Iodine is an essential micronutrient needed for fetal neurodevelopment and thyroid hormone production( Reference Caldwell, Jones and Hollowell 1 ). In early pregnancy, fetal brain development depends on maternal thyroid hormone, whereas iodine deficiency leads to inadequate production of thyroid hormones( Reference Pearce 2 ). A recent medical news and perspectives in 2010 has reported that an estimated 38 million babies born each year in developing countries are at risk for developing brain damage that is associated with iodine deficiency( Reference Hetzel 3 , Reference Mitka 4 ). Furthermore, low birth weight or hearing loss in babies is also associated with prenatal iodine deficiency( Reference Skeaff 5 , Reference Hollowell, Staehling and Hannon 6 ). Therefore, achieving adequate iodine nutrition during pregnancy is an important public health objective.
The key to achieving the best iodine status in the pregnant population is to determine the sources of iodine. Foods are the major sources of the required iodine in the human body, which accounts for more than 90 % of iodine intake, and the largest fraction of dietary iodine is excreted by the kidneys( Reference Nicola, Basquin and Portulano 7 ). The excretion of iodine in the urine is a good measure of iodine intake. Urinary iodine (UI) is considered as a reliable indicator reflecting the latest iodine intake, and as a safety biomarker linking closely to iodine metabolism during pregnancy( Reference Mitka 4 , Reference Kusić, Jukić and Rogan 8 ).
China is one of the countries in which iodine deficiency is due to the deficiency of environmental sources. Since 1996, Shanghai has carried out the universal salt iodisation strategy. In the past, Shanghai was not considered an iodine-deficient area. A recent study in Shanghai has shown that the current dietary iodine intake is sufficient in the general population, but insufficient especially in pregnant women( Reference Zou, Wu and Guo 9 ). However, that study has not reported the prevalence of iodine deficiency in different trimesters of pregnancy or the sources of dietary iodine intake in pregnant women. Therefore, in the present study, we examined dietary factors that influence the iodine level during pregnancy using a questionnaire including sources of dietary iodine intake. Furthermore, we analysed the association between iodine content of salt and secretion in the urine. We intended to clarify the iodine status in the three trimesters of pregnancy, the determinants of UI level, and the association between thyroid hormone and UI levels in pregnant women.
Materials and methods
Subjects
From 2011 to 2012, a total of 1102 pregnant women who lived in Shanghai for at least 3 years were recruited from the clinics of the Maternal and Child Care Service Centre of Minhang District, Shanghai for the present study. Finally, a sample of 916 pregnant women, with 211, 360 and 345 participants in the first, second and third trimesters, respectively, signed the written informed consent form. A first-morning spot urine sample was collected, and a questionnaire was conducted by face-to-face interviews.
Of the 360 participants in the second trimester, 180 were randomly selected and blood samples were collected for the measurement of serum tri-iodothyronine (T3), thyroxin (T4), free tri-iodothyronine (FT3), free thyroxine (FT4) and thyroid-stimulating hormone (TSH). Of the 180 participants, 134 gave their written informed consent for blood sample collection. The present study was approved by the Ethics Committee of Xinhua Hospital, Shanghai Jiao Tong University School of Medicine.
Questionnaire information
From the questionnaire, data on socio-economic status, medical history of thyroid disease (hyperthyroidism, hypothyroidism, goitre, thyroid cyst and thyroid cancer), smoking status and sources of dietary iodine intake in the 24 h recall were collected. Data on the socio-economic status of each participant provided information on residence, occupation, educational levels of couples, and household incomes. Any source of dietary iodine intake in the 24 h recall, such as iodised salt, multivitamin supplement with iodine, cows' milk, yogurt, soya sauce, egg and aquatic foods, was collected.
Measurement of urinary iodine and iodine content of salt
The first-morning spot urine sample was collected from each participant in a clean cup and stored at − 40°C until urine test for iodine. Participants were asked to bring a small bottle of salt from their home. UI was determined by the modified Sandell–Kolthoff reaction, as described previously( Reference Dunn, Crutchfield and Gutekunst 10 ), which was a standard promulgated by the Ministry of Health of the People's Republic of China (WS/T 107-2006). The urine specimen was first digested with chloric acid, and iodine was determined from its catalytic reaction of ceric ammonium sulphate in the presence of arsenious acid( Reference Dunn, Crutchfield and Gutekunst 10 ). Concentrations of urinary creatinine were determined by an automated colorimetric method on a Beckman Synchron LX20 or CX3 clinical analyser, as described previously( Reference Caldwell, Makhmudov and Ely 11 ). Iodine concentrations were adjusted by using creatinine concentrations to correct for variable water excretion rates at the time of spot urine specimen collection. UI concentrations are expressed as μg/g creatinine. Considering the titration method with thiosulphate becomes more complicated because of the interference of Fe in salt( Reference Zimmermann, Zeder and Chaouki 12 ). Iodine content of salt was determined with the modified Sandell–Kolthoff reaction using an aliquot of 10 g salt in distilled water( Reference Pino, Fang and Braverman 13 ).
Serum thyroid-stimulating hormone, free tri-iodothyronine and free thyroxine
In the second trimester of pregnancy, 134 blood samples for serum analysis were collected and stored at − 40°C after serum preparation. Serum T3, T4, FT3, FT4 and TSH were measured by time-resolved fluoroimmunoassay on an auto DELFIA analyser (Wallac Oy)( Reference Burns, Mayne and O'Herlihy 14 ). FT4 testing in pregnancy is challenging, due to a number of related changes such as an alteration in the concentrations of albumin and thyroxine-binding globulin. During pregnancy, FT4 immunoassays are challenging because they may be sensitive to changes in a method-specific manner. Previous studies have proved that immunoassay may yield a FT4 pattern during pregnancy similar to that obtained by equilibrium dialysis isotope dilution–liquid chromatography/tandem MS( Reference Anckaert, Poppe and Van Uytfanghe 15 ).
T3, T4, TSH, FT3 and FT4 are expressed as nmol/l, nmol/l, mIU/l, pmol/l and pmol/l, respectively. In view of the controversial validity of certain immunoassays under conditions of altered serum binding proteins( Reference Demers and Spencer 16 ), trimester-specific reference ranges were used. The second trimester-specific reference ranges for these hormones were as follows: T3 (1·62–3·72 nmol/l); T4 (88·6–213·2 nmol/l); FT3 (3·55–5·25 pmol/l); FT4 (10·6–17·6 pmol/l); TSH (0·05–4·5 μIU/ml)( Reference Yan, Dong and Dong 17 ).
Statistical analyses
The distribution of UI concentrations was skewed towards the left. Thus, we performed log10 transformation before analysis. We first determined the prevalence of UI deficiency in pregnant women (Table 1). Then, we performed a univariate analysis to examine the influencing factors of UI (Table 2), and used multivariable analysis to estimate generalised linear models of UI (Table 3). The prevalence of normal thyroid hormones in the second trimester of pregnant women in Shanghai is presented in Table 4. Finally, the correlation between UI and iodine content of salt was analysed using Pearson's test (Fig. 1). A P value < 0·05 was considered statistically significant. All statistical analyses were performed using Empower(R) (www.empowerstats.com; X&Y Solutions, Inc.) and R (http://www.R-project.org).
Table 1 Median urinary iodine (UI) concentrations and the prevalence of UI deficiency in pregnant women (n 916)
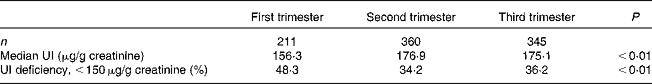
Table 2 Influencing factors of urinary iodine (UI) by the univariate analysis (Mean values and standard deviations, n 916)

Table 3 Generalised linear models of urinary iodine (UI)* (Coefficients with their standard errors, n 916)
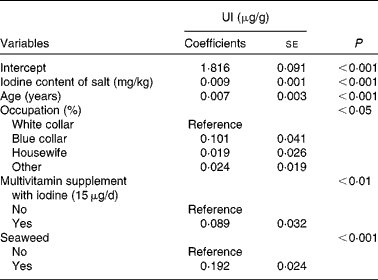
* Other variables included in the linear models were as follows: educational level; husband's educational level; household income; cows' milk; yogurt; soya sauce; egg; river fish; sea fish; clams; medical history of thyroid; smoking status.
Table 4 Prevalence of normal thyroid hormones in the second trimester of pregnancy (Number of women and percentages, n 134)
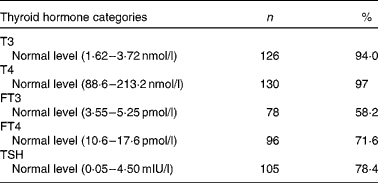
T3, tri-iodothyronine; T4, thyroxin; FT3, free tri-iodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.

Fig. 1 Correlation between iodine content of salt and urinary iodine (UI: r 0·406, P< 0·001). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Results
The median concentrations of UI were 156·3, 176·9 and 175·1 μg/g creatinine in the first, second and third trimesters of pregnancy, respectively, as shown in Table 1. Accordingly, the prevalence of UI deficiency in the first trimester was 48·3 %, which is higher than that in the other two stages (34·2 % in the second trimester and 36·2 % in the third trimester of pregnancy; P< 0·05).
Tables 2 and 3 show unadjusted and adjusted influencing factors of UI, respectively. These risk factors were categorised into socio-economic status, sources of dietary iodine in the 24 h recall, medical history of thyroid and smoking status. Using the generalised linear analysis, the following variables were found to be significant in the model: iodine content of salt (β = 0·009, P< 0·001); age (β = 0·007, P< 0·001); occupation (P< 0·05); multivitamin supplement with iodine (P< 0·01); seaweed intakes (P< 0·001) (Table 3). In addition, there was a linear correlation between iodine content of salt and UI (r 0·406, P< 0·001; Fig. 1).
The proportions of normal T3, T4, FT3, FT4 and TSH were 94·0, 97, 58·2, 71·6 and 78·4 %, respectively, in the second trimester of pregnancy, as shown in Table 4.
Table 5 shows the association between UI and thyroid hormone levels in the second trimester of pregnancy. The association between T3, T4, FT3, FT4, TSH and UI was not found to be significant (P>0·05).
Table 5 Association between urinary iodine (UI) and thyroid hormone levels in the second trimester of pregnancy (n 134)

T3, tri-iodothyronine; T4, thyroxin; FT3, free tri-iodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Discussion
UI is a good biochemical marker for the evaluation of iodine intake and iodine nutrition status. The WHO has recommended the median UI concentration as the indicator of iodine nutrition status in the general population. The recommended median UI concentration for pregnant women is 150–249 g/l( 18 ). In the present study, the median UI concentration in all the three trimesters of pregnancy was found to be within the reference range, but at the low end. However, the prevalence of UI deficiency was high in all the three trimesters of pregnancy. Moreover, the proportion found in the present result was lower than that reported by Zou et al. ( Reference Zou, Wu and Guo 9 ) showing that the prevalence of UI deficiency was 55·4 % for pregnant women in Shanghai.
It is noteworthy that the prevalence of UI deficiency in the first trimester of pregnancy was higher than that in the other trimesters of pregnancy in Shanghai. It is well known that thyroid hormone plays an important role during early brain development in the fetus, whereas fetal thyroid cannot produce thyroid hormones. Normal brain development of the fetus is thus dependent on maternal thyroid hormone via placental transfer in the early stage of pregnancy( Reference Toft 19 ). Iodine is very important for the proper functioning of the thyroid gland. Therefore, iodine deficiency would be considered as one of the influencing factors that interfere with brain development in the fetus during early pregnancy. The high prevalence of UI deficiency in the first trimester of pregnancy may be associated with excess iodine intake resulting in increased thyroid hormone production. (1) Maternal thyroid hormone production during the first trimester of pregnancy obviously increases immediately after its onset, in order to ensure the early surge in circulating FT4, considering, among other factors, that the plasma volume increases rapidly( Reference de Escobar, Obregon and Del Rey 20 ). (2) A transient stimulation of the maternal thyroid gland by elevated levels of human chorionic gonadotropin leads to a rise in thyroid hormone levels during the first trimester of pregnancy( Reference Glinoer 21 ). (3) In Shanghai, some pregnant women began to take multivitamin supplements after their first visit to obstetricians at about 12 weeks of gestation. (4) A lack of the collection of urine samples from the same women may be a possible explanation for the higher prevalence of iodine deficiency in the first trimester of pregnancy.
In the present study, intake of iodised salt, seaweed and iodine supplement was found to be the main sources of iodine. The present study showed that Shanghai, despite being a coastal city, has low iodine content in natural dietary sources. A recent survey in China has demonstrated that although iodised salt was used in some food processing industries, it was not used in household foods, such as soya source, bread, noodles and mineral water. Seaweed is the only seafood that was positively correlated with UI. Similarly, a Korean study has also found a significant correlation between dietary iodine intake primarily from seaweed( Reference Kim, Moon and Kim 22 ) and UI excretion. However, seaweed was not common in the daily diet in Shanghai, and only 15 % of the participants in the present survey consumed seaweed intake. Notably, iodised salt is a main source of iodine, and there is a significant correlation between iodine content of salt and UI. In China, salt production is tightly controlled and the sale of non-iodised salt is restricted. Recently, some coastal cities have been unofficially allowing the sale of non-iodised salt, and there were calls for liberalising provincial control of such sales( Reference Wu, Li and Chang 23 ). According to official reports from Shanghai, the iodine content in iodised salt available in stores ranges from 20 to 50 parts per million. Non-iodised salt is widely used in the food processing industry. In the present study, 14·9 % of pregnant women did not use iodised salt. This might be a threat to universal salt iodisation policy in China, which could raise the possibility of recrudescence of iodine-deficiency disorders, especially in pregnant women. In addition, supplementation of iodine-rich nutrients during pregnancy, especially in early pregnancy, is needed in order to improve the iodine status of pregnant women.
The present study did not find any correlation between T3, T4, FT3, FT4, TSH and UI in the analysis of a small size sample. Although the representativeness of the samples should be considered, the present result is consistent with that of most other studies( Reference Fuse, Ohashi and Yamaguchi 24 – Reference Luton, Alberti and Vuillard 28 ). A recent report from Japan showed no direct correlation between UI and serum TSH or FT4 concentrations during pregnancy( Reference Fuse, Ohashi and Yamaguchi 24 ), nor did a study in northern Spain( Reference Aguayo, Grau and Vela 25 ). It has been speculated that the lack of such correlation could be attributed to the amount of iodine reserve in the thyroid gland in a population that do not have persistent iodine deficiency. Thus, thyroid hormone synthesis could be guaranteed( Reference Aguayo, Grau and Vela 25 ).
Limitations
The present study has some limitations. First, a previous history of chronic thyroiditis and subclinical hypothyroidism cannot be ruled out based on patient's anamnesis in their subclinical course. Serum anti-thyroid peroxidase antibodies, an independent and important predictor of subclinical hypothyroidism and chronic thyroiditis, were not measured in the present study. Second, we neither tested hormone levels nor addressed the association between thyroid hormone levels and UI during pregnancy, especially in the first trimester of pregnancy. Further research is needed to clarify whether UI would be associated with thyroid hormone concentration in the first trimester of pregnancy.
Conclusion
In summary, there is a high prevalence of UI deficiency in pregnant women in Shanghai, especially during the first trimester of pregnancy. Both iodine content of household salt and multivitamin supplement with iodine are the main determinants of UI levels.
Nutritional status of iodine in pregnant women is not well recognised in Shanghai. Thus, it is essential to publicise the knowledge of iodine-deficiency disorders, and its potential risk for fetal neurodevelopment. Regular monitoring of iodine nutrition during pregnancy needs to be considered seriously by health authorities, policy makers and relevant organisations. The use of iodised salt and supplementation of iodine-rich nutrients during pregnancy are recommended.
Acknowledgements
The present study was supported by the Minhang District Maternal and Child Health Hospital, Shanghai, China. The authors are grateful to the researchers X. Z. and L. J. who were responsible for the collection of the sample and data.
The present study was funded by the National Natural Science Foundation, China (grant no. 81373004) and the Establishing a Platform for Clinical Research Data Sharing to Facilitate Multi-Center Collaboration by China–Canada Joint Effort (2014DFG31460).
The authors' contributions are as follows: X. Y. designed the analyses of the study and performed the statistical analyses; Z. W. and W. W. were involved in the writing of the manuscript; J. Z. contributed to the statistical counselling; X. Y., Z. W. and W. W. participated in the discussion of the result and revision of the manuscript; X. Z. and L. J. were responsible for the collection of the sample and data.
There are no conflicts of interest.









