The prevalence of hyperuricaemia is high in Taiwan, especially in the elderly and aborigines, which cannot be completely explained by obesity and alcohol consumption(Reference Chang, Pan and Yeh1–Reference Huang, Peng and Tsai3). Genetic components and environmental factors may play certain roles in this variation of occurrence(Reference Chang, Pan and Yeh1). Typically, hyperuricaemia, i.e. a serum uric acid (UA) level greater than 70 mg/l, is regarded as a marker of gout(Reference Menè and Punzo4). CVD, hypertension and metabolic disorders are also closely associated with hyperuricaemia(Reference Lee, Lin and Chang2, Reference Huang, Peng and Tsai3, Reference Mazzali, Hughes and Kim5–Reference Erdogan, Gullu and Caliskan7). The question of whether hyperuricaemia can induce chronic renal injury has been argued for many years. Recently, UA has been proven to be a mediator of renal disease progression(Reference Mazzali, Hughes and Kim5, Reference Johnson, Titte and Cade6, Reference Kang and Nakagawa8–Reference Ejaz, Mu and Kang10).
In most mammals, UA, a purine metabolite, is degraded by the hepatic enzyme uricase to allontoin. However, mutations in the uricase gene have occurred during human development with the consequence that man has relatively higher levels of serum UA(Reference Johnson, Titte and Cade6). Several studies have demonstrated that hyperuricaemia is associated with glomerular hypertension(Reference Kang and Nakagawa8), lumen obliteration(Reference Nakagawa, Mazzali and Kang11), tubulointerstitial inflammation and fibrosis(Reference Sánchez-Lozada, Tapia and Santamaría12) and end-stage renal disease(Reference Iseki, Ikemiya and Inoue13). Using rats administered with UA and oxonic acid (OA), an inhibitor of uricase capable of reproducing the enzyme deficiency in the human species, Johnson et al. (Reference Johnson, Titte and Cade6) developed an animal model with marked hyperuricaemia and renal crystal deposition. Subsequently, they used OA alone to develop a mild hyperuricaemic rat model and showed that allopurinol treatment, the only US Food and Drug Administration-approved xanthine oxidase inhibitor for the treatment of hyperuricaemia, may prevent blood pressure elevation and tubulointerstitial injury(Reference Mazzali, Hughes and Kim5, Reference Johnson, Titte and Cade6). To mimic clinical situations of hyperuricaemic patients who have developed renal damage, both OA and UA were used to induce hyperuricaemia in rats in the present study.
It has been indicated that eliminating the source and elevating the excretion of UA are useful approaches to attenuate hyperuricaemia(Reference Kutzing and Firestein14). Therefore, a dietary approach might be helpful in preventing hyperuricaemia-associated renal dysfunction and failure. The conventional management of hyperuricaemia includes an energy-adapted diet with moderate amounts of fat, restriction of alcohol, an ovolactovegetarian diet low in purine and adequate amounts of energy-free liquid(Reference Peixoto, Monego and Jardim15, Reference Johnson and Rideout16). Recently, a weight-reducing, purine-unlimited, energy- and carbohydrate-restricted diet with an increased proportional intake of protein and unsaturated fatty acids (UFA) has been proposed(Reference Fam17). For example, in rats with chronic kidney disease, a soya-based diet significantly reduced serum UA, renal fibrosis and renal cyst growth compared with a casein-based diet(Reference Fair, Ogborn and Weiler18). In addition, the favourable effects of various plant oils on glomerular protection have been seen in spontaneously hypertensive rats(Reference Aguila, Pinheiro and Aquino19).
From current studies, it is difficult to conclude whether or not there is a long-term association between the consumption of casein, soya protein and various plant oils with the changes in renal function(Reference Bernstein, Treyzon and Li20). Based on the above studies, we hypothesised that a soya protein-based diet supplemented with UFA may be beneficial to lower serum UA and to attenuate the adverse effects of hyperuricaemia. Therefore, the present study is designed to compare the effects of casein v. soya protein with two commonly used plant oils with different fatty acid components, i.e. palm (palmitic and oleic acid-rich) and safflower-seed (linoleic acid-rich) oils, on various serological parameters and renal damage induced by hyperuricaemia.
Materials
Animals and experimental design
Male Wistar rats, initially weighing 180–200 g and aged 5–6 weeks, were acclimatised to the animal facility with free access to water and a chow diet in a room maintained at 22°C on a 12 h light–12 h dark cycle for several days before the experiment. After fasting overnight, the animals were divided into seven groups (nine rats per group) and fed with modified American Institute of Nutrition (AIN)-93M high-fat diets with approximately 30 % of the total energy from fat (Table 1). Six groups of animals were fed with diets containing 2 % OA (w/w) and 3 % UA (w/w) for the induction of marked hyperuricaemia with renal injury and intratubular crystal deposition based on the pilot studies of Mazzali et al. (Reference Mazzali, Hughes and Kim5). The study design was as follows:
R group: normal control rats fed with a modified AIN-93M high-fat diet, which contained casein and maize oil (ICN Biomedicals, Inc., Cleveland, OH, USA) as the protein and fat sources, respectively.
HY-AL group: hyperuricaemic rats fed with casein and maize oil.
HY+AL group: hyperuricaemic rats fed with casein and maize oil and administered with allopurinol (150 mg/l) in drinking water.
HY+CP group: hyperuricaemic rats fed with casein and palm oil (Fluka 70 905; Sigma-Aldrich Co., St Louis, MO, USA).
HY+CS group: hyperuricaemic rats fed with casein and safflower-seed oil (ICN Biomedicals, Inc.).
HY+SP group: hyperuricaemic rats fed with soya protein (soya protein isolate, ICN no. 905456; ICN Biomedicals, Inc.) and palm oil.
HY+SS group: hyperuricaemic rats fed with soya protein and safflower-seed oil.
All of the diets provided identical amounts of protein (14 % w/w), fat (14 %), maize starch (46·6 %), fibre (5 %), minerals and vitamins (ICN Biomedicals, Inc.), but not sucrose (15·5 or 10·5 %). All of the rats were pair-fed with the HY-AL rats from week 1 to week 8. The animals were individually housed in stainless-steel cages for 8 weeks during the experimental period.
Table 1 Nutrition composition of experimental diets*

R, normal control rats fed with a modified AIN-93M high-fat diet; HY-AL, hyperuricaemic rats fed with casein and maize oil; HY+AL, hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water; HY+CP, hyperuricaemic rats fed with casein and palm oil; HY+CS, hyperuricaemic rats fed with casein and safflower-seed oil; HY+SP, hyperuricaemic rats fed with soya protein and palm oil; HY+SS, hyperuricaemic rats fed with soya protein and safflower-seed oil; AIN, American Institute of Nutrition.
* All of the ingredients were purchased from ICN Biomedical, Inc. (Cleveland, OH, USA), except for palm oil, which was purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Body weight was recorded twice per week and food intake was recorded every day. Approximately 0·5 ml of fasting blood samples were collected from the tail vein for UA and TAG determinations before the treatment (week 0) and on weeks 2, 4, 6 and 8 (on the day before killing). On week 8, animals were killed after being anaesthetised with ketamine (150 mg/kg) and xylazine (15 mg/kg). Blood samples were collected by cardiac puncture and separated into whole blood, serum and plasma for further analysis. The kidneys, liver, heart, lungs and gastrocnemius muscles of each rat were dissected and weighed. The left kidney was collected and fixed with 10 % neutral buffered formalin and the right kidney was put into liquid N2 immediately and stored at − 80°C.
Analytic measurements
The numbers of circulating leucocytes were determined by a haematology analyser (GEN; Beckman Coulter Inc., Miami, FL, USA). Serum concentrations of albumin, blood urea N (BUN) and creatinine were measured by an automatic analyser (Hitachi 747; Hitachi, Tokyo, Japan). Commercially available kits were used to measure serum concentrations of glucose, TAG, cholesterol and UA (Diagnostic Chemicals Ltd, Oxford, CT, USA). A commercially available ELISA kit was used to measure plasma insulin (Mercoda AB, Uppsala, Sweden).
The right kidney was homogenised by polytrone in a 10-fold volume of PBS. The concentrations of NO (represented by nitrite and nitrate) in the serum and renal homogenates were measured by a colorimetric assay kit (lactate dehydrogenase method; Cayman Chemical, Ann Arbor, MI, USA). Concentrations of cytokines, such as TNF-α and interferon (IFN)-γ in the plasma and renal homogenates, and that of transforming growth factor (TGF)-β and nitrotyrosine in the renal homogenates were measured by commercially available ELISA kits (R&D System, Minneapolis, MN, USA and OXIS International Inc., Foster City, CA, USA). Samples were analysed in one assay in duplicate.
At killing, the renal capsule of the left kidney was removed and then immersion fixed in 10 % neutral buffered formalin and embedded in paraffin. Then 4 μm sections of paraffin-embedded kidney were stained with periodic acid–Schiff (PAS). PAS is mainly used for staining structures containing a high proportion of carbohydrate macromolecules, such as glycoproteins. Morphological analyses, including tubulointerstitial nephritis, intralesional calcification, urate crystal and fibrosis, were performed under a light microscope. The extent of injury was graded on a scale of 0 to 4 by an observer blinded to the animal treatment group according to the study of Shih et al. (Reference Shih, Hines and Neilson21). With this method, the extent of injury was graded as 0 for normal, 1 for trace damage, 2 for weak damage, 3 for moderate damage, and 4 for strong damage, which involved 0 %, 1 to 25 %, 25 to 50 %, 50 to 75 %, and 75 to 100 % of the area in the renal sections, respectively.
Statistical analysis
Values were expressed as mean values with their standard errors. Comparisons of each parameter among all of the groups and hyperuricaemic groups were determined by one-way ANOVA using the SAS general linear models program. A repeated-measures analysis was used to determine the main effects of group and time on body weight, serum TAG and serum UA during the experimental period. In addition, two-way ANOVA analysis was used to determine the effects of protein and fat sources for each parameter among the HY+CP, HY+CS, HY+SP and HY+SS groups. Group means were considered to be significantly different at P < 0·05, as determined by the protective least-significant difference technique when the ANOVA indicated an overall significant group effect (P < 0·05). The Kruskal–Wallis test was used for morphological analyses of renal sections, followed by least-significant difference tests to determine whether intergroup differences were significant.
The animal facilities and protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) in Changhua Christian Hospital, Changhua, Taiwan, with approval no. CCH-AE-95 005. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Changhua Christian Hospital.
Results
Whole body growth and food intake
Body weights are shown in Fig. 1(A). Hyperuricaemic rats, i.e. the HY-AL, HY+AL, HY+CP, HY+CS, HY+SP and HY+SS groups, had significantly decreased body weight from week 2 to week 8 compared with normal rats (R group; repeated-measures analysis; P < 0·001). The HY+SP group showed a further decrease in body weight. Two-way ANOVA indicated that hyperuricaemic rats that had ingested palm oil had significantly decreased body weights compared with those that had ingested safflower-seed oil (P < 0·001). Body-weight gain in 8 weeks was also significantly lower in hyperuricaemic rats compared with normal rats (Table 2). Hyperuricaemic rats that had ingested palm oil (HY+CP and HY+SP) had significantly (P < 0·001) decreased body-weight gain compared with those that had ingested safflower-seed oil (HY+CS and HY+SS).

Fig. 1 Body weight (A) and the amount of food intake (B) during 8 weeks. (–▲–), Normal control rats fed with a modified American Institute of Nutrition (AIN)-93M high-fat diet (R); (–△–), hyperuricaemic rats fed with casein and maize oil (HY-AL); (–♦–), hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water (HY+AL); (–●–), hyperuricaemic rats fed with casein and palm oil (HY+CP); (- -○- -), hyperuricaemic rats fed with casein and safflower-seed oil (HY+CS); (–■–), hyperuricaemic rats fed with soya protein and palm oil (HY+SP); (- -□- -), hyperuricaemic rats fed with soya protein and safflower-seed oil (HY+SS). Values are means for nine rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the R group (P < 0·05; one-way ANOVA and least significant differences). † Mean value was significantly different from that of the HY-AL group (P < 0·05; one-way ANOVA and least significant differences). a,b Mean values (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike letters were significantly different (P < 0·05).
Table 2 Relative organ weights in normal and hyperuricaemic rats
(Mean values with their standard errors for nine rats per group)

BW, body weight; R, normal control rats fed with a modified AIN-93M high-fat diet; HY-AL, hyperuricaemic rats fed with casein and maize oil; HY+AL, hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water; HY+CP, hyperuricaemic rats fed with casein and palm oil; HY+CS, hyperuricaemic rats fed with casein and safflower-seed oil; HY+SP, hyperuricaemic rats fed with soya protein and palm oil; HY+SS, hyperuricaemic rats fed with soya protein and safflower-seed oil; AIN, American Institute of Nutrition.
a,b,c Mean values within a column (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike superscript letters were significantly different (P < 0·05).
* Mean value was significantly different from that of the R group (P < 0·05; one-way ANOVA and least significant differences).
† Mean value was significantly different from that of the HY-AL group (P < 0·05; one-way ANOVA and least significant differences).
‡ Values of two-way ANOVA are P values for main effects, such as protein, oil and interaction between protein and oil in the HY+CP, HY+CS, HY+SP and HY+SS groups.
From week 2 to week 8, all of the rats were pair-fed with the HY-AL group; however, several rats in the HY+AL, HY+CP and HY+SP groups did not finish the provided amounts of diets. When calculating the amount of food intake, we did not find a significant difference in weekly food intake among groups (Fig. 1(B)), except for the HY+AL group which had significantly decreased food intake on week 2 to week 4 (one-way ANOVA; P < 0·001). When calculated as grams of body weight gain per kJ of food intake in 8 weeks, feed efficiency was significantly lower in hyperuricaemic rats compared with normal rats (Table 2). The HY+CS group had significantly increased and the HY+SP group had significantly decreased feed efficiency compared with the HY-AL group (one-way ANOVA); and casein was the main factor to increase, and palm oil the main factor to decrease, feed efficiency (two-way ANOVA; P < 0·001).
Relative weights of organs and tissues
The relative weights (g/kg body weight) of the lungs and kidneys (Table 2) were significantly increased in hyperuricaemic rats. Palm oil was the main factor to increase the relative weights of the hearts and lungs (P < 0·01), and soya protein was the main factor to decrease liver weight and to increase gastrocnemius muscle weight in hyperuricaemic rats.
Circulating leucocytes and serum biochemistry
The results presented in Table 3 show that hyperuricaemic rats had significantly decreased albumin and increased leucocytes and BUN compared with normal rats. Soya protein significantly increased while palm oil decreased serum albumin in hyperuricaemic rats. In addition, palm oil increased serum cholesterol and BUN. Serum concentrations of TAG and UA are shown in Fig. 2. On weeks 6 and 8, the HY+CP and HY+SP groups had significantly increased and the HY+CS and HY+SS groups had significantly decreased serum TAG compared with the R and HY-AL groups (one-way ANOVA and least-significant difference; P < 0·05). Serum UA concentration was significantly increased in the HY-AL group compared with the R group from week 2 to week 8. The HY+AL and HY+SS groups had significantly lower serum UA than the HY-AL group. The results of two-way ANOVA indicated that safflower-seed oil was the main factor to decrease serum TAG and UA (P < 0·001).
Table 3 Leucocytes and serum biochemistry in normal and hyperuricaemic rats
(Mean values with their standard errors for nine rats per group)

BUN, blood urea N; R, normal control rats fed with a modified AIN-93M high-fat diet; HY-AL, hyperuricaemic rats fed with casein and maize oil; HY+AL, hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water; HY+CP, hyperuricaemic rats fed with casein and palm oil; HY+CS, hyperuricaemic rats fed with casein and safflower-seed oil; HY+SP, hyperuricaemic rats fed with soya protein and palm oil; HY+SS, hyperuricaemic rats fed with soya protein and safflower-seed oil; AIN, American Institute of Nutrition.
a,b,c Mean values within a column (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike superscript letters were significantly different (P < 0·05).
* Mean value was significantly different from that of the R group (P < 0·05; one-way ANOVA and least significant differences).
† Mean value was significantly different from that of the HY-AL group (P < 0·05; one-way ANOVA and least significant differences).
‡ Values of two-way ANOVA are P values for main effects, such as protein, oil and interaction between protein and oil in the HY+CP, HY+CS, HY+SP and HY+SS groups.

Fig. 2 Serum concentrations of TAG (A) and uric acid (B) during 8 weeks. (–▲–), Normal control rats fed with a modified American Institute of Nutrition (AIN)-93M high-fat diet (R); (–△–), hyperuricaemic rats fed with casein and maize oil (HY-AL); (–♦–), hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water (HY+AL); (–●–), hyperuricaemic rats fed with casein and palm oil (HY+CP); (- -○- -), hyperuricaemic rats fed with casein and safflower-seed oil (HY+CS); (–■–), hyperuricaemic rats fed with soya protein and palm oil (HY+SP); (- -□- -), hyperuricaemic rats fed with soya protein and safflower-seed oil (HY+SS). Values are means for nine rats per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of the R group (P < 0·05; one-way ANOVA and least significant differences). † Mean value was significantly different from that of the HY-AL group (P < 0·05; one-way ANOVA and least significant differences). a,b Mean values (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike letters were significantly different (P < 0·05).
Plasma and renal nitric oxide and cytokines
Plasma concentrations of insulin, NO and cytokines are shown in Table 4. Plasma insulin concentration was significantly lower in the hyperuricaemic rats than in the normal rats and was significantly greater in the HY+CP group than in the HY+SP and HY+SS groups. Plasma NO was not significantly different among groups; however, plasma TNF-α and IFN-γ were significantly greater in the HY+CP group than in the HY+SP and HY+SS groups. Two-way ANOVA indicated that casein was the main factor to increase plasma insulin and IFN-γ.
Table 4 Plasma insulin, nitric oxide and cytokines in normal and hyperuricaemic rats
(Mean values with their standard errors for nine rats per group)
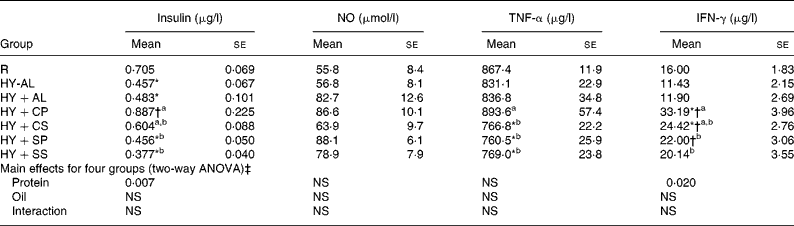
IFN, interferon; R, normal control rats fed with a modified AIN-93M high-fat diet; HY-AL, hyperuricaemic rats fed with casein and maize oil; HY+AL, hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water; HY+CP, hyperuricaemic rats fed with casein and palm oil; HY+CS, hyperuricaemic rats fed with casein and safflower-seed oil; HY+SP, hyperuricaemic rats fed with soya protein and palm oil; HY+SS, hyperuricaemic rats fed with soya protein and safflower-seed oil; AIN, American Institute of Nutrition.
a,b Mean values within a column (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike superscript letters were significantly different (P < 0·05).
* Mean value was significantly different from that of the R group (P < 0·05; one-way ANOVA and least significant differences).
† Mean value was significantly different from that of the HY-AL group (P < 0·05; one-way ANOVA and least significant differences).
‡ Values of two-way ANOVA are P values for main effects, such as protein, oil and interaction between protein and oil in the HY+CP, HY+CS, HY+SP and HY+SS groups.
In addition, renal NO, nitrotyrosine, TNF-α and IFN-γ were significantly decreased, whereas renal TGF-β was significantly increased in hyperuricaemic rats compared with normal rats (Table 5). Soya protein was the main factor to decrease renal NO content; soya protein and palm oil were the main factors to decrease renal nitrotyrosine; and palm oil was the main factor to decrease renal TNF-α and IFN-γ and to increase renal TGF-β.
Table 5 Renal nitric oxide and cytokines in normal and hyperuricaemic rats
(Mean values with their standard errors for nine rats per group)

IFN, interferon; TGF, transforming growth factor; R, normal control rats fed with a modified AIN-93M high-fat diet; HY-AL, hyperuricaemic rats fed with casein and maize oil; HY+AL, hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water; HY+CP, hyperuricaemic rats fed with casein and palm oil; HY+CS, hyperuricaemic rats fed with casein and safflower-seed oil; HY+SP, hyperuricaemic rats fed with soya protein and palm oil; HY+SS, hyperuricaemic rats fed with soya protein and safflower-seed oil; AIN, American Institute of Nutrition.
a,b,c Mean values within a column (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike superscript letters were significantly different (P < 0·05).
* Mean value was significantly different from that of the R group (P < 0·05; one-way ANOVA and least significant differences).
† Mean value was significantly different from that of the HY-AL group (P < 0·05; one-way ANOVA and least significant differences).
‡ Values of two-way ANOVA are P values for main effects, such as protein, oil and interaction between protein and oil in the HY+CP, HY+CS, HY+SP and HY+SS groups.
Morphological assessments of kidney sections
The results of histological assessments of kidney sections in PAS stains and the grades of renal damage are shown in Fig. 3 and Table 6, respectively. In comparison with normal rats (Fig. 3(A)), hyperuricaemic rats (Fig. 3(B)) exhibited extended tubular injury, including severe tubulointerstitial nephritis, lymphoplasmacytosis, fibrosis and intralesional severe renal tubular regeneration and dilation, associated with filled PAS positive material, calcification and feathery urate crystals. In addition, the grades of renal damage, including tubulointerstitial nephritis, intralesional calcification, urate crystallisation and fibrosis were significantly greater in hyperuricaemic rats than in normal rats (Table 6; P < 0·001). The grades of renal damage were not significantly different among the hyperuricaemic groups, except for the HY+CS group, which had significantly attenuated tubular injury, PAS positive material (Fig. 3(E)) and renal damage (Table 6) compared with the other hyperuricaemic groups.

Fig. 3 Light micrographs of kidney sections stained with periodic acid–Schiff (PAS; magnification 200 × ; bars = 100 μm) of: (A) normal control rats fed with a modified American Institute of Nutrition (AIN)-93M high-fat diet (R); (B) hyperuricaemic rats fed with casein and maize oil (HY-AL); (C) hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water (HY+AL); (D) hyperuricaemic rats fed with casein and palm oil (HY+CP); (E) hyperuricaemic rats fed with casein and safflower-seed oil (HY+CS); (F) hyperuricaemic rats fed with soya protein and palm oil (HY+SP); (G) hyperuricaemic rats fed with soya protein and safflower-seed oil (HY+SS). Except for the HY+CS group (E), hyperuricaemic rats (B to D, F and G) exhibited extended tubular injury with PAS positive material, calcification and feathery urate crystals compared with the normal rats (A).
Table 6 Grade of renal damage in normal and hyperuricaemic rats‡
(Mean values with their standard errors for nine rats per group)
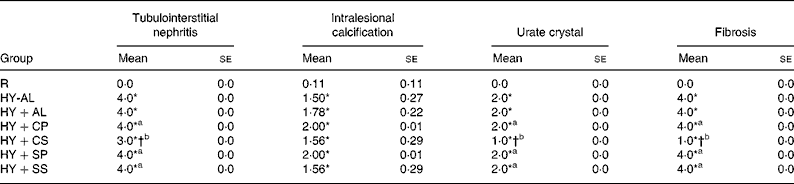
R, normal control rats fed with a modified AIN-93M high-fat diet; HY-AL, hyperuricaemic rats fed with casein and maize oil; HY+AL, hyperuricaemic rats fed with casein and maize oil and administered with allopurinol in drinking water; HY+CP, hyperuricaemic rats fed with casein and palm oil; HY+CS, hyperuricaemic rats fed with casein and safflower-seed oil; HY+SP, hyperuricaemic rats fed with soya protein and palm oil; HY+SS, hyperuricaemic rats fed with soya protein and safflower-seed oil; AIN, American Institute of Nutrition.
a,b Mean values within a column (among the HY+CP, HY+CS, HY+SP and HY+SS groups) with unlike superscript letters were significantly different (P < 0·05).
* Mean value was significantly different from that of the R group (P < 0·05; Kruskal–Wallis test).
† Mean value was significantly different from that of the HY-AL group (P < 0·05; Kruskal–Wallis test).
‡ The extent of injury was graded as 0 for absent; 1 for trace (1 to 25 % of the area); 2 for weak (25 to 50 % of the area); 3 for moderate (50 to 75 % of the area); 4 for strong (75 to 100 % of the area).
Discussion
Epidemiological evidence suggests that a decrease in serum UA may attenuate renal disease progression(Reference Kang and Nakagawa8). Using rats administered with OA, a uricase inhibitor, and UA, we confirmed that hyperuricaemia results in renal damage, whereas allopurinol treatment did not attenuate this damage. So far, there is no dietary consensus for individuals with hyperuricaemia, especially for those with renal damage. A soya-based diet supplemented with UFA is hypothesised to be beneficial in decreasing serum UA and attenuating the complications of hyperuricaemia. Therefore, we investigated the effects of casein v. soya protein with two commonly used plant oils with different fatty acid components, i.e. palm (palmitic and oleic acid-rich) and safflower-seed (linoleic acid-rich) oils, on several serological parameters and renal damage in hyperuricaemic rats. The rationale for choosing these two oils is because of their notably diverse fatty acid components; for example, the UFA:SFA ratio of palm oil is close to 1(Reference Bayorh, Abukhalaf and Ganafa22) and that of safflower-seed oil is close to 10(Reference Cosge, Gurbuz and Kiralan23).
In the present study, after being administered with OA and UA for 8 weeks, the rats had significantly increased serum UA and BUN and decreased body-weight gain, feed efficiency and serum albumin. These results suggest that hyperuricaemia may interfere with anabolism. Human and animal studies have demonstrated that hyperuricaemia may result in the deposition of urate crystals in the tubular system, leading to renal failure(Reference Johnson, Titte and Cade6, Reference Kutzing and Firestein14, Reference Avram and Krishnan24). Prolonged hyperuricaemia may worsen the progression of renal disease by inactivating NO synthase and augmenting inflammatory responses(Reference Menè and Punzo4, Reference Mazzali, Hughes and Kim5, Reference Kang and Nakagawa8, Reference Ejaz, Mu and Kang10). In the present study we found that hyperuricaemic rats had significantly elevated circulating leucocytes, kidney weights and renal TGF-β (a pivotal protein in the pathogenesis of renal fibrosis), and recognisable morphological changes in the kidneys, such as extended tubular injury and elevated PAS positive material (Fig. 3 and Table 6). In addition, the renal contents of NO and nitrotyrosine, an indicator of NO production, were significantly decreased in hyperuricaemic rats. These results suggest that UA inactivates NO synthase(Reference Khosla, Zharikov and Finch25) and further causes renal damage and cell dysfunction, as shown in the decreased productions of TNF-α and IFN-γ in the kidneys. Taken together, the present results clearly indicate that hyperuricaemia may result in decreased anabolism and extended renal damage.
Allopurinol, an inhibitor of xanthine oxidase that mainly works to decrease UA production, is recommended as a first-line UA-lowering therapy in patients with hyperuricaemia(Reference Kang and Nakagawa8). Recent clinical studies have shown that allopurinol has serious toxicity in a small number of patients with renal impairment(Reference Dalbeth and Stamp26, Reference Markel27). In the present study, the allopurinol-treated hyperuricaemic rats had significantly decreased amounts of food intake on weeks 2 to 4. This decrease might be related to the bitter taste of allopurinol in drinking water. However, the food intake was increased and the catch-up growth occurred on week 5. We also found that allopurinol administration did not improve renal damage in rats treated with OA and UA, even though serum UA was significantly decreased. These results imply that allopurinol may effectively maintain serum UA concentrations via decreasing UA production and/or increasing UA excretion; however, the formation of urate crystals may occur in the renal tubules and interstitial tissue to induce renal damage (Fig. 3(C) and Table 6).
Accumulating evidences has demonstrated that soya-based diets may improve hyperlipidaemia(Reference Ogborn, Nitschmann and Weiler28–Reference Azadbakht, Atabak and Esmaillzadeh30), renal function(Reference Teixeira, Tappenden and Carson31, Reference Azadbakht, Shakerhosseini and Atabak32) and progression in chronic renal disease(Reference Fair, Ogborn and Weiler18, Reference Chen, Yang and Huang33), which may be related to soya protein-induced increases in NO generation and decreases in oxidative stress and TGF-β expression(Reference Trujillo, Cruz and Tovar34). Recently, soya-based diets with UFA have been promoted as a way to alleviate hyperuricaemia and to halt the progression of renal disease. In the present study, we found that both casein and soya protein combined with safflower-seed oil significantly decreased serum TAG in hyperuricaemic rats. In addition, casein plus safflower-seed oil significantly increased body-weight gain, feed efficiency and renal contents of NO, nitrotyrosine, TNF-α and IFN-γ, as well as significantly decreasing serum cholesterol, BUN, and UA and renal TGF-β content. The morphological results further demonstrated the beneficial effects of casein plus safflower-seed oil in improving hyperuricaemia-induced renal damage, as shown in the attenuated tubular injury, dilation, and PAS positive material (Fig. 3). Moreover, soya protein plus safflower-seed oil significantly decreased serum TAG and UA (Fig. 2). These anti-hyperlipidaemic and anti-hyperuricaemic activities were closely associated with the consumption of safflower-seed oil (two-way ANOVA; P < 0·001). In the present study, we excluded the possibility that energy restriction is the main factor to result in the serological changes and renal damage in hyperuricaemic rats. One of the reasons is that the palm oil-fed rats tended to have lower food intake but had significantly increased serum TAG and UA compared with the safflower-seed oil-fed rats. In addition, animals with casein combined with safflower-seed oil had significantly attenuated hyperuricaemia-induced renal injury and their food intake and body weight were similar to those with soya protein combined with safflower-seed oil. The mechanisms of casein plus safflower-seed oil in improving renal damage and soya protein plus safflower-seed oil in decreasing serum TAG in hyperuricaemia need to be further investigated.
Reports in the literature suggest that palm oil may attenuate arterial thrombosis, atherosclerosis(Reference Ganafa, Socci and Eatman35) and hypertension via elevations in endothelial NO and a reduction in oxidative stress(Reference Bayorh, Abukhalaf and Ganafa22). However, we found inconsistent results in OA- and UA-induced hyperuricaemic rats. For example, the body-weight gain, food intake and renal nitrotyrosine, TNF-α and IFN-γ contents were significantly decreased, whereas heart weight, serum TAG (Fig. 2(A)), cholesterol, BUN, creatinine (Table 3) and renal TGF-β content (Table 5) were significantly increased in rats with palm oil. These adverse effects are possibly because the palm oil was melted by heat for 5 min when we prepared the semi-purified powdered diet. This oxidised palm oil may have worsened lipid profiles and have been toxic to organs, as shown in previous studies(Reference Aguila, Pinheiro and Aquino19, Reference Edem36, Reference Leong, Aishah and Nor Aini37).
It has been indicated that fatty acid compositions of the kidneys are associated with renal function and morphological changes in Han:SPRD-cy heterozygous rats(Reference Ogborn, Nitschmann and Bankovic-Calic38). In addition, these changes may be influenced by the dietary contents. For example, diets rich in conjugated linoleic acid significantly reduce fibrosis, macrophage infiltration, tissue oxidised LDL content and proliferation of epithelial cells in the kidneys(Reference Ogborn, Nitschmann and Goldberg39). The pitfall of the present study is that fatty acid compositions of the plasma and kidneys were not determined to explain the possible mechanism of soya protein plus safflower-seed oil in attenuating hyperuricaemia and casein plus safflower-seed oil in improving hyperuricaemia-induced renal damage. The effects of dietary contents on circulating and tissue fatty acid composition and tissue function require further investigation.
In summary, the present study indicates that OA- and UA-induced hyperuricaemia may decrease anabolism and result in renal damage, as shown in the significantly elevated leucocytes, BUN and renal TGF-β, decreased renal NO, and extended tubular injury and urate crystals. Allopurinol may decrease serum UA; however, the hyperuricaemia-induced renal damage was not improved. In hyperuricaemic rats, safflower-seed oil had an anti-hyperlipidaemic effect, whereas palm oil had a hyperlipidaemic effect and may have augmented renal dysfunction, as evidenced by increased TAG, BUN, creatinine and renal TGF-β. Moreover, soya protein combined with safflower-seed oil significantly attenuated hyperuricaemia and casein combined with safflower-seed oil significantly attenuated hyperuricaemia-induced renal injury. The utilisation of casein or soya protein plus safflower-seed oil in improving hyperuricaemia-associated complications in human subjects needs to be investigated further.
Acknowledgements
The present study is supported by Changhua Christian Hospital (grant no. 96-CCH-IPI-09).
H.-C. L. and Y. Y. designed the study. H.-C. L. conducted the animal study and wrote the manuscript. Y.-H. W. and S.-H. L. contributed to sample analyses and statistical analyses. H.-Y. C. conducted the tissue section analysis and picture preparation. Y. Y. critically reviewed the manuscript. H.-C. L. was the principal investigator who supervised aspects of the whole study.
The authors declare that there are no conflicts of interest.











