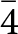Introduction
Tetrahedrite-group minerals are the most common sulfosalts in different kinds of hydrothermal ore deposits. They form a complex isotypic series, with the general structural formula M (2)A6M (1)(B4C2)X (3)D4S(1)Y12S(2)Z, where A = Cu+, Ag+, □ (vacancy); B = Cu+ and Ag+; C = Zn2+, Fe2+, Hg2+, Cd2+, Ni2+, Mn2+, Cu2+, Cu+, In3+ and Fe3+; D = Sb3+, As3+, Bi3+ and Te4+; Y = S2– and Se2–; and Z = S2–, Se2– and □. Thus, tetrahedrite-group minerals are characterised by several homo- and heterovalent substitutions. The classification of tetrahedrite-group minerals is based on the different combinations of chemical constituents, identifying distinct series according to different A, B, D and Y+Z constituents. At the species level, the C chemical constituents are then considered (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020).
Among the currently known species, those having Sb (Z = 51) and As (Z = 33) as dominant D chemical constituents are the most widespread, whereas species with predominance of Te (Z = 52) are rare and those with Bi (Z = 83) are exceptional. The name annivite was formerly used to indicate the Bi-dominant analogue of tetrahedrite-group minerals. However, chemical analyses of type annivite from the Anniviers Valley (Switzerland – Fellenberg, Reference Fellenberg1854) corresponded to a Bi-rich variety of tennantite-(Fe). For this reason, annivite was considered as a questionable mineral (Moëlo et al., Reference Moëlo, Makovicky, Mozgova, Jambor, Cook, Pring., Paar, Nickel, Graeser, Karup-Møller, Balić-Žunić, Mumme, Vurro, Topa, Bindi, Bente and Shimizu2008) and it was later discredited by Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020). However, some compositions corresponding to annivite are known (e.g. Lur´ye et al., Reference Lur´ye, Tsepin and Vyal’sov1974, Bortnikov et al., Reference Bortnikov, Kudryavtsev and Troneva1979; Kieft and Eriksson, Reference Kieft and Eriksson1984; Spiridonov et al., Reference Spiridonov, Chvileva, Borodaev, Vinogradova and Kononov1986; Gołębiowska et al., Reference Gołębiowska, Pieczka and Parafiniuk2012; Velebil and Sejkora, Reference Velebil and Sejkora2018). According to Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020), annivite and its name could be re-validated if samples having isotypic relations with tetrahedrite-group minerals and showing Bi > As and Bi > Sb are found. This is the case described in this paper. In agreement with the current nomenclature of tetrahedrite-group minerals (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020), the hyphenated suffix ‘-(Zn)’ is added to the root-name ‘annivite’ in order to stress the dominance of Zn as C chemical constituent.
The new mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, under the voting number IMA 2023–124 (Sejkora et al., Reference Sejkora, Biagioni, Dolníček, Velebil and Škácha2024). Its mineral symbol, in accord with Warr (Reference Warr2021), is Anv-Zn. Holotype material (polished section) of annivite-(Zn) is deposited in the collections of the Department of Mineralogy and Petrology, National Museum in Prague, Cirkusová 1740, 193 00 Praha 9, Czech Republic, under the catalogue number P1P 50/2023 whereas the grain used for single-crystal X-ray diffraction study (extracted from the above mentioned polished section) is kept in the collections of the Museo di Storia Naturale of the Università di Pisa, Via Roma 79, Calci (PI), under catalogue number 20069. This polished section was taken from the sample deposited in the collections of the Department of Mineralogy and Petrology, National Museum in Prague, under catalogue number P1N 39199.
Experimental
Occurrence and physical properties
Annivite-(Zn) was found on historical samples originally labelled ‘walpurgite’ or ‘tennantite’ originating from Jáchymov (St. Joachimsthal), Krušné hory Mountains, Czech Republic, which were stored in a mineralogical collection of the National Museum, Prague.
The Jáchymov ore district is a classic example of five element (Ag+As+Co+Ni+Bi) and U vein-type hydrothermal mineralisation (i.e Kissin Reference Kissin1992; Markl et al., Reference Markl, Burisch and Neumann2016). The ore veins cut a complex of medium-grade metasedimentary rocks of Cambrian to Ordovician age, in the contact aureole of a Variscan granite pluton. The majority of the ore minerals was deposited during the Variscan mineralising epoch from mesothermal to epithermal fluids (Ondruš et al., Reference Ondruš, Veselovský, Gabašová, Drábek, Dobeš, Malý, Hloušek and Sejkora2003a, Reference Ondruš, Veselovský, Gabašová, Hloušek, Šrein, Vavřín, Skála, Sejkora and Drábek2003b, Reference Ondruš, Veselovský, Gabašová, Hloušek, Šrein, Vavřín, Skála, Sejkora and Drábek2003d). Primary and supergene mineralisation in this district resulted in extraordinarily varied associations; more than 440 mineral species have been reported from there (Ondruš et al., Reference Ondruš, Veselovský, Hloušek, Skála, Frýda, Čejka and Gabašová1997a, Reference Ondruš, Veselovský, Skála, Císařová, Hloušek, Frýda, Vavřín, Čejka and Gabašová1997b and Reference Ondruš, Veselovský, Gabašová, Hloušek and Šrein2003c, Reference Ondruš, Veselovský, Gabašová, Hloušek, Šrein, Vavřín, Skála, Sejkora and Drábek2003d; Hloušek et al., Reference Hloušek, Plášil, Sejkora and Škácha2014; Škácha et al., Reference Škácha, Plášil and Horák2019).
The type sample (P1N 39199) of annivite-(Zn) comes from the vein Geister, the 7th Geister level of the Rovnost I (Werner) shaft (GPS coordinates: 50°22ʹ18.33ʹʹN, 12°53ʹ32.79ʹʹE), western part of the Jáchymov ore district. The sample originally labelled ‘walpurgite’ (6 × 4 cm) is formed of a massive aggregate of ore composed of tetrahedrite-group minerals, emplectite and bismuth, and is partly supergene altered (bismite and walpurgite). The predominant mineral of the tetrahedrite group is Bi-rich tennantite-(Zn) accompanied by annivite-(Zn) and more rarely also Bi-rich tennantite-(Fe).
Annivite-(Zn) was also identified in other samples from the Geister vein with localisations 3rd Geister level (120 m under surface), 7th Geister level (232 m) and below Barbora level (262 m). All these samples probably come from one ore body of the Geister vein exposed in vertical extent at about 150 m; for a description of samples see Table S1 (Supplementary Material). The mineral association includes, in addition to the annivite-(Zn) proposed, Bi-rich tennantite-(Zn), tennantite-(Fe), tetrahedrite-(Zn), the not-yet approved ‘annivite-(Fe)’, bismuth, emplectite, wittichenite and supergene bismite, walpurgite, arsenates of the vivianite group and metazeunerite. The occurrence of potential annivite-(Zn) at the Geister vein on the basis of electron microprobe data was previously mentioned by Velebil and Sejkora (Reference Velebil and Sejkora2018). The tetrahedrite-group minerals form strongly chemically zoned aggregates and annivite-(Zn) only occurs as tiny growth zones and isometric domains (Fig. 1).

Figure 1. Back-scattered electron images of the chemically zoned grains of Bi-rich tennantite-(Zn) from the Geister vein, Jáchymov, Czech Republic, with zones and domains of annivite-(Zn) marked by red points; white is bismite and other supergene Bi and U minerals. Field of view of all images is 265 μm (sample P1N38896, National Museum Prague, image reproduced from Velebil and Sejkora, Reference Velebil and Sejkora2018).
In holotype material, annivite-(Zn) occurs as anhedral grains, up to 50 μm in size, and growth zones (up to 100 μm in thickness) in zoned annivite-(Zn)/tennantite-(Zn) grains (Fig. 2). It is dark grey in colour, with a grey streak, and metallic lustre. Mohs hardness was not measured, but it should be close to 4, in agreement with other members of the tetrahedrite group. Annivite-(Zn) is brittle, with a conchoidal fracture and indistinct cleavage. Owing to the small amount of available material, density was not measured; based on the empirical formula and unit-cell parameters derived from single-crystal X-ray diffraction, the calculated density is 5.259 g/cm3. In reflected light, annivite-(Zn) is isotropic, pale grey, with brownish shade. Internal reflections are very rare in pale brown tints. Reflectance values (Table 1) were measured in air using a spectrophotometer MSP400 Tidas at Leica microscope, with a 50× objective and WTiC Zeiss 370 standard. These values are plotted in Fig. 3, whereas Fig. 4 compares them with published data for tennantite-(Zn) and tetrahedrite-(Zn).

Figure 2. Reflected light (a) and back-scattered electron (b) images of the chemically zoned crystal of tetrahedrite-group minerals (TGM). The white domain marked by red points within the grain of TGM corresponds to annivite-(Zn). The grain used from single-crystal X-ray diffraction study was extracted from the red box. Holotype sample (polished section) P1P 50/2023; this polished section was taken from hand-size sample P1N39199, National Museum Prague.

Figure 3. Reflectance curve for annivite-(Zn) from Jáchymov, Czech Republic.

Figure 4. Reflectance curve for annivite-(Zn) from Jáchymov, Czech Republic (this paper) compared with published data for tennantite-(Zn) with 0.56 apfu Bi from Bicknoller Quarry, Somerset, UK (Criddle and Stanley, Reference Criddle and Stanley1993); tennantite-(Zn) from Tsumeb (Criddle and Stanley, Reference Criddle and Stanley1993) and tetrahedrite-(Zn) from Fresney d´Oisans, Isère, France (Criddle and Stanley, Reference Criddle and Stanley1993).
Table 1. Reflectance data for annivite-(Zn) from Jáchymov, Czech Republic

Note: the four COM values are shown in bold.
Recently, annivite-(Zn) was also identified during the investigation of samples from dump material of the abandoned Mauritius mine. This tin mine is situated about 1 km N of the Hřebečná village, 16 km N of Karlovy Vary, Krušné hory Mountains, Czech Republic (GPS coordinates: 50°23ʹ13.49ʹʹN, 12°49ʹ51.72ʹʹE). The mineralisation studied, in coarse-grained quartz gangue with abundant fluorapatite, differs significantly from the usual fine-grained greisens mined in this area. The history, geology and mineralogy of the Hřebečná deposit can be found elsewhere (Jangl et al., Reference Jangl, Hašková and Lisková1989; Urban, Reference Urban2014; Sejkora et al., Reference Sejkora, Pauliš, Urban, Dolníček, Ulmanová and Pour2021b). Annivite-(Zn) was determined in one sample of quartz gangue as anhedral domains 5–20 μm in size enclosed in grains of Bi-rich tennantite-(Zn) in close association with arsenopyrite and pyrite (Fig. 5). Other associated primary minerals are chalcopyrite, sphalerite, Cu sulfides (anilite, digenite, geerite, spionkopite and covellite), aikinite, bismuthinite, berryite, cuprobismutite, emplectite and wittichenite (Sejkora et al., Reference Sejkora, Pauliš, Urban, Dolníček, Ulmanová and Pour2021b).

Figure 5. Back-scattered electron image of annivite-(Zn) domains (white) hosted in a zonal aggregate of Bi-rich tennantite-(Zn) in association with arsenopyrite (grey) and pyrite (black); Hřebečná, Czech Republic.
Chemical data
Quantitative chemical analyses on type material from Jáchymov and from the new finding at Hřebečná were carried out using a Cameca SX 100 electron microprobe (National Museum, Prague, Czech Republic) and the following experimental conditions: WDS mode, accelerating voltage 25 kV, beam current 20 nA and beam diameter 0.7 µm. Standards (element, emission line) were: Ag (AgLα), Bi2Se3 (BiMβ), chalcopyrite (CuKα and SKα), NiAs (AsLβ), PbS (PbMα), pyrite (FeKα), Sb2S3 (SbLα) and ZnS (ZnKα). The contents of other sought elements (Au, Cd, Co, Ga, Ge, Hg, In, Mn, Cl, Ni, Se, Sn, Te and Tl) were below detection limits. Matrix correction using the PAP procedure (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the data. Analytical data for annivite-(Zn) from Jáchymov (average of 5 spot analyses) and Hřebečná (average of 8 spot analyses) are given in Table 2. The full dataset of EPMA data is deposited as Tables S2 and S3 (Supplementary Material).
Table 2. Chemical data (in wt.%) for annivite-(Zn) from Jáchymov (grain used for single crystal X-ray diffraction study) and Hřebečná, in the Czech Republic

(σ) – estimated standard deviation; n = number of spot analyses.
X-ray crystallography
Powder X-ray diffraction data could not be collected, owing to the small amount of the available material. Consequently, these data, given in Table 3, were calculated through the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) using the structural model of the sample from Jáchymov discussed below.
Table 3. Calculated X-ray powder diffraction data for annivite-(Zn)*

* Relative intensity and d hkl (Å) were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model given in Tables 4 and 5. Only reflections with I calc > 2 are listed. The five strongest reflections are given in bold.
Single-crystal X-ray diffraction intensity data were collected using a Bruker D8 Venture four-circle diffractometer equipped with an air-cooled Photon III detector, and microfocus MoKα radiation (Centro per l’Integrazione della Strumentazione scientifica dell’Università di Pisa, Università di Pisa, Italy). The detector-to-crystal distance was set to 38 mm. Data were collected using φ scan modes, in 0.5° slices, with an exposure time of 10 s per frame. A total of 560 frames were collected and they were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data were corrected for Lorentz-polarisation, absorption and background. Unit-cell parameters were refined on the basis of the XYZ centroids of 1652 reflections above 20 σI with 5.564° < 2θ < 62.73° as a = 10.3545(6) Å, V = 1110.16(19) Å3 and space group I ![]() $\bar{4}$3m.
$\bar{4}$3m.
The crystal structure of annivite-(Zn) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the atomic coordinates of Johnson and Burnham (Reference Johnson and Burnham1985). The occurrence of a racemic twin was modelled. The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used: Cu vs. □ (vacancy) at the M(2) and M(1) sites; Bi vs. As at X(3); and S at the S(1) and S(2) sites. The site occupancies at the M(2) and M(1) positions did not deviate from full occupancy by Cu and were fixed to one; taking into account the similarity of site scattering between Cu (Z = 29) and Zn (Z = 30), the latter replacing Cu at the M(1) site, their ratio was fixed in accord with electron microprobe data. After several cycles of isotropic refinement, the R 1 converged to 0.0868, confirming the correctness of the structural model. The anisotropic model for both cations and anions converged to R 1 = 0.0555. Residuals were located around the M(2) and X(3) sites, suggesting their possible splitting. Allowing the splitting of these positions, the structural model converged to R 1 = 0.0493 for 278 reflections with F o > 4σ(F o) and 23 refined parameters. Details of data collection and refinement are given in Table 4. Fractional atom coordinates and equivalent isotropic displacement parameters are reported in Table 5. Table 6 reports selected bond distances, whereas weighted bond-valence sums, calculated according to Brese and O’Keeffe (Reference Brese and O’Keeffe1991), are shown in Table 7. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Crystal and experimental data for annivite-(Zn) from Jáchymov, Czech republic

1 w = 1/[σ2(F o2)+(0.0521P)2+67.1698P].
2 Flack (Reference Flack1983).
Table 5. Site, site occupancy (s.o.), fractional atom coordinates and equivalent isotropic displacement parameters (Å2) for annivite-(Zn)

Table 6. Selected bond distances (in Å) for annivite-(Zn)

Table 7. Weighted bond-valence sums (in valence units) in annivite-(Zn)*

* Note: bond-valence sums were weighted according to the site occupancies discussed in the text and using the bond parameters of Brese and O’Keeffe (Reference Brese and O’Keeffe1991). Left and right superscripts indicates bonding involving cations and anions, respectively.
Results and discussion
Chemical formula
As discussed in previous papers (e.g. Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021a), there are different approaches to recalculate the chemical formulae of tetrahedrite-group minerals. The two better ones normalise the number of atoms on the basis of ΣMe = 16 atoms per formula unit (apfu) or on the basis of (As + Sb + Te + Bi) = 4 apfu. The former approach assumes that no vacancies occur at the M(2), M(1), and X(3) sites, whereas the latter is based mainly on the results discussed by Johnson et al. (Reference Johnson, Craig and Rimstidt1986) who revealed that negligible variations in the ideal number of atoms hosted at the X(3) position usually occur.
The first approach gives the chemical formulae Cu10.13Ag0.02Zn1.93Fe0.03Pb0.02(Bi1.79As1.43Sb0.66)Σ3.88S13.10 (holotype material from Jáchymov) and Cu9.87Ag0.02Zn1.85Fe0.34(Bi1.91As1.72Sb0.30)Σ3.93S13.34 (Hřebečná) whereas the other normalisation strategy corresponds to the formulae Cu10.44Ag0.02Zn1.99Fe0.03Pb0.02(Bi1.84As1.48Sb0.68)Σ4.00S13.50 (holotype Jáchymov) and Cu10.05Ag0.02Zn1.89Fe0.34(Bi1.95As1.75Sb0.30)Σ4.00S13.60 (Hřebečná). The simplified formula of annivite-(Zn) is (Cu,Ag)6(Cu,Zn,Fe,Pb)6(Bi,As,Sb)4S13 corresponding to the end-member formula Cu6(Cu4Zn2)Σ6Bi4S13. This corresponds to (in wt.%) Cu 31.48, Zn 6.48, Bi 41.40, S 20.64, total 100.00.
Chemical variability of Bi-rich members of the tetrahedrite group from Jáchymov and Hřebečná
The composition for all studied samples of Bi-rich members of the tetrahedrite group from the Geister vein, Jáchymov are shown in Figs 6 and 7 (based on ΣMe = 16 apfu, Table S2). Tennantite-(Zn) is the most common (345 spot analyses) and shows a wide range of Bi contents (0.43–1.86 apfu) accompanied by dominant As (1.53–3.07 apfu) and subordinate Sb (up to 0.95 apfu). The C chemical constituent is dominated by Zn ranging between 0.93–2.02 apfu. There are 171 spot analyses that correspond to annivite-(Zn) with dominant Bi (1.54–2.37 apfu) accompanied by As (0.87–1.83 apfu) and Sb (0.09–1.10 apfu); the Zn contents range between 0.98 and 2.00 apfu. We also observed thin (5–10 μm) zones (16 spot analyses) with chemical composition corresponding to the not-yet approved ‘annivite-(Fe)’ with dominant Bi contents in the range 1.58–1.98 apfu and Fe (0.93–1.16 apfu) prevailing over Zn (0.68–0.94 apfu). Relatively abundant (67 spot analyses) tennantite-(Fe) shows, in addition to the dominant As (1.61–2.67 apfu), also elevated Bi (0.50–1.74 apfu) and Sb (0.02–1.06 apfu) contents; Fe content ranges between 0.94 and 1.42 apfu. The Bi-rich (0.80–1.20 apfu) tetrahedrite-(Zn) was found only rarely in the samples studied (9 spot analyses). For all the studied members of the tetrahedrite group, the (Cu + Ag) content is very close to the ideal one, i.e. 10.00 apfu, ranging between 9.81 and 10.31 apfu, whereas Ag contents are negligible (up to 0.16 apfu). The C chemical constituent is represented by dominant Zn or Fe; however, Zn is clearly more abundant than Fe, and compositions close to Fe end-members were not detected (Fig. 7a,b). Minor Pb (locally up to 0.54 apfu) is here also considered as a C constituent, even if its actual structural role in tetrahedrite-group minerals is currently unknown (e.g. Biagioni et al., Reference Biagioni, Voudouris, Moëlo, Sejkora, Dolníček, Musetti and Mauro2024). The contents of Bi distinctly correlate negatively with As contents (Fig. 7c), on the contrary, no correlations were found between Bi-Sb, As-Sb, Fe-Bi, Fe-As, Pb-Bi, Pb-Sb and Pb-Fe.

Figure 6. Ternary Bi–Sb–As diagram (at.%) for tetrahedrite-group minerals from the Geister vein, Jáchymov, Czech Republic.

Figure 7. Compositional variations Zn vs. Fe (in apfu) of Bi-bearing tetrahedrite-group minerals from the Geister vein, Jáchymov, Czech Republic. (a) Zn vs. Fe (in apfu) for Bi-dominant members; (b) Zn vs. Fe (in apfu) for As- and Sb-dominant members; (c) Bi vs. As (in apfu).
Annivite-(Zn) from Hřebečná is much rarer (Fig. 8, Table S3) and displays Bi contents between 1.85–2.20 apfu (8 spot analyses); the most abundant tennantite-(Zn) with 0.05–1.73 apfu Bi (143 spot analyses) is accompanied by rare tennantite-(Fe) with 0.05–1.32 Bi (10 spot analyses). All members of the tetrahedrite group from Hřebečná are poorer in Sb than the Jáchymov ones (Fig. 8), as the observed contents reached up 0.36 Sb apfu only. The contents of Ag are negligible (up to 0.07 apfu); the dominant C-cation is mainly Zn, and Fe is present only in sporadic cases, accompanied in a part of the analyses by some formally divalent Cu (up to 0.77 apfu).

Figure 8. Ternary Bi–Sb–As diagram (at.%) for tetrahedrite-group minerals from the Hřebečná deposit, Czech Republic.
Crystal structure description
The crystal structure of annivite-(Zn) agrees with the general features of the members of the tetrahedrite isotypic group. The electron density associated with the M(2) site is split into two sub-positions, i.e. M(2a) and M(2b), with M(2a)–M(2b) = 0.53(4) Å. The former has a triangular coordination, whereas the latter has a flat trigonal pyramidal coordination. Average <M(2a)–S> and <M(2b)–S> distances are 2.256 and 2.31 Å. The refinement of the site occupancy points to a pure Cu site, in agreement with electron microprobe data indicating a negligible amount of Ag. The bond-valence sum, i.e. 1.04 valence units (vu), is in accord with such an occupancy by formally monovalent Cu.
The tetrahedrally coordinated M(1) site has an average <M(1)–S> bond distance of 2.339 Å, similar to those reported by Wuensch (Reference Wuensch1964) and Wuensch et al. (Reference Wuensch, Takéuchi and Nowacki1966) for tetrahedrite-(Fe/Zn) and tennantite-(Zn), i.e. 2.342 and 2.337 Å, respectively. Electron microprobe analysis indicates that it has an ideal (Cu0.67Zn0.33) site occupancy. On the basis of this proposed site occupancy, the bond-valence sum at the M(1) site is 1.40 vu, to be compared with a theoretical value of 1.33 vu.
The X(3) site was found to be split into two sub-positions, separated by a distance of 0.29(3) Å. The X(3a) sub-site has a <X(3a)–S> distance of 2.477 Å, whereas the X(3b) has an average bond distance of 2.330 Å. Refined site scattering at the X(3a) + X(3b) site corresponds to 55.00 electrons per site. The site occupancy, based on electron microprobe data, would be (Bi0.448As0.358Sb0.165□0.029), corresponding to a mean atomic number of 57.41 electrons. Observed bond distances suggest that X(3a) is a mixed (Bi,As) position whereas X(3b) is a mixed (As,Sb) position. On the basis of the observed average bond distances, one can calculate the ratios for Bi/As and As/Sb. The ideal As–S and Sb–S distances can be calculated on the basis of bond parameters of Brese and O’Keeffe (Reference Brese and O’Keeffe1991) and correspond to 2.26 and 2.45 Å, respectively; these values are close to those observed for As–S (2.246 Å) and Sb–S (2.446 Å) bonds by Wuensch et al. (Reference Wuensch, Takéuchi and Nowacki1966) and Wuensch (Reference Wuensch1964), respectively. Some comments are necessary for the Bi–S ideal bond in trigonal pyramidal coordination. By using the bond parameters of Brese and O’Keeffe (Reference Brese and O’Keeffe1991), the ideal Bi–S value of 2.55 Å can be calculated. However, in some species showing trigonal–pyramidally coordinated Bi, the Bi–S is longer, e.g. 2.61 Å in emplectite (Kyono and Kimata, Reference Kyono and Kimata2005), and 2.592 Å in wittichenite (Kocman and Nuffield, Reference Kocman and Nuffield1973). Moreover, the results of Klünder et al. (Reference Klünder, Karup-Møller and Makovicky2003) indicated a possible maximum Bi–S bond length of 2.511 Å in tetrahedrite-group minerals. As the observed unit-cell parameters for the sample studied agree with the trend expected for Bi-bearing tetrahedrite-group minerals, as suggested by these latter authors, we used their proposed Bi–S distance of 2.51 Å (only two digits are considered). The Bi/As and As/Sb ratios at X(3a) and X(3b), calculated on the basis of these ideal bond distances, are 0.88/0.12 and 0.63/0.37. Considering these ratios and the refined site scatterings at X(3a) (= 36.52 electrons) and X(3b) (= 18.48 electrons), one achieves the site occupancies (Bi0.42As0.06) and (As0.29Sb0.17), resulting in an occupancy at X(3a) + X(3b) of (Bi0.42As0.35Sb0.17□0.06), in accord with chemical data. On the basis of the proposed site occupancies, the bond-valence sum at X(3a) + X(3b) is 2.97 vu.
The S(1) and S(2) sites are fully occupied by S. Their bond-valence sums are 1.97 and 1.86 vu, respectively.
The structural formula of annivite-(Zn), based on electron microprobe data and crystal structure refinement, assuming that no vacancy occurs at the X(3) site, could be written as M (2)Cu6.00M (1)(Cu4.00Zn2.00)Σ6.00X (3)(Bi1.80As1.48Sb0.72)S(1)+S(2)S13.00 (Z = 2).
Relationship between Bi content and unit-cell parameter
The results of electron microprobe analysis, (Cu6.11Ag0.02)Σ6.13(Cu4.02Zn1.93Fe0.03Pb0.02)Σ6.00(Bi1.79As1.43Sb0.66)Σ3.88S13.10, as well as the structural analysis, suggest the possible simplified formula Cu6(Cu4Zn2)(Bi1.8As1.5Sb0.7)S13. This formula can be used for calculating the expected unit-cell parameter of the sample studied.
Johnson et al. (Reference Johnson, Craig and Rimstidt1987) proposed the following relation between the unit-cell parameter a and the chemistry of the sample studied: a (Å) = 10.379 + 0.082(Ag) – 0.01(Ag2) – 0.009(Cu*) + 0.066(Hg) – 0.038(As) + 0.144(Bi). On the basis of the simplified formula given above, the calculated unit-cell parameter a would be 10.59 Å, very different from the observed value of 10.35 Å. Indeed, Johnson et al. (Reference Johnson, Craig and Rimstidt1987) stated that the data for Bi used in their regression equation were anomalous, limiting the usefulness of the Bi term in their equation. If the data of synthetically prepared Bi-rich members (Klünder et al., Reference Klünder, Karup-Møller and Makovicky2003) are used to derive the new coefficient for Bi in the Johnson et al. (Reference Johnson, Craig and Rimstidt1987) formula, the value 0.0075(Bi) is found; for this coefficient the calculated unit-cell parameter for the composition of type material is a = 10.34 Å, which corresponds very well to the observed value of 10.35 Å.
Another approach is that of Klünder et al. (Reference Klünder, Karup-Møller and Makovicky2003) who proposed an increase in the a unit-cell parameter of 0.036 Å for every Bi atom in tennantite and 0.011 Å in tetrahedrite. As the studied sample is a mixed (As/Sb) term, the composition Cu6(Cu4Zn2)(As3.3Sb0.7)S13, that is with all Bi replaced by As, could be initially used. According to the relation of Johnson et al. (Reference Johnson, Craig and Rimstidt1987), this composition should have the unit-cell parameter a = 10.25 Å; following Charlat and Lévy (Reference Charlat and Lévy1975), the a value should be 10.26 Å. Considering the occurrence of 1.8 Bi apfu, the unit-cell should increase to ∼10.32 Å, to be compared with the observed value of 10.35 Å.
Bismuth in tetrahedrite-group minerals
A survey of the available literature reveals that Bi-dominant members of tetrahedrite-group have been reported since the end of the 1970s. They have been mentioned from five occurrences (Table 8): Vindfall, Sweden with 2.26 apfu Bi (Kieft and Eriksson, Reference Kieft and Eriksson1984); Tary-Ekan, Central Asia with 1.57 apfu Bi (Lur´ye et al., Reference Lur´ye, Tsepin and Vyal’sov1974; Bortnikov et al., Reference Bortnikov, Kudryavtsev and Troneva1979; Spiridonov et al., Reference Spiridonov, Chvileva, Borodaev, Vinogradova and Kononov1986); Rędziny, Poland with 2.65 apfu Bi (Gołębiowska et al., Reference Gołębiowska, Pieczka and Parafiniuk2012); Jáchymov (Velebil and Sejkora, Reference Velebil and Sejkora2018) and Hřebečná, both Czech Republic (Sejkora et al., Reference Sejkora, Pauliš, Urban, Dolníček, Ulmanová and Pour2021b). The material from Jáchymov and Hřebečná was newly investigated and is the subject of this paper. Other published chemical compositions (e.g. Oen and Kieft, Reference Oen and Kieft1976; Sergeyeva and Shatagin, Reference Sergeyeva and Shatagin1980, Vinogradova et al., Reference Vinogradova, Kononov, Borodayev, Bochek and Dvortsova1985; Igumnova, Reference Igumnova1986; Borisova et al., Reference Borisova, Borodaev and Bocharova1986; Förster et al., Reference Förster, Hunger and Grimm1986; Dobosi and Nagy, Reference Dobosi and Nagy1991; Breskova and Tarkian, Reference Breskovska and Tarkian1994 and references therein; Kemkina, Reference Kemkina2007; Voudouris et al., Reference Voudouris, Melfos, Spry, Bonsall, Tarkian and Solomos2008; Staude et al., Reference Staude, Mordhorst, Neumann, Prebeck and Markl2010) correspond, in fact, to Bi-rich tennantite-, tetrahedrite- or goldfieldite-series minerals, respectively.
Table 8. Published data for Bi-dominant members of tetrahedrite group, recalculated on the basis of ΣMe = 16

* Chemical analysis is not given; sample from Rędziny corresponds to the not-yet approved ‘argentoannivite-(Fe)’; other samples are annivite-(Zn)
It is worth noting that among samples of ‘annivite’ given in Table 8, arsenic is usually more abundant than Sb, with As/(As+Sb) atomic ratios between 0.54 and 0.87. The only sample with Sb >As, having As/(As+Sb) = 0.13, has a high Ag content (3.41 Ag apfu). It is possible that the small As atoms and the large Bi atoms may approximate together the size of the Sb atoms, thus favouring the solubility of Bi in As-rich members of tetrahedrite group. On the contrary, the substitution of Sb by Bi could cause an expansion of the crystal structure of tetrahedrite-group minerals, balanced by the introduction of the large Ag atoms replacing Cu. However, synthetic experiments do not fully support this hypothesis.
Indeed, attempts to synthesise a Bi-dominant analogue of tetrahedrite or tennantite have not yet been successful; Klünder et al. (Reference Klünder, Karup-Møller and Makovicky2003) report Bi contents in synthetically prepared tetrahedrites and tennantites up to 0.8 apfu at 350°C and up to 1 apfu at 450 and 520°C, but no meaningful differences between Sb- and As-rich phases were observed. The synthetic Bi-doped members of tetrahedrite group were prepared many times for studies of their promising thermoelectric properties but with maximum Bi content of 0.80 apfu (Kumar et al., Reference Kumar, Chetty, Femi, Chattopadhyay, Malar and Mallik2017; Peccerillo and Durose, Reference Peccerillo and Durose2018).
Conclusion
Annivite-(Zn) is a new member of the tetrahedrite group. Its discovery and comparison with other members of this group confirm the fundamental role of studies devoted to natural mineral assemblages to reveal novel crystal structures so far not obtained in synthesis experiments (e.g. Klünder et al., Reference Klünder, Karup-Møller and Makovicky2003).
Moreover, tetrahedrite-group minerals are currently actively studied for their high-tech properties (e.g. Suekuni et al., Reference Suekuni, Tomizawa, Ozaki and Koyano2014; Chetty et al., Reference Chetty, Bali and Mallik2015; Levinsky et al., Reference Levinsky, Candolfi, Dauscher, Tobola, Hejtmánek and Lenoir2019). Among the chemical compositions showing interesting properties, synthetic Bi-doped members have potential thermoelectric properties which have been the subject of several research works in the last decade (e.g. Goncalves et al., Reference Goncalves, Lopes, Villeroy, Monnier, Godart and Lenoir2016; Kumar et al., Reference Kumar, Chetty, Femi, Chattopadhyay, Malar and Mallik2017; Peccerillo and Durose, Reference Peccerillo and Durose2018; Kwak et al., Reference Kwak, Pi, Lee and Kim2020; Lee and Kim, Reference Lee and Kim2020; Baláž et al., Reference Baláž, Guilmeau, Achimovičová, Baláž, Daneu, Dobrozhan and Kaňuchová2021).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.54.
Acknowledgements
We appreciate the many constructive comments of an anonymous reviewer, Panagiotis Voudouris, Peter Leverett, the Associate Editor and Principal Editor Stuart Mills that helped to improve this paper significantly. The study was supported financially by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2024-2028/1.II.a; National Museum, 00023272) and by the Slovak Research and Development Agency under the contract APVV-22-0041. CB acknowledges the financial support by the Ministero dell’Istruzione, dell’Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32. The Centro per l’Integrazione della Strumentazione scientifica dell’Università di Pisa (C.I.S.U.P.) is thanked for the access to the single-crystal X-ray diffraction laboratory.
Competing interests
The authors declare none.