selected with a backward selection procedure (α = 0·05) and their inclusion/deletion were assessed at each step by using a likelihood ratio test (LRT). Farm and nest-box identities were also included in initial models and tested for significance as random variables with LRTs. Only nest-box identity was significant and kept in nestling models. For individuals with repeated records (N = 306), we used only the data obtained at first capture. All variables that were highly correlated (r > 0·8) or with high variance inflation factors (VIF > 3) were removed from models to avoid collinearity (see Table A4 and A5 for correlation coefficients). All statistical analyses were performed in R 3·1·2 (R Core Team, 2016), with the lme4 package (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). The estimation of variance explained (R 2) by the mixed models was obtained with the methods described in Nakagawa and Schielzeth (Reference Nakagawa and Schielzeth2013), while the method presented in Nagelkerke (Reference Nagelkerke1991) was used for the other models.
RESULTS
Prevalence and lineage diversity
We found a low haemosporidian prevalence in adult Tree swallows for all parasite genera combined between 2012 and 2015 (19% of 1562 individuals; Table 1 and Fig. 2). Prevalence for Plasmodium and Leucocytozoon species were 7·9% (N = 125) and 9·9%, respectively (N = 155; Table 1). We found no infection by Haemoproteus species (Table 1). A relatively similar overall prevalence was observed for nestlings (17% of 217 individuals) and for each parasite genus (Plasmodium species: 9·2%; Leucocytozoon species: 5·5%; Table 1). Detection of haemosporidian parasites in nestlings confirmed its transmission on the breeding sites. Overall, 312 lineages were confirmed (from 87% of sequences analysed; Table 2). We found 8 and 2 known lineages for the genus Plasmodium and Leucocytozoon, respectively (Table 2). CB1 (haplotype of Leucocytozoon majoris) and SEIAUR01 (haplotype of Plasmodium cathemerium) were the most common known lineages in both adults and nestlings (Table 2). A total of 22 individuals (18 adults and four nestlings) had a mixed infection by parasites either of the same or of a different genus (e.g. Plasmodium–Leucocytozoon; see Table 3).
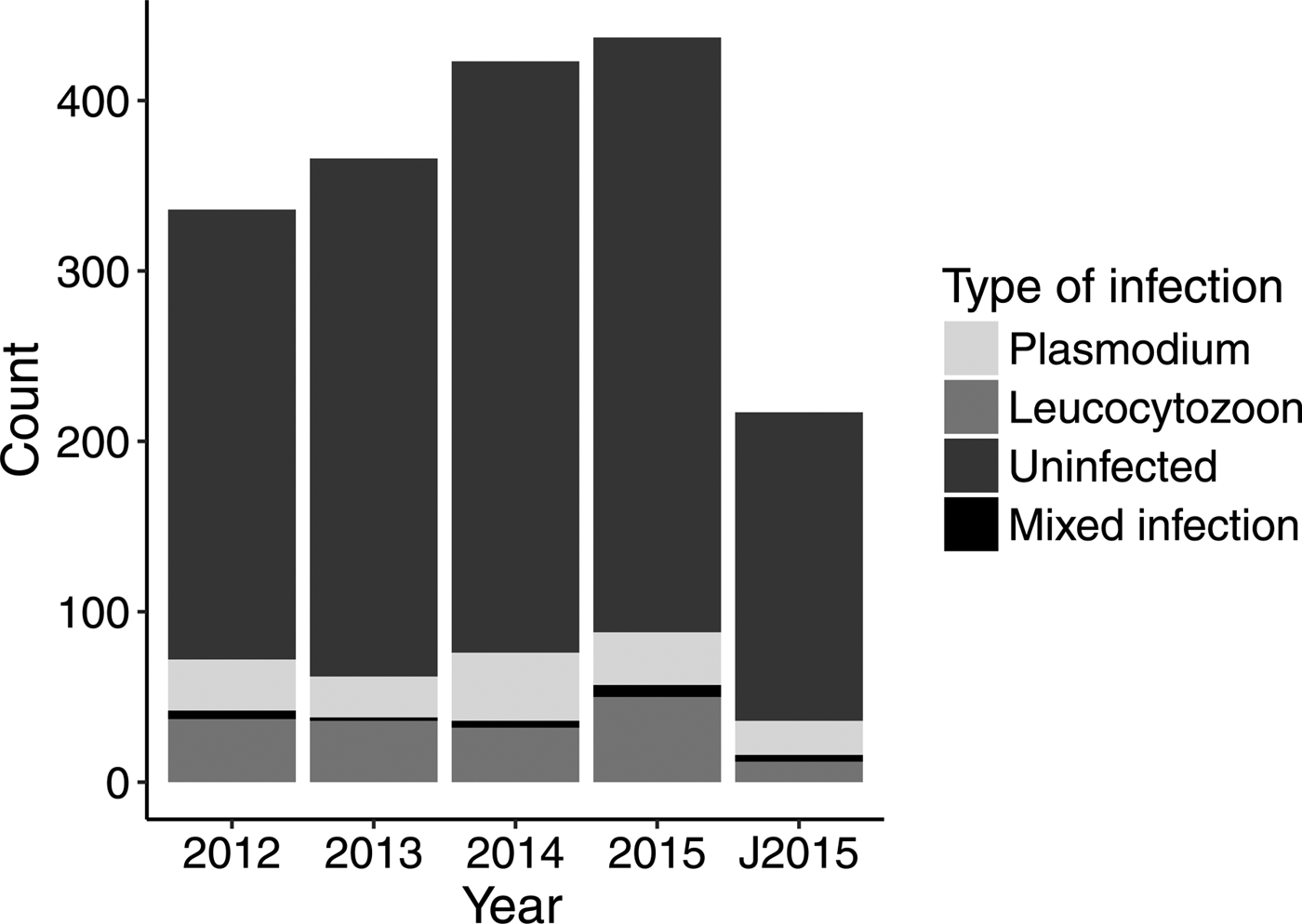
Fig. 2. Annual count of each haemosporidian genus detected in Tree swallow adults (2012–2015) and nestlings (J2015) in southern Québec, Canada.
Table 1. Summary of individuals analysed and infected by haemosporidian parasites in breeding Tree swallows (Tachycineta bicolor) sampled in southern Québec, Canada. Adults were sampled from 2012 to 2015 and nestlings in 2015 only. Prevalence corresponds to the overall proportion of the infected individuals
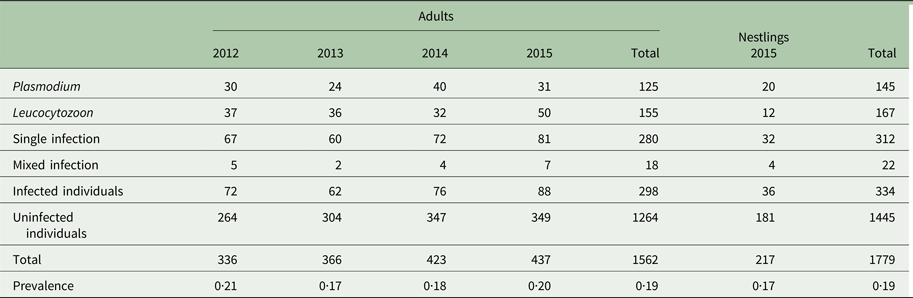
Table 2. Summary of haemosporidian parasite lineages detected in adult Tree swallows (Tachycineta bicolor) from 2012 to 2015 and in nestlings for 2015
a SEIAUR01: P. cathemerium; SGS1: P. relictum; CB1: L. majoris
Table 3. Number of individuals (and lineages) for which each type of mixed infections by haemosporidian parasites were detected in Tree swallow (Tachycineta bicolor) for adults from 2012 to 2015 and for nestlings from 2015
Environmental effects
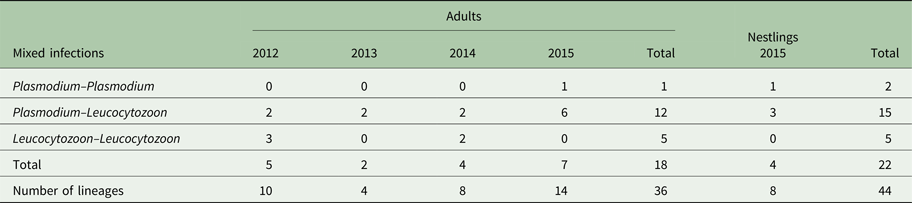
Plasmodium parasite infection status in adult Tree swallows was negatively related to the percentage of anthropic areas within 500 m (Fig. 3A, Table A6). On the other hand, the probability to be infected by Leucocytozoon parasites was negatively affected by the mean daily temperature variation (Fig. 3B, Table A6). However, birds located in landscapes with a higher percentage of extensive cultures or of forested areas within 500 m were more likely to be infected by Leucocytozoon species (Fig. 3C and D and Table A6). Conducting all analyses without dead individuals and with only the most prevalent lineages (CB1 and SEIAUR01 separately) provided similar results (see Figure A1, A2 and Table A7).
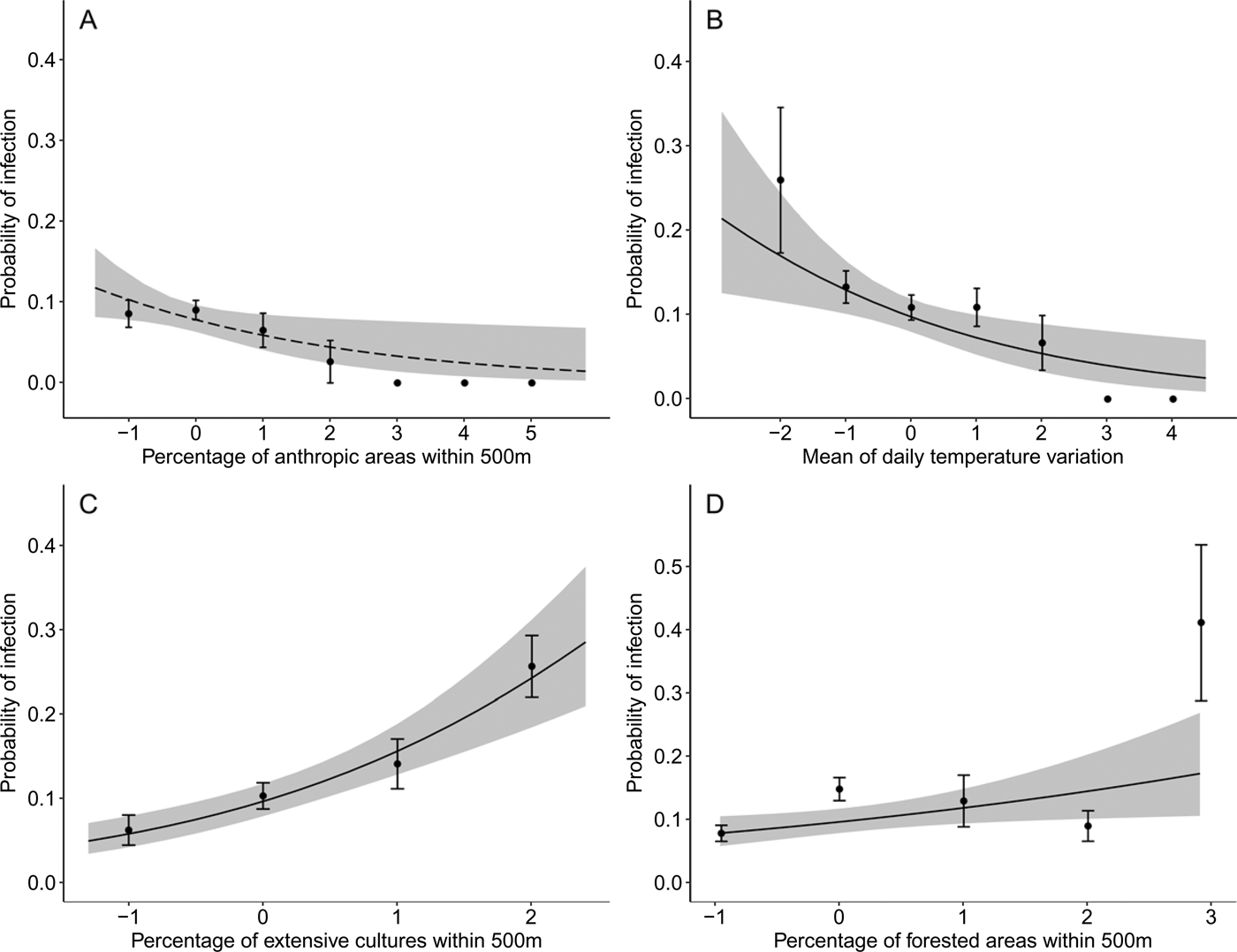
Fig. 3. Relationship between the probability that the birds were infected by Plasmodium [final model R 2 = 0·01, (A); dashed line] or Leucocytozoon parasites [final model R 2 = 0·09, (B–D); solid line] separately, modelled by logistic regressions (black dots represent mean ± s.d.), and standardized environmental variables: (A) percentage of anthropic areas within 500 m; (B) mean of daily temperature variation; (C) percentage of extensive cultures within 500 m; and (D) percentage of forested areas within 500 m in adult Tree swallows, southern Québec, Canada, 2012–2015. Grey areas depict the 95% confidence intervals of predictions.
In nestlings, the mean amount of precipitation increased the probability to be infected by all genera combined (Fig. 4 and Table A8). In this analysis, nest-box identity explained 32% of the total variance in the infection status. Analyses of environmental effects conducted separately for Plasmodium and Leucocytozoon species revealed that none of the variables we considered had a significant effect on haemosporidian prevalence in nestlings (Table A8).
Fig. 4. Relationship between the probability that birds were infected by all haemosporidian genera combined (Plasmodium and/or Leucocytozoon species), modelled by a mixed logistic regression (black dots represent mean ± s.d.), and the standardized mean amount of precipitation (mm) in nestling Tree swallows, southern Québec, Canada, 2015. Grey areas depict the 95% confidence intervals of predictions. Nest-box identity was used as a random effect. Final model marginal R 2 = 0·10.
DISCUSSION
Understanding how anthropogenic activities and environmental changes interact to influence parasite prevalence is critical given the increasing number of wild populations affected by human activities. Here, we detected a low prevalence and a moderate lineage diversity of haemosporidian parasites in a declining wild population of Tree swallows. In addition to being the most prevalent lineages found in the adult population, CB1 and SEIAUR01 were also detected in nestlings. Our results thus provide strong evidence that transmission can occur on the breeding grounds of this migratory species. We also showed that the infection status was related to several environmental variables on a fine spatial scale, including the relative amount of anthropic areas and extensive cultures surrounding the birds’ nest. However, these environmental characteristics affected haemosporidian prevalence differently depending on the parasites’ genus.
Parasite prevalence
The Tree swallow population we studied showed a low prevalence of infection by all haemosporidian parasite genera combined. There is little-published information available to compare the prevalence observed in our study system with that of other Tree swallow populations. Yet, most studies that assessed haemosporidian prevalence in Tree swallows in North America reported low prevalence or absence of infections (Table A9). Similar patterns of relatively low prevalence were also reported in other species of the Hirundinidae family [prevalence range : (0–33%); Oakgrove et al. Reference Oakgrove, Harrigan, Loiseau, Guers, Seppi and Sehgal2014; Medeiros et al. Reference Medeiros, Ricklefs, Brawn and Hamer2015; Moens et al. Reference Moens, Valkiūnas, Paca, Bonaccorso, Aguirre and Pérez-Tris2016; Ellis et al. Reference Ellis, Medeiros, Collins, Sari, Coffey, Dickerson, Lugarini, Stratford, Henry, Merrill, Matthews, Hanson, Roberts, Joyce, Kunkel and Ricklefs2017]. This is concordant with the observation that aerial insectivores tend to have low prevalence, likely as a result of the difficulties that insect vectors experience in order to feed on these very mobile birds [prevalence range for other insectivorous passerines: (0–75%); review from Greiner et al. Reference Greiner, Bennett, White and Coombs1975; see also: Szymanski and Lovette, Reference Szymanski and Lovette2005]. However, it should be noted that most of the previous studies conducted on Tree swallows in North America were located at lower latitudes and had very low sample sizes (N < 10) to assess prevalence with confidence (see Table A9). One exception is the study by Szymanski and Lovette (Reference Szymanski and Lovette2005) who used a sampling design comparable with our study in terms of bird capture technique and parasite detection method (see Table A9). Despite a lower sample size (N = 89) and a shorter monitoring period (i.e. 1 year) than in our study, they found a higher global prevalence in Tree swallows breeding near the centre of New York state, USA (48%; see Table A9; Szymanski and Lovette, Reference Szymanski and Lovette2005). Given that local prevalence tends to be stable over time (Fallon et al. Reference Fallon, Ricklefs, Latta and Bermingham2004; Ricklefs et al. Reference Ricklefs, Swanson, Fallon, Martínez-Abraín, Scheuerlein, Gray and Latta2005), these results suggest large-scale spatial variation in haemosporidian prevalence among Tree swallow populations.
We also documented a low prevalence in nestling Tree swallows supporting that haemosporidian parasite transmission can occur during the breeding season at this northern latitude and even in subarctic regions (see also: Oakgrove et al. Reference Oakgrove, Harrigan, Loiseau, Guers, Seppi and Sehgal2014). Since prevalence in nestlings was obtained from a small sample size collected early in the breeding season, our assessment may slightly underestimate the prevalence that could be observed/detected at the end of the season. Indeed, haemosporidian parasites may not be detected in 16 days old nestlings given the presence of a prepatent period [which may vary between 1 and 3 weeks; see Valkiūnas (Reference Valkiūnas2005)] in the parasite life cycle. Previous studies also documented avian malaria transmission in North America by the identification of parasites in juvenile migratory birds (Ricklefs et al. Reference Ricklefs, Swanson, Fallon, Martínez-Abraín, Scheuerlein, Gray and Latta2005; Szymanski and Lovette, Reference Szymanski and Lovette2005; Medeiros et al. Reference Medeiros, Ricklefs, Brawn and Hamer2015). In particular, the study of Szymanski and Lovette (Reference Szymanski and Lovette2005), conducted approximately 500 km south of our study system (Freeville, NY, USA), also detected avian malaria in juvenile Tree swallows. Furthermore, some avian malaria lineages detected in blood meals of trapped mosquitoes in the study of Kimura et al. (Reference Kimura, Darbro and Harrington2010), in Ithaca, NY, USA, were also identified in our Tree swallow population (e.g. PADOM11 and SEIAUR01, see Table 2), suggesting that some common vectors in North America can transmit parasite lineages detected in our study system.
Parasite diversity
The higher diversity of Plasmodium lineages detected, compared to Leucocytozoon, is concordant with its general low degree of host specificity that promotes infection of a large array of migratory birds (Valkiūnas, Reference Valkiūnas2005; Hellgren et al. Reference Hellgren, Waldenström, Peréz-Tris, Szöllösi, Hasselquist, Krizanauskiene, Ottosson and Bensch2007). Leucocytozoon species, on the other hand, tend to be more specialized through particular feeding preferences and developmental environmental conditions of their specific vectors, which limit their contact with numerous avian species (Malmqvist et al. Reference Malmqvist, Strasevicius, Hellgren, Adler and Bensch2004; Hellgren et al. Reference Hellgren, Bensch and Malmqvist2008; Svensson-Coelho et al. Reference Svensson-Coelho, Loiselle, Blake and Ricklefs2016). However, the degree of specificity can vary greatly between lineages of a given genus ranging from a parasite lineage specializing on a single host species to a lineage found in a large diversity of hosts (e.g. CB1 is found in different species from both Europe and North America; see Table A10) (Hellgren, Reference Hellgren2005; Medeiros et al. Reference Medeiros, Hamer and Ricklefs2013). Based on the MalAvi database, all the lineages we amplified were detected in North America, except for SGS1 (haplotype of Plasmodium relictum; South America), and detected in Passeriformes, except for STVAR04 (Strigiformes) (Table A10). The two most abundant lineages in our study system, CB1 and SEIAUR01, were also detected in other Tree swallow populations (e.g. CB1: Murdock, Reference Murdock2005; SEIAUR01: Beadell et al. Reference Beadell, Ishtiaq, Covas, Melo, Warren, Atkinson, Bensch, Graves, Jhala, Peirce, Rahmani, Fonseca and Fleischer2006). Six lineages (e.g. STVAR04, TABI09, CATUST05, PADOM11, SGS1 and WW3) were rare in our study system, each represented <2% of all lineages detected. The presence of rare lineages can be the result of sporadic spillovers if competent generalist vectors and permanent reservoir of generalist lineage populations are present in contact zones (Power and Mitchell, Reference Power and Mitchell2004; Ricklefs et al. Reference Ricklefs, Fallon and Bermingham2004; Moens et al. Reference Moens, Valkiūnas, Paca, Bonaccorso, Aguirre and Pérez-Tris2016). Contact zones on both breeding sites and wintering grounds can act like a transmission corridor between host species (Reullier et al. Reference Reullier, Pérez-Tris, Bensch and Secondi2006). Lineage competition could also explain the presence of rare lineages that were excluded by more abundant ones in the host organism (Svensson-Coelho et al. Reference Svensson-Coelho, Loiselle, Blake and Ricklefs2016). Also, more virulent lineages can lead to higher mortality rates of migratory birds before their arrival on breeding sites, resulting in lower prevalence during reproduction (Bensch et al. Reference Bensch, Waldenström, Jonzén, Westerdahl, Hansson, Sejberg and Hasselquist2007). However, our findings need to be interpreted cautiously given that they are based only on a PCR detection method (Valkiūnas et al. Reference Valkiūnas, Palinauskas, Ilgūnas, Bukauskaitė, Dimitrov, Bernotienė, Zehtindjiev, Ilieva and Iezhova2014). Indeed, haemosporidian parasites detected with PCR methods can represent abortive infections with incomplete development (Valkiūnas et al. Reference Valkiūnas, Palinauskas, Ilgūnas, Bukauskaitė, Dimitrov, Bernotienė, Zehtindjiev, Ilieva and Iezhova2014). Additional evidence such as detection of haemosporidian parasites through microscopy analysis and in blood-sucking insects would be necessary to further support the results we obtained.
We found very few mixed infections in our study system, which was rather surprising given that those are generally common in wild avian populations (Pérez-Tris and Bensch, Reference Pérez-Tris and Bensch2005; Loiseau et al. Reference Loiseau, Iezhova, Valkiūnas, Chasar, Hutchinson, Buermann, Smith and Sehgal2010; Palinauskas et al. Reference Palinauskas, Valkiūnas, Bolshakov and Bensch2011). The low rate of mixed infections can be the result of many types of biological mechanisms such as synergistic interactions and competitions between cohabiting lineages (Bensch et al. Reference Bensch, Waldenström, Jonzén, Westerdahl, Hansson, Sejberg and Hasselquist2007; Marzal et al. Reference Marzal, Bensch, Reviriego, Balbontin and de Lope2008; Palinauskas et al. Reference Palinauskas, Valkiūnas, Bolshakov and Bensch2011; Zehtindjiev et al. Reference Zehtindjiev, Križanauskienė, Bensch, Palinauskas, Asghar, Dimitrov, Scebba and Valkiūnas2012b). However, it is possible that the rate of mixed infections detected can be underestimated due to the PCR methods we used, as preferential amplifications could occur when using primers with greater affinity and/or high intensity of infection is present in some lineages only (Pérez-Tris and Bensch, Reference Pérez-Tris and Bensch2005; Valkiūnas et al. Reference Valkiūnas, Bensch, Iezhova, Križanauskienė, Hellgren and Bolshakov2006; Bensch et al. Reference Bensch, Waldenström, Jonzén, Westerdahl, Hansson, Sejberg and Hasselquist2007; Martínez et al. Reference Martínez, Martínez-De La Puente, Herrero, Del Cerro, Lobato, Rivero-De Aguilar, Vásquez and Merino2009; Zehtindjiev et al. Reference Zehtindjiev, Križanauskienė, Bensch, Palinauskas, Asghar, Dimitrov, Scebba and Valkiūnas2012b; Synek et al. Reference Synek, Albrecht, Vinkler, Schnitzer, Votýpka and Munclinger2013; Bernotienė et al. Reference Bernotienė, Palinauskas, Iezhova, Murauskaitė and Valkiūnas2016). Thus, complementary blood analysis by microscopy and qPCR would increase confidence in detections of mixed infections, while providing information on the parasitaemia (Richard et al. Reference Richard, Sehgal, Jones and Smith2002; Valkiūnas et al. Reference Valkiūnas, Bensch, Iezhova, Križanauskienė, Hellgren and Bolshakov2006; Zehtindjiev et al. Reference Zehtindjiev, Križanauskienė, Bensch, Palinauskas, Asghar, Dimitrov, Scebba and Valkiūnas2012b; Bernotienė et al. Reference Bernotienė, Palinauskas, Iezhova, Murauskaitė and Valkiūnas2016).
Environmental effects
Haemosporidian prevalence was modulated at a fine spatial scale by different environmental factors depending on the parasite genus. This is not surprising given that vector species differ in their optimal environmental requirements during their larval and adult stages of development (Patz et al. Reference Patz, Graczyk, Geller and Vittor2000; Gonzalez-Quevedo et al. Reference Gonzalez-Quevedo, Davies and Richardson2014; Sehgal, Reference Sehgal2015). For example, weather factors have been previously identified as major determinants of vector-borne parasite infection risks (Paaijmans et al. Reference Paaijmans, Read and Thomas2009, Reference Paaijmans, Blanford, Bell, Blanford, Read, Thomas and Denlinger2010; Garamszegi, Reference Garamszegi2011; Colinet et al. Reference Colinet, Sinclair, Vernon and Renault2015). In the population of Tree swallows that we studied, Leucocytozoon parasites prevalence was negatively related to daily temperature fluctuations. Mosquitoes undergo spatial and temporal temperature variations across all of their life stages and those fluctuations may affect these vectors’ biology depending on their intensity (Carrington et al. Reference Carrington, Seifert, Willits, Lambrechts and Scott2013; Zhao et al. Reference Zhao, Chen, Feng, Li and Zhou2014). For instance, Paaijmans et al. (Reference Paaijmans, Blanford, Bell, Blanford, Read, Thomas and Denlinger2010) associated a decrease in vector and parasite development rates and an increase in mosquito mortality rates with wide fluctuations around the mean high temperatures in tropical regions. These results were subsequently supported by many other studies that also showed low malaria prevalence (Plasmodium spp.) under similar climatic conditions (Blanford et al. Reference Blanford, Blanford, Crane, Mann, Paaijmans, Schreiber and Thomas2013; Paaijmans et al. Reference Paaijmans, Heinig, Seliga, Blanford, Blanford, Murdock and Thomas2013; Estay et al. Reference Estay, Lima and Bozinovic2014; Colinet et al. Reference Colinet, Sinclair, Vernon and Renault2015; Davies et al. Reference Davies, Coetzee and Lyons2016; Rund et al. Reference Rund, O'Donnell, Gentile and Reece2016). However, none of these studies were conducted in temperate regions, and specifically on Leucocytozoon species, making it difficult to compare with our study system. Further research is needed to assess if similar effects of temperature fluctuations on prevalence are observed in other populations evolving under temperate climatic conditions.
Anthropic areas were associated with lower prevalence of Plasmodium parasites suggesting that parasite transmission patterns were altered by human presence or activities. This supports the findings of previous studies that observed a decrease of haemosporidian infection rates due to anthropogenic perturbations that led to a deterioration of ecosystems and potentially affected the abundance and richness of hosts and vectors (Patz et al. Reference Patz, Graczyk, Geller and Vittor2000; Bradley and Altizer, Reference Bradley and Altizer2007; Okanga et al. Reference Okanga, Cumming, Hockey and Peters2013; Fourcade et al. Reference Fourcade, Keišs, Richardson and Secondi2014; Sehgal, Reference Sehgal2015). In our study system, higher levels of drainage, generally associated with human activities, may have contributed to the diminution of transmission rates due to the loss of appropriate breeding sites for vectors (Sérandour et al. Reference Sérandour, Girel, Boyer, Ravanel, Lemperière and Raveton2007). Habitat perturbations can also alter interspecific competition and feeding habits of vectors implicated in parasite transmission, resulting in decreased prevalence (Bonneaud et al. Reference Bonneaud, Sepil, Milá, Buermann, Pollinger, Sehgal, Valkiūnas, Iezhova, Saatchi and Smith2009; Chasar et al. Reference Chasar, Loiseau, Valkiūnas, Iezhova, Smith and Sehgal2009). Other landscape characteristics, such as the percentage of forested areas and extensive cultures within 500 m, were associated with higher Leucocytozoon parasites prevalence. These types of land covers correspond to less perturbed environments and are characteristic of heterogeneous agricultural landscapes with low pesticide inputs (Ghilain and Bélisle, Reference Ghilain and Bélisle2008). The vegetation covers of these habitats tend to support greater vector and host abundances, and may thereby facilitate parasite transmission (Mercer et al. Reference Mercer, Sheeley and Brown2005; Bonneaud et al. Reference Bonneaud, Sepil, Milá, Buermann, Pollinger, Sehgal, Valkiūnas, Iezhova, Saatchi and Smith2009; Crowder et al. Reference Crowder, Dykstra, Brauner, Duffy, Reed, Martin, Peterson, Carrière, Dutilleul and Owen2013; Roiz et al. Reference Roiz, Ruiz, Soriguer and Figuerola2015). For example, Bonneaud et al. (Reference Bonneaud, Sepil, Milá, Buermann, Pollinger, Sehgal, Valkiūnas, Iezhova, Saatchi and Smith2009) have previously suggested that high vegetation density may provide protective cover against predation for weak birds infected by Plasmodium species when breeding under poor environmental conditions. Finally, the spatial heterogeneity in infection by haemosporidian parasites in our study system supports the idea that transmission occurs locally over breeding sites as suggested by Knowles et al. (Reference Knowles, Wood, Alves and Sheldon2014).
Contrary to our expectations, the relative amount of water bodies, as well as avian population density and diversity, did not affect the prevalence of haemosporidian parasites in Tree swallows. The relatively large spatial scales used in our analysis may have prevented the detection of an effect of water bodies on infection status. Indeed, Roiz et al. (Reference Roiz, Ruiz, Soriguer and Figuerola2015) highlighted the importance of temporary pools in vector development and the difficulty to detect them at large spatial scales. Usually, avian density and diversity are key factors in the transmission of vector-borne parasites (Keesing et al. Reference Keesing, Holt and Ostfeld2006; Fourcade et al. Reference Fourcade, Keišs, Richardson and Secondi2014; Ellis et al. Reference Ellis, Medeiros, Collins, Sari, Coffey, Dickerson, Lugarini, Stratford, Henry, Merrill, Matthews, Hanson, Roberts, Joyce, Kunkel and Ricklefs2017). Dense host populations increase vector–host encounter rates that result in contagion enhancement (Ellis et al. Reference Ellis, Medeiros, Collins, Sari, Coffey, Dickerson, Lugarini, Stratford, Henry, Merrill, Matthews, Hanson, Roberts, Joyce, Kunkel and Ricklefs2017). However, an over or under-utilization of hosts by vectors and a lack of parasitic compatibility with some avian species may also limit parasite transmission in specific regions despite a high avian density (Ellis et al. Reference Ellis, Medeiros, Collins, Sari, Coffey, Dickerson, Lugarini, Stratford, Henry, Merrill, Matthews, Hanson, Roberts, Joyce, Kunkel and Ricklefs2017).
The amount of precipitation was the only environmental factor that positively affected the probability to be infected by haemosporidian parasites in nestlings. Rainfall provides the humid environment and temporary pools that are essential for the survival and reproduction of vectors, and subsequently, for haemosporidian transmission (Patz et al. Reference Patz, Graczyk, Geller and Vittor2000; Mercer et al. Reference Mercer, Sheeley and Brown2005; Galardo et al. Reference Galardo, Zimmerman, Lounibos, Young, Galardo, Arruda and D'Almeida Couto2009; Hernández-Lara et al. Reference Hernández-Lara, González-García and Santiago-Alarcon2017). It should be noted that nest-box identity explained 32% of the total variance in this analysis, suggesting a high amount of variation among nest boxes, which may reflect fine-scale environmental variation that can impinge on the haemosporidian prevalence. The fact that different environmental variables affected juveniles and adults should be interpreted with caution given our limited sampling of nestlings. Further analysis of nestling samples covering the whole study area across multiple years is needed for a better understanding of the mechanisms responsible for haemosporidian parasite transmission at this stage.
Conclusion
Overall, a low prevalence and a moderate lineage diversity of haemosporidian parasites were observed in our Tree swallow population. We also found that climatic and landscape characteristics are important determinants of haemosporidian parasite prevalence and that the effect of these variables differs according to the parasite genus. Interestingly, we showed that some landscapes associated with human activities, such as agriculture and urbanization, can influence the spatial distribution of haemosporidian prevalence. Host–parasite relationships are very complex and our results generate many questions regarding the factors determining the status of haemosporidian infections. A large part of the variation in infection status remained unexplained in our models, suggesting that other variables such as intrinsic factors (e.g. age and sex) are at play in determining haemosporidian prevalence in our study system. Further studies are thus needed before we can better understand the role of vector-borne parasites on the decline of avian populations in temperate environments.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017002128
Acknowledgements
We want to thank the graduate students and field and laboratory assistants who have contributed to the collection of data since the beginning of the Tree swallow Project. We also thank three anonymous reviewers for their comments on a previous version of this manuscript. Special thanks to Hélène Presseault-Gauvin, Nicolas Bousquet and Chloé Martineau who have participated in the laboratory analysis specific to this project. This research would not have been possible without the participation of the 40 farmers who generously provide access to their land each year.
Financial support
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant to DG (grant number: 327312), by a Fonds de Recherche du Québec—Nature et Technologies (FRQNT) team research project grant to DG, FP and MB (grant number: 167001), and by the Canada Research Chairs program to FP. AT was supported by funding from Université de Sherbrooke.










