Cognitive decline and dementia have a significant impact on the independence and quality of life of sufferers and carers, and research into modifiable risk factors is paramount.
The most prevalent form of dementia is Alzheimer's disease (AD) which accounts for up to 70 % of cases(Reference Smith1), with other common forms including dementia with Lewy bodies, frontotemporal dementia and vascular dementia(Reference Camicioli and Rockwood2). Risk factors for dementia include advanced age, genetics, low educational level as well as CVD, and its component vascular risk factors(Reference Smith1, Reference Camicioli and Rockwood2). The most important known genetic risk factor for the development of dementia is possession of the apoE4 allele which substantially increases the risk of AD by two to three times(Reference Corder and Beaumont3).
Poor vitamin B12 status has been linked to cognitive decline for at least 50 years(Reference Droller and Dossett4) but the role of vitamin B12 in this process is not clear. Vitamin B12 deficiency causes neurological degeneration with demyelination of the spinal cord and some initial studies have described a reversible dementia related to vitamin B12 deficiency(Reference Martin, Francis and Protetch5–Reference Osimani, Berger and Friedman10).
A link between vitamin B12 status and cognitive decline is biologically plausible. Vitamin B12 is required for DNA and myelin synthesis, and it is a cofactor for the methylation of total homocysteine (tHcy) to methionine and for the conversion of methylmalonyl-CoA to succinyl-CoA(Reference Reynolds11). Both tHcy and methylmalonic acid (MMA) accumulate while holotranscobalamin (holoTC), the active transport protein carrying vitamin B12, decreases with inadequate vitamin B12 status.
The suggested aetiologies behind any association between cognitive decline related to low vitamin B12 status include inadequate methylation in the central nervous system(Reference Morrison, Smith and Kish12), the accumulation of tHcy and/or MMA(Reference McCracken, Hudson and Ellis13), the effects on the cerebral vasculature and brain atrophy and white matter damage(Reference Smith1).
Recent reviews have linked high tHcy concentrations with an increased risk of cognitive decline and dementia(Reference Wald, Kasturiratne and Simmonds14–Reference Ho, Cheung and Fu18); however, it is not known whether tHcy is a marker of disease or a causative factor in the dementing process. A large number of studies have investigated the association between vitamin B12 status and cognition, but there is no consistency in outcomes and the independent role of vitamin B12 status in the development of neurocognitive decline is uncertain.
Cross-sectional studies showed positive associations between serum vitamin B12 and scores on cognitive tests, but cohort studies did not(Reference Balk, Chung and Raman19, Reference Vogel, Dali-Youcef and Kaltenbach20). Balk et al. (Reference Balk, Chung and Raman19) assessed seven cohort studies, with only two showing associations of improved cognition with higher serum vitamin B12 concentrations. In three recent reviews, it was found that seven of fifteen, one of six and zero of three cohort studies showed associations between low serum vitamin B12 status and increased rates of cognitive decline(Reference Vogel, Dali-Youcef and Kaltenbach20–Reference Rosenberg22). These reviews, although finding no association between vitamin B12 and cognitive decline, have highlighted the methodological limitations of studies(Reference Werder15, Reference Balk, Chung and Raman19–Reference Raman, Tatsioni and Chung21).
The most recent systematic reviews include studies published before 2007(Reference Balk, Chung and Raman19, Reference Raman, Tatsioni and Chung21) and only include studies assessing vitamin B12 concentrations. Other studies that utilise sensitive biomarkers of vitamin B12 status, holoTC and MMA, have since been published. The aim of the present systematic literature review was to provide an up-to-date identification and critical appraisal of published studies of the longitudinal association between vitamin B12 status and the spectrum of cognitive decline and dementias in older adults.
Methods
A review protocol including library search strategy, inclusion and exclusion criteria, use of data extraction and quality tool templates was determined before the review.
Search strategy
A literature search was undertaken on 6 June 2010 and an update was performed on 12 August 2011. Database searches included Medline (1950–present), Pre-medline, Psyc INFO (1806–present), all EBM Reviews (ACP Journal Club, DARE and CCTR (1991–present), and Cochrane DSR (2005–present)), Cinahl (1982–present) and Embase (1980–present). Both medical subject headings and text words were used for dietary supplement terms, vitamin B12 and biomarkers (e.g. homocysteine, methylmalonic acid and holotranscobalamin) and common terms for cognition and dementia (e.g. cognition, dementia, memory and Alzheimer's disease) with appropriate truncation. The full search terms for the Medline database can be seen in the Appendix. Filters to limit publications to English language and to middle- and older-aged human subjects were applied at the end of each database search if available.
Study selection
Citations from each literature database search were downloaded into referencing software Endnote X1. Titles and abstracts were assessed for inclusion criteria. Inclusion criteria were prospective cohort studies assessing the association of serum vitamin B12, MMA or holoTC and cognition or dementia in older adults. Studies that measured vitamin B12 status but only reported a lack of association in the text of the results were also included. All other studies were excluded. The reference lists of included studies and reviews identified in the literature search were checked for relevant articles. Studies with more than one publication from the same subject sample were reported as one study and studies reporting more than one outcome, e.g. dementia and AD, were reported separately.
Data extraction and synthesis
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2009 statement was used to guide the conduct and reporting of the present systematic review(Reference Liberati, Altman and Tetzlaff23). Data were extracted by two reviewers using the American Dietetic Association's Evidence Analysis data extraction template(24), with adjudication by a third reviewer if required. For each study, the following information was extracted: study description; participant selection and characteristics (including age, disease characteristics, baseline cognitive status and method of diagnosis); inclusion and exclusion criteria; subject numbers and withdrawals; statistical methods used; ascertainment and length of exposure; outcome measure characteristics; funding arrangements. No attempt was made to contact authors of included studies as only published data were included. A meta-analysis was deemed inappropriate due to the variability of baseline populations, cognitive and vitamin B12 status tests and reported outcome statistics.
Study quality
The quality of the studies was assessed by two reviewers using the American Dietetic Association Study Quality criteria guidelines(24), with a third reviewer resolving any disagreements. The quality assessment tool comprises ten questions related to the soundness and reporting of study design, methods and results and returns one of three scores of ‘positive’, ‘neutral’ or ‘negative’. An overall positive score requires that the majority of the questions be answered ‘yes’ and that four essential validity questions be answered ‘yes’. These questions assess bias in subject selection, comparability of subject groups, intensity and duration of exposure and the validity and reliability of the outcome measurements. For the present review, two of these questions were not able to be answered as the information is not known, so the questions were designated as ‘not applicable’ as outlined in the National Institute for Health and Clinical Excellence (NICE) guideline development manual(25). These questions (numbers 6 and 7) relate to the ‘intensity and duration of exposure’ to low vitamin B12 status required to show an effect and the ‘validity and reliability of outcome measures’, i.e. of cognitive assessment tools. A neutral score is given if the answers to the four essential validity questions do not indicate that the study is exceptionally strong and a negative is awarded if most (six or more) of the answers to the validity questions are ‘No’.
Results
A total of 3772 citations were downloaded for review with thirty-five cohort studies fulfilling the selection criteria. Fig. 1 illustrates the study selection process.
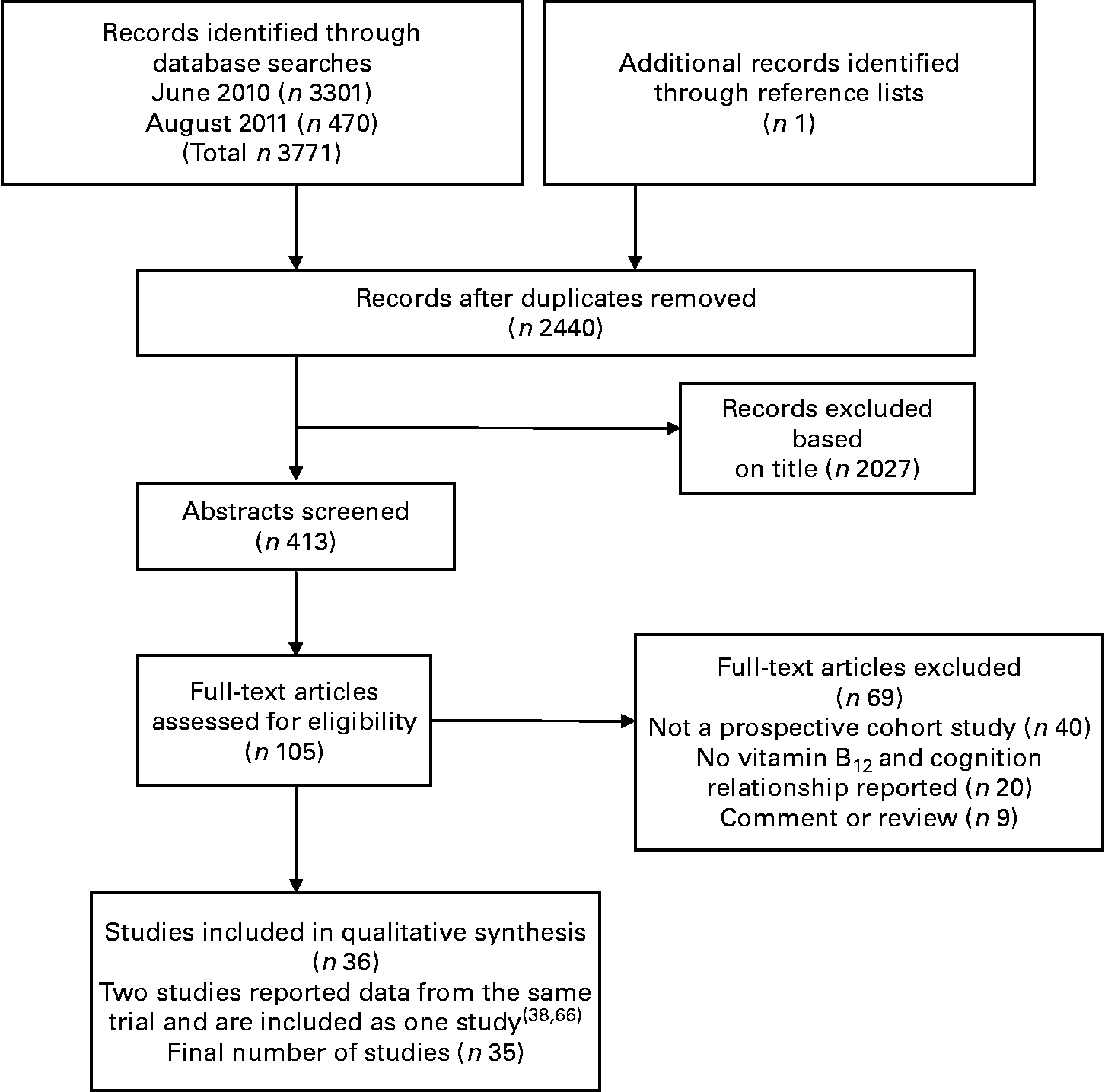
Fig. 1 Flow chart of literature search and study selection.
Summary of included studies
The studies were from ten countries encompassing the areas of North America (11), Europe (16), the UK (3), Asia (4) and Israel (1), and assessed a total of 14 325 subjects. Subject ages ranged from 47 to 101 years with a mean sample size of 409 subjects (median 271; range 24–1405), followed for a mean of 5·4 years (median 4·4 years; range 0·5–35 years).
Vitamin B12 status was determined predominantly by serum vitamin B12 concentrations alone (thirty-one studies). However, four studies used MMA(Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27) and/or holoTC(Reference Clarke, Birks and Nexo26, Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29) and one study used MMA in combination with serum vitamin B12 (Reference Tangney, Tang and Evans27). The majority of studies used multiple regression to assess the association of serum vitamin B12 and cognition with three studies applying cut-points of 110–251 pmol/l(Reference Crystal, Ortof and Frishman30–Reference Ravaglia, Forti and Maioli32). Moreover, seven studies reported only unadjusted data(Reference Crystal, Ortof and Frishman30, Reference Annerbo, Wahlund and Lökk33–Reference Tu, Huang and Chen38).
Cognitive decline was assessed in seventeen studies(Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27, Reference Eussen, Ferry and Hininger35, Reference La Rue, Koehler and Wayne37, Reference Kim, Kim and Shin39–Reference Dufouil, Alperovitch and Ducros51), the development of dementia or AD was determined in thirteen studies(Reference Hooshmand, Solomon and Kareholt28–Reference Annerbo, Wahlund and Lökk34, Reference Haan, Miller and Aiello40, Reference Zylberstein, Lissner and Bjorkelund52–Reference Bowirrat, Friedland and Farrer56) and five studies assessed cognitive deterioration in subjects with diagnosed dementia or AD(Reference Huang, Chang and Lui36, Reference Tu, Huang and Chen38, Reference Oulhaj, Refsum and Beaumont57–Reference Small, Viitanen and Winblad59). Futhermore, five studies reported two outcomes, one for dementia and one for AD(Reference Kivipelto, Annerbo and Hultdin29–Reference Ravaglia, Forti and Maioli32, Reference Seshadri, Beiser and Selhub54).
Quality assessment and outcome
Quality assessment found that twenty-one studies were positive, ten were neutral and four were negative. Of the twenty-one positive studies with a low risk of bias, seven studies found positive associations between vitamin B12 status and cognitive decline(Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27, Reference Elias, Sullivan and D'Agostino42, Reference Tucker, Qiao and Scott47), dementia(Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31) or AD(Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31) and fourteen studies did not(Reference Ravaglia, Forti and Maioli32, Reference Annerbo, Wahlund and Lökk34, Reference Kim, Kim and Shin39, Reference Kado, Karlamangla and Huang41, Reference Teunissen, Blom and Van Boxtel43–Reference Kang, Irizarry and Grodstein46, Reference Garcia, Haron and Pulman50, Reference Zylberstein, Lissner and Bjorkelund52–Reference Seshadri, Beiser and Selhub54, Reference Oulhaj, Refsum and Beaumont57, Reference Ravaglia, Forti and Maioli60). In addition, nineteen(Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27, Reference Wang, Wahlin and Basun31, Reference Ravaglia, Forti and Maioli32, Reference Annerbo, Wahlund and Lökk34, Reference Kim, Kim and Shin39, Reference Kado, Karlamangla and Huang41–Reference Tucker, Qiao and Scott47, Reference Garcia, Haron and Pulman50, Reference Zylberstein, Lissner and Bjorkelund52–Reference Seshadri, Beiser and Selhub54, Reference Oulhaj, Refsum and Beaumont57, Reference Ravaglia, Forti and Maioli60) of the twenty-one studies of positive quality used serum vitamin B12 with three finding significant associations with cognitive decline(Reference Tangney, Tang and Evans27, Reference Elias, Sullivan and D'Agostino42, Reference Tucker, Qiao and Scott47). All four studies using the markers holoTC and/or MMA were of positive quality and all showed significant associations with cognitive decline, dementia or AD(Reference Clarke, Birks and Nexo26–Reference Kivipelto, Annerbo and Hultdin29).
Neuropsychological assessment was performed using standardised tools. Dementia and AD diagnosis were defined by the Diagnostic and Statistical Manual of Mental Disorders (third and fourth edition), the Mattis Dementia Rating Scale and the Mental Deterioration Battery of the Neuroepidemiology Branch of the National Institute of Neurological Disorders and Stroke criteria. Studies assessing cognitive decline used a variety of neuropsychological tool sets or individual tests, singly or in combination, and included the Mini Mental State Examination (MMSE) Score, the Wechsler Adult Intelligence Scale subsets, the Boston Naming Test, the Stroop Colour Word Test and the Wechsler Memory Scale.
Cognitive decline and vitamin B12 status in non-demented subjects
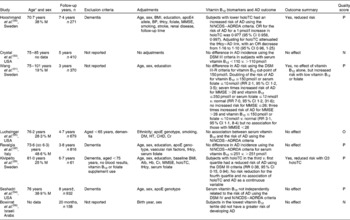
The association of cognitive decline with vitamin B12 status in non-demented subjects was assessed in six studies (Table 1). Haan et al. (Reference Haan, Miller and Aiello40) found that vitamin B12 was associated with an increased hazard ratio for the development of dementia or cognitive impairment (hazard ratio 1·07, 95 % CI 1·02, 1·11). Interactions between vitamin B12 and tHcy were also found, with vitamin B12 modifying the association between tHcy and the development of dementia or cognitive impairment. For those in the first tertile of serum vitamin B12 ( < 340 pg/ml), the rates of dementia or cognitive impairment associated with tHcy were higher, and for those in the third tertile of vitamin B12 ( ≥ 498 pg/ml), the rates of dementia or cognitive impairment were lower compared with the second tertile(Reference Haan, Miller and Aiello40). Elias et al. (Reference Elias, Sullivan and D'Agostino42) found a small significant association of serum vitamin B12 with the Wechsler Memory Scale composite score and its subsets. However, four studies showed no associations of cognitive decline and serum vitamin B12(Reference Kim, Kim and Shin39, Reference Kado, Karlamangla and Huang41, Reference Teunissen, Blom and Van Boxtel43, Reference McCaddon, Hudson and Davies44) after a mean follow-up period of 5·1 years.
Table 1 Relationship between vitamin B12 and cognitive decline in non-demented subjects (Mean values, ranges and medians)

M, male; MMSE-K, Mini Mental State Examination – Korean; P, positive; CIND, cognitive impairment no dementia; tHcy, total homocysteine; HR, hazard ratio; O, neutral; WAIS-R, Wechsler Adult Intelligence Scale-Revised; PD, Parkinson's disease; MMSE, Mini Mental State Examination Score; HT, hypertension.
* Mean or range (years).
† Median.
Cognitive decline and vitamin B12 status in subjects with unspecified cognition
The association of cognitive decline in subjects with normal or unspecified cognition was assessed in eleven studies using multiple tests of cognition. Of these eleven studies, four found an association of vitamin B12 status and at least one test of cognition (Table 2). Tucker et al. (Reference Tucker, Qiao and Scott47) studied male subjects for 3 years and found a positive association of serum vitamin B12 and construction praxis (a neuropsychological assessment tool), but no other cognitive measures. Further, two studies followed subjects for 6 years, with Nurk et al. (Reference Nurk, Refsum and Tell49) finding a trend for increasing risk of memory deficit with decreasing quintiles of baseline vitamin B12 and the Kendrick Object Learning Test, and Tangney et al. (Reference Tangney, Tang and Evans27) found associations between higher serum vitamin B12 and a slower decline in memory, and a faster decline in memory with higher MMA concentrations. Clarke et al. (Reference Clarke, Birks and Nexo26) followed 472 subjects for 10 years and reported no association of cognitive decline (MMSE score) with serum vitamin B12 concentrations, but found that a doubling of holoTC or MMA was associated with a slower and faster cognitive decline, respectively. Moreover, seven studies(Reference Eussen, Ferry and Hininger35, Reference La Rue, Koehler and Wayne37, Reference van den Kommer, Dik and Comijs45, Reference Kang, Irizarry and Grodstein46, Reference Mooijaart, Gussekloo and Frolich48, Reference Garcia, Haron and Pulman50, Reference Dufouil, Alperovitch and Ducros51) found no associations between serum vitamin B12 and individual or composite cognition scores or the MMSE after a follow-up ranging between 2·3 and 6·0 years.
Table 2 Relationship between vitamin B12 and cognitive decline in subjects with unspecified cognition (Mean values and ranges)

M, male; Cr, creatinine; HT, hypertension; ACT, α-1-antichymotrysin; tHcy, total homocysteine; MMSE, Mini Mental State Examination; P, positive; MMA, methylmalonic acid; holoTC, holotranscobalamin; BP, blood pressure; F, female; DM, diabetes; TC, total cholesterol; HRT, hormone replacement therapy; PA, physical activity; FA, folic acid; O, neutral; Q, quintile; N, negative.
* Mean or range (years).
† For global score, n 389; for telephone interview for cognitive status, n 391; for verbal score, n 391.
‡ For construction praxis, n 280–284; for language, n 239–243; for working memory, n 236–240; for recall memory, n 235–239; for MMSE n 271–275.
Development of dementia in subjects with mild cognitive impairment
The development of dementia or AD in subjects with cognitive impairment was assessed in three studies. Of these, one study from Sweden(Reference Annerbo, Wahlund and Lökk33) analysed unadjusted data and found that females who developed AD had lower baseline vitamin B12 concentrations compared with those without AD, but no effect was found in the other two studies assessing subjects with cognitive impairment(Reference Annerbo, Wahlund and Lökk34, Reference Ravaglia, Forti and Maioli60) (Table 3).
Table 3 Relationship between vitamin B12 and dementia or Alzheimer's disease (AD) in subjects with mild cognitive impairment (Mean values and ranges)

M, male; MCI, mild cognitive impairment; P, positive; MMSE, Mini Mental State Examination; ADL, activities of daily living; HR, hazard ratio, O, neutral.
* Mean or range (years).
Development of dementia in subjects with no dementia at baseline
A further eight studies assessed non-demented subjects and the development of dementia (Table 4). Of these, three studies found associations between serum vitamin B12 or holoTC and the development of dementia(Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31, Reference Haan, Miller and Aiello40), and five found no associations(Reference Crystal, Ortof and Frishman30, Reference Ravaglia, Forti and Maioli32, Reference Zylberstein, Lissner and Bjorkelund52–Reference Seshadri, Beiser and Selhub54). The development of AD was assessed in eight studies, with three finding associations with holoTC or serum vitamin B12 alone or in combination with low serum folate concentrations(Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31) (Table 5). Wang et al. (Reference Wang, Wahlin and Basun31) detected no association between serum vitamin B12 or folate and AD alone but found a doubling of risk of AD with serum vitamin B12 ≤ 150 pmol/l or serum folate ≤ 10 nmol/l compared with normal concentrations. A 7-fold additional risk was found for subjects with a MMSE >26 and serum vitamin B12 ≤ 250 pmol/l or folate ≤ 12 nmol/l v. normal(Reference Wang, Wahlin and Basun31). Kivipelto et al. (Reference Kivipelto, Annerbo and Hultdin29) found that subjects with baseline holoTC concentrations in the third, compared with the first, quartile had a reduced relative risk for the development of AD after nearly 7 years (relative risk 0·38, 95 % CI 0·15, 0·94). However, no difference between the fourth and first quartiles was found(Reference Kivipelto, Annerbo and Hultdin29). Hooshmand et al. (Reference Hooshmand, Solomon and Kareholt28) also found that subjects with lower holoTC had a reduced OR of 0·977 for each 1 pmol/l increase in holoTC.
Table 4 Relationship between vitamin B12 and development of dementia in subjects without dementia at baseline (Mean values, medians and ranges)

M, male; MMSE, Mini Mental State Examination; TC, total cholesterol; BP, blood pressure; DSM III-R, Diagnostic and Statistical Manual of Mental Disorders (third edition revised); P, positive; tHcy, total homocysteine; holoTC, holotranscobalamin; Alb, albumin; Cr, creatinine; RR, relative risk; Q, quartile; PA, physical activity; MMSE-K, Mini Mental State Examination – Korean; CIND, cognitive impairment no dementia; HR, hazard ratio; O, neutral; NINCDS–ADRDA, National Institute of Neurological and Communicative Disease and Stroke–Alzheimer's Disease and Related Disorders Association; FA, folic acid; N, negative.
* Mean or range (years).
† Median.
Table 5 Relationship between vitamin B12 and the development of Alzheimer's disease (AD) in subjects with no dementia (Mean values, medians and ranges)

M, male; MMSE, Mini Mental State Examination; BP, blood pressure; tHcy, total homocysteine; holoTC, holotranscobalamin; NINCDS–ADRDA, National Institute of Neurological and Communicative Disease and Stroke–Alzheimer's Disease and Related Disorders Association; P, positive; DSM III and DSM IV, Diagnostic and Statistical Manual of Mental Disorders (third and fourth edition); N, negative; DSM III-R, Diagnostic and Statistical Manual of Mental Disorders (third edition Revised); RR, relative risk; DM, diabetes; HT, hypertension; Cr, creatinine; O, neutral; Alb, albumin.
* Mean or range (years).
† Median.
Cognitive decline in subjects with existing dementia
The association of baseline vitamin B12 and cognitive deterioration was assessed in five studies of subjects with dementia (Table 6). No associations between vitamin B12 and cognition were observed(Reference Huang, Chang and Lui36, Reference Tu, Huang and Chen38, Reference Oulhaj, Refsum and Beaumont57–Reference Small, Viitanen and Winblad59).
Table 6 Relationship of vitamin B12 and cognitive decline in subjects with dementia or Alzheimer's disease (AD) (Mean values and ranges)

M, male; CAMCOG, Cambridge Cognitive Assessment; P, positive; O, neutral; MMSE, Mini Mental State Examination; Cr, creatinine; LFT, liver function tests; FA, folic acid.
* Mean or range (years).
Discussion
The present review finds that there is insufficient evidence to determine whether vitamin B12 status is associated with cognitive decline or dementia. The assessment of the thirty-five cohort studies or, more particularly, of the twenty-one studies of positive quality, does not support a role for the association of serum vitamin B12 concentrations in the aetiology of cognitive impairment or dementia. Of the twenty-one studies of positive quality, seven found significant associations between vitamin B12 status and cognitive decline(Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27, Reference Elias, Sullivan and D'Agostino42, Reference Tucker, Qiao and Scott47), dementia(Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31) or AD(Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31). An interesting finding of the review is that the markers of vitamin B12 status with greater specificity, i.e. holoTC and MMA, showed consistent results with all four studies finding associations with cognitive decline(Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27), dementia(Reference Kivipelto, Annerbo and Hultdin29) and AD(Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29). However, the study by Kivipelto et al. (Reference Kivipelto, Annerbo and Hultdin29) found associations of holo TC and dementia development only for the third quartile.
The subject's age at recruitment and the length of time of exposure to a low vitamin B12 status for a change (if any) in cognition to be noted are not known; however, cognitive impairment and dementia generally develop over many years and studies of inadequate duration may not show any effect. The median duration of studies was 4 years, with only seven of the thirty-five studies assessing cognition for more than 6 years. The majority of studies recruited subjects aged greater than 75 years at baseline, with only two studies recruiting subjects from a midlife stage, with a follow-up of 35 and 6 years, respectively; however, neither showed any association with serum vitamin B12 and cognition(Reference Teunissen, Blom and Van Boxtel43, Reference Zylberstein, Lissner and Bjorkelund52). Of the nine studies recruiting subjects from the seventh decade or earlier, five(Reference Annerbo, Wahlund and Lökk33, Reference Haan, Miller and Aiello40, Reference Elias, Sullivan and D'Agostino42, Reference Tucker, Qiao and Scott47, Reference Nurk, Refsum and Tell49) found associations between cognition and vitamin B12 status, while only five(Reference Clarke, Birks and Nexo26–Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31) of the twenty-five studies commencing in later life found associations. The effect of vitamin B12 status is likely to commence in midlife with a long period for disease development, hence the baseline age of subjects needs consideration(Reference Smith1).
In order to ascertain whether any effect exists, the correct diagnosis of vitamin B12 status must be made; however, there is no ‘gold standard’ for the determination of vitamin B12 status and each diagnostic assay has limitations. High vitamin B12 concentrations generally indicate sufficiency, but the interpretation of the lower concentrations of vitamin B12 concentrations is unclear(Reference O'Leary and Samman61, Reference Green62). Vitamin B12 is carried by two proteins, with the active form of holoTC making up only 20–30 % of the total serum vitamin B12 measured. Studies have shown that the determination of the active form of vitamin B12, holoTC and/or MMA, an indicator of tissue stores, improves the prediction of low vitamin B12 status(Reference Green62), and a recent review has found the use of serum vitamin B12 concentrations alone unreliable in diagnosing a vitamin B12 deficiency(Reference Willis, Elshaug and Milverton63). Of the thirty-two studies that assessed serum vitamin B12, ten showed associations with cognition, while four of four studies assessing the newer and more specific markers of vitamin B12 status showed an effect. Of the two studies that assessed holoTC or MMA in addition to serum vitamin B12 (Reference Clarke, Birks and Nexo26, Reference Tangney, Tang and Evans27), only the study by Tangney et al. (Reference Tangney, Tang and Evans27) showed an association with serum vitamin B12, and this was in a folate-fortified population using neuropsychological assessment tools and after a follow-up of 6 years. Futhermore, one cohort study published after the literature evaluation for the present review was completed has been located. This study assessed non-demented subjects from the Cardiovascular Risk Factors, Aging and Dementia study(Reference Hooshmand, Solomon and Kareholt64) and supports the association of low holoTC concentrations with a decline in cognition.
The present review of papers indicates that the direction of any likely effect is consistent, with low vitamin B12 status being associated with increased rates of dementia or cognitive impairment. There was one study showing that both low and high serum vitamin B12 concentrations were associated with an increased risk of dementia or cognitive impairment. However, this result was confounded by an interaction effect between vitamin B12 and tHcy that was not included in the reported statistical model(Reference Haan, Miller and Aiello40).
The cognitive assessment tools that are chosen also need to have the sensitivity to detect changes in cognition affected by low vitamin B12 status. The majority of studies in the present review used neuropsychological tests for the detection of cognitive decline or dementia. However, nine studies used the MMSE alone or in combination with other cognitive tests. The MMSE is a validated screening, rather than a diagnostic tool, and, as such, may be less sensitive to cognitive change(Reference Folstein, Folstein and McHugh65). Vitamin B12 status was found to be associated with the MMSE in only one study, and this study used vitamin B12 markers with greater specificity, i.e. holoTC and MMA(Reference Clarke, Birks and Nexo26). A combination of the MMSE and other cognitive tests was used in three studies(Reference Tucker, Qiao and Scott47, Reference Mooijaart, Gussekloo and Frolich48, Reference Dufouil, Alperovitch and Ducros51), with one finding an association with vitamin B12(Reference Tucker, Qiao and Scott47) but none with the MMSE. The sensitivity of cognitive tests to changes in tHcy has been investigated(Reference Schafer, Glass and Bolla66) but there is little information on the sensitivity of cognitive tests to vitamin B12 status, and the development of this knowledge is an important area of research. Studies using brain scans to assess brain atrophy and white matter changes have shown associations with vitamin B12 status and may be a more sensitive outcome measure than tests of cognition(Reference Smith, Smith and de Jager67–Reference de Lau, Smith and Refsum69).
The study populations assessed in the present review varied with populations drawn from ten countries, with differing levels of cognitive decline, chronic disease profiles and folate fortification strategies. Some studies excluded subjects with dementia or cognitive impairment while others included all subjects without assessment of cognition. Baseline vitamin B12 concentrations were not always described as a number of studies were primarily assessing the effect of tHcy on cognition. All measures of vitamin B12 status are confounded by diseases such as renal and liver disease(Reference Green62), and the adjustment for confounders based on the chronic disease profile, e.g. stroke and renal diseases, is required to ensure that the true effect of vitamin B12 status can be seen.
Numerous genes have been implicated in the development of cognitive decline and dementia(Reference Payton70), with the most common genetic polymorphism being the apoE4 genotype which doubles the risk of AD development(Reference Raber, Huang and Ashford71). Low vitamin B12 concentrations have been found to increase the risk of cognitive decline in apoE4 carriers in some(Reference Bunce, Kivipelto and Wahlin72, Reference Bunce, Kivipelto and Wahlin73) but not all studies(Reference Dufouil, Alperovitch and Ducros51, Reference Brown, Huang and Karlamangla74). Of the studies reviewed, eight(Reference Clarke, Birks and Nexo26, Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29, Reference Ravaglia, Forti and Maioli32, Reference Elias, Sullivan and D'Agostino42, Reference Nurk, Refsum and Tell49, Reference Seshadri, Beiser and Selhub54, Reference Luchsinger, Tang and Shea55) controlled for apoE4 in statistical analysis, with five(Reference Clarke, Birks and Nexo26, Reference Hooshmand, Solomon and Kareholt28, Reference Kivipelto, Annerbo and Hultdin29, Reference Elias, Sullivan and D'Agostino42, Reference Nurk, Refsum and Tell49) showing associations of vitamin B12 status and cognitive decline after adjustment for confounders. Due to the potential impact of genotype on the risk of disease, known genetic traits must be controlled for in any analysis assessing the role of vitamin B12 status.
Higher folate status has been associated with improved cognition(Reference Smith1), but in subjects with low vitamin B12 status, high serum folate concentrations have been associated with increased concentrations of MMA and tHcy(Reference Selhub, Morris and Jacques75) and increased cognitive decline(Reference Morris, Jacques and Rosenberg76). In the present review, half (four out of seven) of the studies performed in folate-fortified subjects found negative associations between vitamin B12 status and cognitive decline(Reference Tangney, Tang and Evans27, Reference Haan, Miller and Aiello40, Reference Elias, Sullivan and D'Agostino42, Reference Tucker, Qiao and Scott47) compared with approximately one-third of studies overall(Reference Clarke, Birks and Nexo26–Reference Kivipelto, Annerbo and Hultdin29, Reference Wang, Wahlin and Basun31, Reference Annerbo, Wahlund and Lökk33, Reference Haan, Miller and Aiello40, Reference Elias, Sullivan and D'Agostino42, Reference Tucker, Qiao and Scott47, Reference Nurk, Refsum and Tell49). This is consistent with the postulated detrimental effect of high folate concentrations on the progression of cognitive impairment(Reference Smith, Kim and Refsum77).
The studies may have been underpowered to show an effect of vitamin B12 with only ten studies having sample sizes of more than 500. Power calculations were generally not performed or reported; however, many studies cited inadequate power as a possible explanation for the lack of effect seen. The population standard deviation for serum vitamin B12 and biomarkers can be large and any effect is only likely to be seen in subjects with a low vitamin B12 status which is found in 10–20 % of older people(Reference Carmel, Green and Rosenblatt78), indicating that the number of study subjects may need to be larger.
The present systematic review has limitations, as it assessed only cohort studies and did not attempt to locate unpublished results or include publications not in English. A meta-analysis was deemed not appropriate due to the variability of subjects, vitamin B12 status and outcome measures used. The strengths of the study include the inclusion of multiple database and hand searches. The present review included all located studies, including those primarily interested in tHcy, with any reported associations between vitamin B12 and cognition, and included studies with only in-text associations of no effect between vitamin B12 and cognition. This increased the number of included studies and thus reduced reporting bias. The use of a quality tool strengthened the study, but the lack of knowledge of the time frame of cognitive decline or dementia development and the inability to determine vitamin B12 adequacy limited its application.
Future studies should aim to use MMA and/or holoTC as well as serum vitamin B12 and describe the analysis fully, including both significant and non-significant outcome results for any tested cut-points and continuous measures of vitamin B12 status. Studies should be of adequate duration (more than 6 years) and choose sensitive cognitive assessment rather than screening tools. Further research into the sensitivity and specificity of these tests is needed. Where possible, studies should aim to include the assessment and adjustment for confounders of age, sex, smoking, physical activity, socio-economic status, vitamin and genetic factors, and others known to be associated with the increased risk of cognitive decline, e.g. CVD, diabetes and chronic kidney disease.
In summary, current studies examined in the present review do not show a clear link between serum vitamin B12 concentrations and cognitive decline, but these studies were limited by recruitment age, inadequate subject numbers, lack of adjustment for confounders, study duration and, in some studies, the choice of cognitive outcome measure. The biomarkers of vitamin B12 status showed consistent significant associations between lower vitamin B12 status and increased rates of cognitive decline or dementia diagnosis, and these biomarkers deserve more study.
Acknowledgements
F. O'L. completed the literature search, quality assessment, data extraction and interpretation and drafted the manuscript. M. A.-F. assisted with quality assessment and the interpretation of the data, and commented on drafts of the manuscript. S. S. completed the quality assessment, data extraction and interpretation, and commented on drafts of the manuscript. None of the authors has any conflict of interest. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Appendix: Medline search protocol
1. vitamin B?12.mp. or exp Vitamin B 12/
2. vitamin B?12 deficiency.mp. or exp Vitamin B 12 Deficiency/
3. transcobalamin.mp. or exp Transcobalamins/
4. exp homocysteine/ or exp s-adenosylhomocysteine/
5. homocysteine.mp.
6. homocystine.mp.
7. exp Homocystine/
8. exp Hyperhomocysteinemia/
9. hyperhomocysteinemia.mp.
10. hyperhomocysteinaemia.mp.
11. methylmalonic acid.mp.
12. methylmalonate.mp.
13. holotranscobalamin.mp.
14. cognition/ or exp awareness/ or exp comprehension/
15. cognition.mp.
16. cognit*.mp. [mp = title, original title, abstract, name of substance word, subject heading word, unique identifier]
17. dementia.mp. or exp Dementia/
18. exp Dementia, Vascular/ or exp Dementia, Multi-Infarct/ or Dementia/ or exp Frontotemporal Dementia/
19. memory/ or memory, short-term/ or mental recall/or “recognition (psychology)”/ or “retention (psychology)”/
20. memory.mp. [mp = title, original title, abstract, name of substance word, subject heading word, unique identifier]
21. alzheimer's disease.mp. or exp Alzheimers Disease/
22. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
23. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
24. dietary supplements.mp. or Dietary Supplements/
25. 22 or 24
26. 23 and 25
27. limit 26 to (english language and humans)
28. limit 27 to “middle aged (45 plus years)”