Elevated concentrations of plasma LDL-cholesterol are recognised as a major risk factor for the development of premature CVD. The first step towards reducing LDL-cholesterol levels in individuals with mild-to-moderate hypercholesterolaemia is a modification of the lifestyle that should include dietary changes, and in particular, a reduction in the intake of total and saturated fats(1). Among other recommendations, the daily consumption of phytosterols (PS) has been shown to reduce the plasma concentration of LDL-cholesterol by about 10 % when administered at a dose of 2 g/d(Reference Katan, Grundy and Jones2–Reference Demonty, Ras and van der Knaap4). PS act at the intestinal level, reducing cholesterol absorption by both biliary and dietary sources by competing with cholesterol molecules for uptake into mixed micelles(Reference von Bergmann, Sudhop and Lutjohann5). Despite the important hypocholesterolaemic benefit of PS consumption, a potential side effect is a slight reduction in plasma carotenoid levels(Reference Richelle, Enslen and Hager6). Some reports have endorsed adding carotenoids to products enriched with PS to compensate for this undermining of carotenoid levels(Reference Quílez, Rafecas and Brufau7, Reference Brufau, Quílez and Canela8), whereas others have reported excellent results after supplementing an enriched diet of fruits and vegetables with PS(Reference Noakes, Clifton and Ntanios9–Reference Colgan, Floyd and Noone11).
Factors that might influence the cholesterol-lowering action of PS include their form, matrix and frequency of intake and type of diet. Plant sterols and stanols produce similar reductions in plasma cholesterol(Reference Vanstone, Raeini-Sarjaz and Parsons12). Most previous reports have suggested that the dispersion of PS in different food forms substantially affects the degree of LDL-cholesterol reduction achieved(Reference Katan, Grundy and Jones2). In relation to frequency, most studies have shown that PS are more effective when consumed in two to three portions per d(Reference De Graaf, De Sauvage Nolting and Van Dam13–Reference Thomsen, Hansen and Christiansen15) than when a single dose is administered(Reference Plat, van Onselen and van Heugten16, Reference AbuMweis, Vanstone and Ebine17). Plat et al. (Reference Plat, van Onselen and van Heugten16) showed that a single dose of PS with lunch had an effect that was the same as that produced when a dose was provided at each meal time (three times per d), whereas a single dose of different preparations of PS administered in the morning was reported to lower LDL levels(Reference AbuMweis, Vanstone and Ebine17). It is a matter of controversy whether the effect of PS on lipoproteins depends on diet composition, particularly with respect to cholesterol and saturated fat. While some investigators have reported that low levels of dietary cholesterol intake attenuate the effectiveness of PS(Reference Denke18, Reference Mussner, Parhofer and Von Bergmann19), others have shown that PS lower cholesterol concentration, even in subjects with a strict restriction of cholesterol intake(Reference Hallikainen and Uusitupa20, Reference Kassis, Vanstone and AbuMweis21).
In this context, the purpose of the present study was to compare the effect of low-fat milk enriched with PS on lipid profile in a population of moderately hypercholesterolaemic patients who followed either a standard healthy diet or free diet. Our aim was to evaluate whether the efficacy of PS is related to the composition of saturated fat and dietary cholesterol, and to determine the effect of PS on carotenoid content after dietary intervention.
Experimental methods
Patients
Patients with untreated moderate hypercholesterolaemia were recruited from the Service of Endocrinology and Nutrition of University Hospital Dr Peset (Valencia, Spain). Patients between the ages of 18 and 76 years (inclusive) were eligible for inclusion into the study. Further inclusion criteria were a serum LDL-cholesterol concentration of 4·14–4·92 mmol/l in patients with less than two cardiovascular risk factors and 3·37–4·14 mmol/l in patients who presented two or more cardiovascular risk factors, and a TAG concentration < 4·52 mmol/l in any case. Cardiovascular risk factors were defined as age ( ≥ 45 years in men and ≥ 55 years in women), a smoking habit, hypertension ( ≥ 140/90 mmHg), diabetes mellitus, a HDL-cholesterol concentration < 1·04 mmol/l and a family history of CVD. Exclusion criteria were pregnancy or lactation, change of oral contraceptives, severe disease, a history of CVD or chronic inflammatory disease, hypersensitivity to milk proteins and lipid-lowering medication.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committee of the hospital. Written informed consent was obtained from all the patients. On the basis of these criteria, eighty-eight patients were included in the study and were randomly assigned to a healthy diet (n 24), free diet plus PS (n 29) or healthy diet plus PS (n 31) group. Four patients dropped out of the study for personal reasons, including lack of time and difficulties in attending the research clinic.
Study design
The study consisted of a randomised parallel trial with a three-arm design, and took place during a period of 3 months. Before dietary therapy was initiated, in order to stabilise dietary patterns before intervention in the groups that were to follow a healthy diet (healthy diet or healthy diet plus PS), patients were submitted to a 3-month run-in period of a standard healthy diet recommended by ATP III(1). After this adaptation period, three intervention groups were evaluated: a healthy diet group that followed the dietetic guidelines of the ATP III and whose diet included 500 ml/d of standard low-fat milk; a free diet plus PS group, whose members consumed 2 g of PS per d in 500 ml of low-fat milk, and were encouraged to maintain their dietetic habits; and a healthy diet plus PS group that received 2 g of PS per d in 500 ml of low-fat milk, and followed the dietetic guidelines of the ATP III.
Patients following a controlled diet were monitored throughout the trial by an experienced dietitian, and received detailed written and oral instructions about their diet, including the precise amounts to be eaten and the quality of the food products to be consumed. The recommended diet included total cholesterol ( < 200 mg/d), saturated fat ( < 7 % of daily total energy), low contents of simple sugars ( < 10 %), MUFA mainly from olive oil (up to 20 % of MUFA with respect to total energy), n-3 fatty acids from fish, two to three pieces of fruits and unlimited vegetables. Insofar as daily energy intake, 8368 kJ (2000 kcal) were proposed for men and 7112·8 kJ (1700 kcal) for women, of which 18–19 % was proteins, 52–53 % was carbohydrates and 29–30 % was fats, and which included 20–30 g of dietary fibre.
Adherence to the diet was monitored by means of 3 d food records (compiled on weekdays) and 24 h diet recall at baseline, after the adaptation period and at the end of the study (carried out during appointments with the dietitian). Food intake was converted into energy and nutrients with the help of the Spanish Food Composition Table(Reference Mataix, Mañas and Llopis22). The composition database was created with AYS44 Diet Analysis software obtained from ASDE, SA (Valencia, Spain). Patients were encouraged to maintain their normal pattern of activity.
The PS-enriched milk was produced by Unilever (Barcelona, Spain) and packed in white containers. In addition to 0·4 g of vegetable sterols, every 100 ml of milk provided 3·2 g of protein, 4·7 g of carbohydrates, 1·8 g of fat (0·25 g saturated, 0·50 g monounsaturated and 1·05 g polyunsaturated) and 200·83 kJ (48 kcal) of energy. The PS consisted of vegetable oil-based sterols esterified with sunflower oil fatty acids, and their specific content was analysed in our laboratory. The PS content of the enriched milk was 70·7 % β-sitosterol, 15·0 % campesterol, 9·6 % β-sitostanol, 2·6 % brassicasterol, 1·0 % campestanol and 0·9 % stigmasterol.
The diet group received commercially available low-fat milk with a macronutrient composition and energy intake similar to that consumed by the PS group but without vegetal sterols. Patients were recommended to consume the milk twice per d with meals.
Compliance was evaluated by interviewing the patients and counting the unopened and unconsumed product packages returned to the clinic, and it was recorded as the percentage of scheduled servings consumed. Non-compliance was defined as the consumption < 80 % of the scheduled servings during the study period.
Lipid analysis
Venous blood samples were collected from patients after 12 h overnight fasting at baseline and 3 months. To separate serum from blood cells, samples were immediately centrifuged at 2000 g for 15 min at 4°C. Freshly separated serum was employed for the determination of the lipid profile, and the remaining aliquots of serum were stored at − 80°C until they were assessed for the measurement of PS and major carotenes.
Total cholesterol and TAG were measured by means of enzymatic assays(Reference Bucolo and David23–Reference Allain, Poon and Chan24), and HDL-cholesterol concentrations were recorded using a direct method(Reference Sugiuchi, Uji and Okabe25) with a Beckman LX-20 autoanalyser (Beckman Coulter, La Brea, CA, USA). The intraserial variation coefficient was < 3·5 % for all the determinations. When TAG values were below 3·39 mmol/l, LDL-cholesterol concentration was calculated using the Friedewald method(Reference Friedewald, Levy and Fredrickson26). Non-HDL-cholesterol concentration was obtained by calculating the difference between total and HDL-cholesterol. Apo A-I and apo B-100 were determined by immunonephelometry (Dade Behring BNII, Marburg, Germany), for which the intraassay variation coefficient was < 5·5 %.
Plasma campesterol and β-sitosterol concentrations were measured by GLC as described by Wolthers et al. (Reference Wolthers, Walrecht and van der Molen27), with minor modifications. In short, 2 μg of internal standard 5β-cholestan-3α-ol (Sigma-Aldrich, St Louis, MO, USA) were added to 0·1 ml serum samples that were then saponified with 1 ml of methanolic potassium hydroxide (0·71 m; Scharlau, Barcelona, Spain). Non-saponified materials were then extracted twice with hexane (Sigma-Aldrich) and distilled water. The extracts were derivatised and injected into a gas–liquid chromatograph equipped with a flame ionisation detector (Perkin Elmer Autosystem XL, Norwalk, CT, USA) and a 30 m column (Supelco equity, Bellefonte, MO, USA). Campesterol and β-sitosterol plasma concentrations were determined in triplicate by determining their peaks and expressing them in relation to the 5β-cholestan-3α-ol internal standard.
To analyse the PS content of the PS-enriched milk, a previous fat extraction was performed as described by Calvo et al. (Reference Calvo, Ramos and Fontecha28). In summary, 200 μg of internal standard 5β-cholestan-3α-ol (Sigma-Aldrich) were added to 1 g of the enriched milk, and then extracted once with 3·0 ml of isopropanol (Scharlau, Barcelona, Spain) and twice with 3·5 ml of hexane (Sigma-Aldrich). The extracted fat was then saponified, and the non-saponified materials were extracted and derivatised to be analysed as described earlier.
Serum concentrations of hydrocarbon carotenoids (β-carotene and lycopene) and oxygenated carotenoids (lutein and β-cryptoxanthin) were determined at the beginning and at the end of the trial by means of a HPLC method described by Olmedilla et al. (Reference Olmedilla, Granado and Rojas-Hidalgo29), with slight modifications. In brief, 400 μl of serum samples were mixed with an equivalent quantity of ethanol (Prolabo, Fontenay, France) and 90 ng of an internal standard solution of β-apo-8-carotenal (Roche, Basel, Switzerland). Fat-soluble compounds were extracted twice with n-hexane (1 ml each time stabilised with 0·01 % butylated hydroxytoluene; Sigma-Aldrich), and the extract was evaporated to dryness under nitrogen. The samples were dissolved once more in 150 μl of tetrahydrofuran (Sigma-Aldrich) and stabilised with 0·01 % butylated hydroxytoluene for HPLC reversed-phase analysis (Waters, Eschborn, Germany). We employed an isocratic method with dichloromethane–acetonitrile–methanol (20:70:10, by vol.) (all purchased from JT Baker, Deventer, The Netherlands) with a flow of 1·3 ml/min as a mobile phase. Detection was performed at 450 nm with absorbance units full scale 0·005.
Statistical analysis
The total sample size was eighty-four patients. The present study was designed to have a power of 80 % in order to be able to detect differences among the groups in relation to the primary efficacy criterion (LDL-cholesterol variation) ≥ 3·89 mmol/l, assuming a common standard deviation of 5·96 mmol/l.
Data are expressed as means and standard deviations (for tables), or as means with their standard errors (for figures) for parametric data and as median and interquartile ranges for non-parametric data. Statistical analyses were conducted using GRAPHPAD PRISM software (version 4; GraphPad Software, San Diego, CA, USA). Baseline characteristics among the groups were analysed by one-way ANOVA or a Kruskal–Wallis test for parametric or non-parametric data, respectively. Between-group and within-group differences were analysed using a two-factor repeated-measures ANOVA followed by paired Student's t test or one-way ANOVA for parametric data when no interaction was found. For non-parametric variables, data were normalised using a log transformation. P < 0·05 was considered significant.
Results
A total of eighty-four individuals with an age range of 18–76 years completed the study. The diet recalls and records revealed no deviations from the guidelines during the course of the study (Table 1). Baseline anthropometric parameters of the patients are presented in Table 2. Men and women were distributed similarly among the three groups, with men constituting 25 % of the healthy diet group, 29 % of the healthy diet plus PS group and 35 % of the free diet plus PS group. At onset of the study, no significant differences were observed between the anthropometric parameters of the patients receiving/not receiving a diet or beverages enriched with PS (Table 2). Moreover, 6·0 % of the subjects were found to be hypertriglyceridemic (TAG>2·26 mmol/l), 33·3 % exhibited fasting glucose ≥ 5·55 mmol/l, 3·6 % were diabetic and 19·0 % were diagnosed with metabolic syndrome (data not shown).
Table 1 Nutrient composition of a standard healthy diet and a free diet for mildly hypercholesterolaemic patients*
(Mean values and standard deviations)
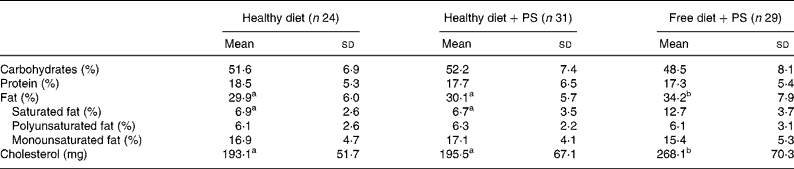
PS, phytosterols.
a,b Mean values with unlike superscript letters were significantly different when comparing the values of different treatments by one-way ANOVA (P < 0·05).
* Average daily nutrient intake as percentage of total dietary energy intake; 3 d food records (data collected on weekdays) and 24 h diet recall.
Table 2 Baseline characteristics of mildly hypercholesterolaemic patients*
(Mean values and standard deviations)
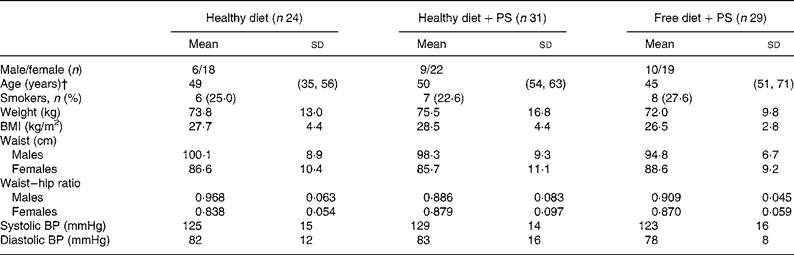
PS, phytosterols; BP, blood pressure.
* One-way ANOVA or Kruskal–Wallis test for non-parametric data revealed no significant differences among the groups.
† For age, results are represented as median and interquartile range.
After 3 months of PS therapy, patients on the free diet and on the healthy diet supplemented with PS exhibited a significant reduction in total cholesterol, LDL-cholesterol and non-HDL-cholesterol, with a similar hypolipaemic effect being displayed in both the groups (Table 3). Apo B-100 and the apo B-100/apo A-I ratio, considered an even better marker of cardiovascular events than lipid concentration per se (Reference Ridker, Rifai and Cook30), were also reduced to the same extent in the patients receiving low-fat milk enriched with PS (Table 3). On the contrary, these lipid parameters were unaltered in patients undergoing dietary therapy only. No changes were found with respect to HDL-cholesterol, TAG (all TAG values were < 3·39 mmol/l) or apo A-I in any of the groups (Table 3).
Table 3 Lipoprotein profile of mildly hypercholesterolaemic patients at baseline and after 3 months of the corresponding intervention†
(Mean values and standard deviations)

PS, phytosterols.
a,b Mean values with unlike superscript letters were significantly different with those obtained from different treatments by one-way ANOVA (P < 0·05).
* Mean values were significantly different when compared using paired Student's t test (P < 0·05).
† Data were analysed by a two-factor repeated-measures ANOVA followed by paired Student's t test and one-way ANOVA to compare the within-group effect and between-group effect, respectively.
‡ Values of plasma TAG concentrations were normalised using a log transformation.
A standard healthy diet did not affect the levels of serum PS during the 3-month monitoring period. However, intake of PS increased serum levels of campesterol and β-sitosterol to a similar extent independently of the type of diet followed (Table 4).
Table 4 Concentration of phytosterols (PS) – campesterol and sitosterol – in serum of mildly hypercholesterolaemic patients at baseline and after 3 months of the corresponding intervention†
(Mean values and standard deviations)
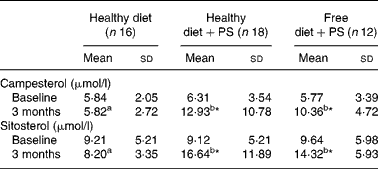
a,b Mean values with unlike superscript letters were significantly different with those obtained from different treatments by one-way ANOVA (P < 0·05).
* Mean values were significantly different when compared using paired Student's t test (P < 0·05).
† Data were analysed by two-factor repeated-measures ANOVA followed by paired Student's t test and one-way ANOVA to compare the within-group effect and between-group effect, respectively.
Serum concentration of liposoluble antioxidants (measured as carotenoid levels) in patients at the beginning and at the end of the trial are shown in Fig. 1. Lutein and cryptoxanthin concentrations were similar in the three groups at baseline and at the end of the study, and showed no change after the corresponding intervention. However, β-carotene was significantly increased by 26·9 % by the standard healthy diet and was reduced by 21·0 % by the free diet enriched with PS, whereas no change was observed in the group that followed a standard healthy diet with a low-fat milk enriched with PS. A moderate though non-significant increase of the other hydrocarbon carotenoid, lycopene, was observed in the patients who followed the dietetic guidelines, whereas the difference was reduced significantly by 22·8 % in the patients who consumed PS without modifying their dietary pattern, and was maintained at baseline levels in the group that received the combined therapy.
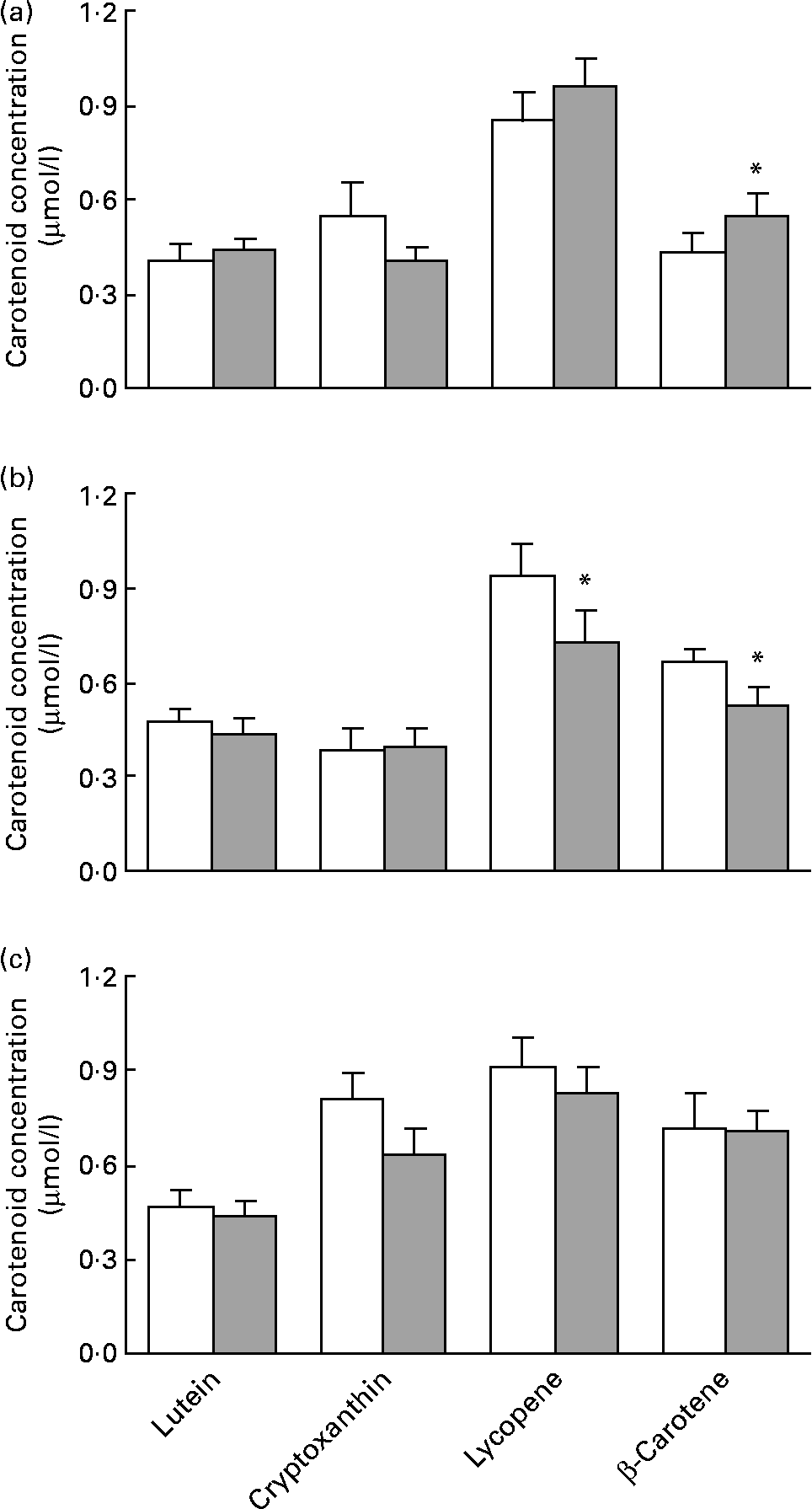
Fig. 1 Concentration of carotenoids in the serum of mildly hypercholesterolaemic patients at baseline and after 3 months of the corresponding intervention. (a) Levels of lutein, cryptoxanthin, lycopene and β-carotene in subjects who followed a standard healthy diet at baseline and after 3 months of intervention. Data are expressed as means with their standard errors of sixteen subjects. (b) Levels of lutein, cryptoxanthin, lycopene and β-carotene in subjects who followed a free diet complemented with 2·0 g of phytosterols (PS) at baseline and after 3 months of intervention. Data are expressed as means with their standard errors of twelve subjects. (c) Levels of lutein, cryptoxanthin, lycopene and β-carotene in subjects who followed a standard healthy diet complemented with 2·0 g of PS at baseline and after 3 months of intervention. Data are expressed as means with their standard errors of eighteen subjects.* Mean values were significantly different when compared using paired Student's t test (P < 0·05). (![]() ) Baseline; (
) Baseline; (![]() ) 3 months.
) 3 months.
After adjusting the carotenoid data for total cholesterol, we found that addition of PS to a free diet produced a significant decrease in lycopene, but that this effect was absent when PS were combined with a healthy diet. Levels of β-carotene also fell when PS were administered in the absence of dietary guidelines. A healthy diet had a positive effect on this carotenoid, which was increased by 8·9 and 31·4 % in the groups that followed a healthy diet with or without PS, respectively (data not shown).
Discussion
The results of the present study show that dietary cholesterol levels do not have an impact on the efficacy of PS as cholesterol-lowering agents in mildly hypercholesterolaemic human subjects. Furthermore, we have demonstrated that serum carotenoid concentrations are not reduced when PS are added to a healthy diet in the same way as they are when these liposoluble compounds are added to a free diet.
Patients with hypercholesterolaemia are usually advised to limit their intake of saturated fat and dietary cholesterol – in particular, those obtained from dairy products – in order to reduce LDL concentration and, therefore, reduce the risk of cardiovascular complications(1). In the present study, no change occurred in any of the lipoprotein profile parameters (including total and LDL-cholesterol) after 3 months of healthy diet ingestion. At first sight, these results may be surprising; however, we must take into account that in order to stabilise dietary patterns before intervention, patients were previously submitted to a 3-month run-in period of a standard healthy diet. We have shown previously that changes in cholesterol and LDL-cholesterol take place only during the first 3 months of healthy diet ingestion(Reference Bañuls, Martínez-Triguero and López-Ruiz31). This result is in accordance with a report by McCaffrey's group, who reported a similar pattern of response after 6 weeks of dietary intervention but observed no further significant decrease in the following 12 weeks(Reference Bae, Keenan and Wenz32). This experimental design allows us to evaluate the effects of PS according to the type of diet that patients follow – free or healthy – but it avoids the positive effect that a standard healthy diet has on reduction of total and LDL-cholesterol.
PS are included in the guidelines of the National Cholesterol Education Program(1) and the American Heart Association/American College of Cardiology(Reference De Backer, Ambrosioni and Borch-Johnsen33) for secondary prevention of CVD, since for each percentage of LDL-cholesterol reduction, the risk of CVD is reduced by 2–3 %. As far as we know, this is the first human clinical study to compare the effects of esterified PS when administered as part of a diet in which the levels of saturated fats and cholesterol intake are significantly different. Moreover, we have employed a three-arm, randomised, parallel study design in which the diet was controlled by an experienced dietitian throughout the trial. In line with previous studies(Reference Katan, Grundy and Jones2–Reference Demonty, Ras and van der Knaap4), we observed that ingestion of approximately 2 g of PS per d was associated with a 7·0–10·0 % reduction in LDL-cholesterol levels. In addition, we have shown that 3-month ingestion of low-fat milk enriched with PS produced similar reductions in total and LDL-cholesterol concentrations (5·5 and 6·7 % for total cholesterol and 7·0 and 9·6 % for LDL-cholesterol in free or healthy diet, respectively). Only a few studies have analysed the efficacy of PS in relation to dietary cholesterol intake. Jones' group did not observe variations in the effectiveness of free sterols when a single dose was administered in the morning, which is in accordance with our findings(Reference Kassis, Vanstone and AbuMweis21). On the other hand, other studies have shown that low levels of dietary cholesterol attenuate the efficacy of a low-dose PS therapy or have no effect at all(Reference Denke18, Reference Mussner, Parhofer and Von Bergmann19). Therefore, it would appear that in moderately hypercholesterolaemic subjects, the efficacy of PS with respect to lipoprotein profile is not influenced by the dietary composition of saturated fat or cholesterol intake, at least under the conditions of our experimental design. This finding could be of special relevance, since these patients are likely to continue their free diet, which can be supplemented with PS-enriched food. On the basis of the present results, we conclude that PS when added to low-fat milk may be as effective in lowering LDL-cholesterol as traditional fat matrices such as spreads and dressings(Reference Noakes, Clifton and Ntanios9, Reference Colgan, Floyd and Noone11, Reference Judd, Baer and Chen34), and that the fat content of the enriched product or the diet itself does not interfere with the efficacy of PS.
Recent data suggest that an increase in apo B-100 and apo B-100/apo A-I ratio and a decrease in apo A-I are important risk factors for CVD, proving to be even more relevant than lipid concentration per se (Reference Ridker, Rifai and Cook30). Most studies have reported that a daily intake of approximately 2 g of PS has no effect on apo A-I, but that it significantly affects apo B-100 levels, which is in the line with our findings(Reference Colgan, Floyd and Noone11, Reference Madsen, Jensen and Schmidt35). We observed that the apo B-100/apo A-I ratio dropped by 11·5 % (free diet) and 11·6 % (healthy diet) in the PS groups independently of the dietary intervention followed. This suggests that there is a reduction in cardiovascular risk following the intake of PS.
The increases in serum campesterol and sitosterol levels recorded in our study are within the normal range reported for the general population(Reference Kritchevsky and Chen36), although lower concentrations have been reported by other authors(Reference Mannarino, Pirro and Cortese37–Reference Jones, Vanstone and Raeini-Sarjaz40). Previous reports have described increments in serum campesterol and sitosterol of 8·4–38·8 and 26·7 %, respectively, while others have observed no variations in the levels of these sterols following the ingestion of a low-fat milk-based beverage. The fact that our study population was hypercholesterolaemic and that the amount and duration of PS consumption were smaller than those in the above-mentioned studies ( < 2 g/d and < 3 months) could explain these differences.
Measurement of serum PS has revealed that campesterol and sitosterol concentrations increase during the consumption of PS independently of diet, suggesting that dietary cholesterol has no effect on the absorption of PS. Due to cholesterol being absorbed via the same pathway as PS(Reference Trautwein, Duchateau and Lin41), we expect the stronger competition due to a higher cholesterol intake to reduce the amount of PS in circulation. However, it has been shown that exogenous cholesterol makes a very slight contribution to the level of cholesterol in the blood, reaching 20 % of that of endogenously synthesised cholesterol. Moreover, the absorption of cholesterol and PS varies in the different parts of the intestine(Reference Trautwein, Duchateau and Lin41). Therefore, a change in dietary cholesterol is unlikely to induce a change in PS bioavailability.
It is worth noting that campesterol seems to be absorbed to a greater degree than sitosterol; though the level of the former is about four times less than that of the latter in milk, its increase in serum is approximately twice as great. In addition, clearance of campesterol is lower than that of sitosterol, indicating that the two PS possess different kinetics(Reference Sudhop, Sahin and Lindenthal42).
The precise mechanism by which PS reduce plasma carotenoid concentration is not well defined, but it has been suggested that their incorporation into the mixed micelles(Reference Brufau, Canela and Rafecas43) and their variable bioavailability, which is influenced by many dietary and physiological factors(Reference Yonekura and Nagao44), could explain the conflicting results reported in the literature.
In our study, β-carotene and lycopene serum concentrations dropped following PS intake when the patients followed their regular diet, whereas oxygenated carotenoid levels (lutein and β-cryptoxanthin) were not modified. This may be due to the distribution of these antioxidants in the mixed micelles (surface core). It is thought that apolar hydrocarbon carotenoids (β-carotene and lycopene) are solubilised in the core of the mixed micelles, whereas oxygenated carotenoids are located mainly on the surface(Reference Borel, Grolier, Armand and Partier45). This suggests that PS replace not only cholesterol from the core of the mixed micelles but also other compounds present in the micelles. Most reports associate intake of PS with a decrease in the levels of β-carotene(Reference Colgan, Floyd and Noone11, Reference Judd, Baer and Chen34, Reference Hansel, Nicolle and Lalanne39, Reference Noakes, Clifton and Doornbos46, Reference Polagruto, Wang-Polagruto and Braun47), whereas an additional daily serving of fruits and vegetables (with high levels of carotenoids) or products enriched with both carotenoids and PS ameliorates any reductions in plasma carotenoid concentration induced by PS(Reference Brufau, Quílez and Canela8–Reference Colgan, Floyd and Noone11). This is compatible with the present results, and reinforces the idea that the enrichment of diet with fruits and vegetables has a positive effect on antioxidant levels.
We should point out that results may change over long periods of intake of PS, which represents a limitation with respect to the conclusions of the present study. Furthermore, the present results do not exclude a greater effect of diets richer in cholesterol and saturated fat than that followed by our population. It should also be emphasised that this type of diet is not recommended for mildly hypercholesterolaemic patients.
To summarise, the present study demonstrates that the positive effect of PS on the lipoprotein profile of moderately hypercholesterolaemic patients is not influenced by dietary composition of saturated fat or dietary cholesterol. Moreover, the present results confirm and extend the scope of the positive influence of dietary therapy based on the consumption of fruits and vegetables on the negative effects that PS exert on carotenoid levels.
Acknowledgements
We kindly thank M. M. Jarabo, L. García-Pareja, A. López-Ruiz, B. Normanly and I. Soria-Cuenca for their contribution to the present study. We also thank all the volunteers who participated. The study was supported by Unilever and grants UVEG2004-ALIFUNC-01-03 from the University of Valencia, 027/2005 from the Valencian School for Health Studies, ACOMP/2009/009 from the Regional Ministry of Education of Valencian Community, GE 049/09 from the Regional Ministry of Health of Valencian Community, and PI07/0091, PI09/01025 from Fondo de Investigación Sanitaria (FIS) and CONSOLIDER INGENIO 2010 programme, FUNC FOOD CSD2007-00063. V. M. V. and M. R. are the recipients of FIS contracts CP07/00171 and CA07/00366, respectively. None of the authors has any personal or financial conflict of interest. A. H.-M., R. F. and M. L. M.-T. designed the research; C. M., C. B., M. J. L., A. A., R. B. and R. L. conducted the research; C. B., V. M. V. and M. R. performed the statistical analysis; C. B., V. M. V. and M. R. prepared the manuscript; and A. H.-M. and M. J. L. were responsible for its final content. All authors have read and approved the final manuscript.







