INTRODUCTION
Coxiella burnetii is the infectious agent responsible for Q fever. A manifestation of one of the most infectious organisms to humans, this disease has long been considered a disease associated with particular industries, namely those that bring labourers into close contact with the organism's primary reservoir, domesticated herd animals [Reference Parker, Barralet and Bell1–Reference Woldehiwet3]. Examples include agriculture, animal slaughter, and veterinary practice. National surveillance over the last quarter century has shown that Q fever incidence has more than doubled, and has transitioned to endemicity across parts of the country. However, Q fever is still a rare disease in the USA with less than one case per million people annually [Reference McQuiston4]. A previous report on the largest survey of C. burnetii prevalence yet undertaken in the USA, showed that Mexican Americans were at particular risk for infection [Reference Anderson5]. However, this study only considered the excess burden of disease in the context of basic socioeconomic status and pet ownership. Nevertheless, uncertainty remains regarding this infection's distribution in high-risk occupations in the USA. Moreover this same study population can be used to illuminate such uncertainty, which may ultimately come to bear on the identification of an important occupational hazard. As such, this report seeks to enquire whether or not infection with C. burnetii is differentially experienced by those working in the agricultural industry.
METHODS
To more clearly elucidate the relationship between C. burnetii infection and occupation, this study examined data from the National Health and Nutrition Examination Survey (NHANES), conducted between the years 2003 and 2004 by the National Center for Health Statistics at the Centers for Disease Control and Prevention. Methods describing this national survey have been described previously [6]. Briefly, the survey was designed to obtain nationally representative information on the health and nutritional status of the population of the USA through interviews and direct physical and laboratory examinations. Self-reported health data as well as physiological measures were collected in either the Mobile Examination Center or at the participants' homes. As described in the NHANES laboratory documentation, an enzyme-linked immunosorbent assay (ELISA) was used to initially screen all sera specimens for IgG phase II antibody seropositivity, a marker of acute infection. The ELISA was performed in 96-well flat-bottomed polystyrene microtitre plates with sonicated purified antigens (Pan-Bio, USA). Any sera samples positive by ELISA were tested by immunofluorescence antibody assay (IFA) in order to obtain endpoint titres for IgG to both phase I and phase II antigens. The IFA test was performed by the method of Philip et al. [Reference Philip7] and adapted to C. burnetii (purified phases I and II, strain Nine Mile; Rocky Mountain Laboratories, USA) [6, Reference Péter8]. Serial twofold dilutions of sera were prepared in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 1% normal goat serum. After incubation at 37°C for 30 min, the slides were washed with PBS and normal yolk sac and fluorescein isothiocyanate-conjugated goat anti-human IgG (gamma-chain specific) added at optimal dilution. This was incubated and washed as before. The slides were counterstained using Eriochrome black T and coverslipped with an antifade mounting medium. The wells were examined under 400×magnification and any wells with distinct fluorescence of the organisms were scored as positive [6].
Ethnicity was determined by self-report and was represented by three categories: African Americans, Mexican Americans, and Whites. Birthplace was determined by the answer to the question: ‘Were you born in the United States?’, and was categorized as born in the USA, born in Mexico, or born elsewhere. The poverty income ratio (PIR) was used as the measure of socioeconomic status. The total number of people in the household and the household density (ratio of the number of people in the household to the number of rooms in the household) were household characteristics that were included in the present study. Occupational status was determined by self-report of employment history. Participants described their occupation and this response was categorized according to the industry worked in and the job conducted in that industry. Those who performed regular work in the agricultural industry were listed as agricultural workers. As described previously [Reference Anderson5], there were a total of 6916 individuals sampled from the US population for the 2003–2004 cycle of NHANES. Of these, 5041 agreed to participate in the interview. Of those interviewed, 4742 were examined with 4437 of the examined participants submitting blood samples and having adequate serum to test for antibodies to C. burnetii infection. Because of the substantially smaller sample sizes of the other ethnic groups, the current report included only Mexican Americans, African Americans and White Americans in the analyses, which reduced the sample from 4437 participants to 4152 participants. Finally, a total of 2587 of the 4152 eligible participants had complete data on their occupational experience and these constituted the final study population for this work. Statistical analyses proceeded as follows. Sample population proportions are presented with 95% confidence intervals and means are presented with linearized standard errors. Bivariate associations between infection status and risk factors were assessed using Fisher's exact tests to test differences in the categorical data, and t tests to test differences in the continuous data. Multiple logistic regression was used to assess the relationship between C. burnetii infection and agricultural work, while also controlling for gender, age and PIR. We additionally assessed ethnicity and birthplace. However, all of the infection cases in agricultural workers occurred in US-born White participants. As such additional stratification on ethnicity and/or birthplace, would yield unstable results. Therefore ethnicity and birthplace were not included in the full model in Table 3, although the findings with these factors included are described briefly in the Results section. The PIR was included as a continuous variable to capture more of the variability experienced across the spectrum of socioeconomic status, rather than dichotomizing a classification of living in poverty or not. Three models were fitted to systematically assess these Q fever risk factors. Model 1 evaluated the bivariate relationship between agricultural work and C. burnetii infection. Model 2 assessed the relationship between infection and agricultural work while controlling for gender and age. Finally, model 3 further examined the association between infection and agricultural work, while controlling for gender, age and PIR. The svymean, svytab, and svylogit (for the logistic regression) commands in Stata were used in order to account for the NHANES weighted sampling design. Stata version 10 was the software used for all statistical analyses (StataCorp LP, USA).
RESULTS
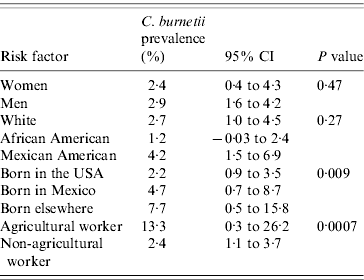
Of the 2587 participants described for this analytical sample, 63 were identified as positive for evidence of C. burnetii infection. There were 98 individuals working in the agricultural industry. The weighted prevalence of infection in the sample overall was 2·9%, which was nearly the same as that reported in the previous report that used much more of the full sample [Reference Anderson5]. The prevalence was 2·7%, 1·2% and 4·2% in the White, African American and Mexican American US population, respectively (P=0·27, Table 1), in this reduced sample. Men had a half percent higher prevalence than women (2·9% vs. 2·4%), which was not significant (P=0·47). Birthplace was associated with significant disparity, with US-born participants experiencing 2·2%, Mexican-born participants experiencing 4·7%, and participants born elsewhere experiencing 7·7% prevalence, respectively (P=0·009). However, the largest disparity observed in this study was between agricultural workers and those of other occupations (13·3% vs. 2·4%, respectively; P=0·0007), with agricultural workers experiencing approximately six times the occurrence of the general population. Interestingly, as demonstrated in Table 2, there were no significant differences in either economic status or housing characteristics between those who were positive and those who were negative for C. burnetii infection. However, those participants with evidence of C. burnetii infection were older (P=0·006) as expected.
Table 1. Categorical risk factors by C. burnetii prevalenceFootnote *
CI, Confidence interval.
* Prevalence estimates of C. burnetii are presented by gender, ethnicity, country of birth, and occupation along with their 95% CIs. P values are based on Fisher's exact tests.
Table 2. Continuous risk factors by C. burnetii statusFootnote *
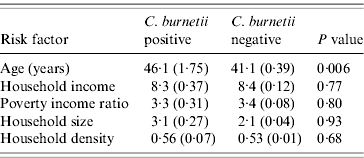
* Mean age (in years), household income (in US dollars), poverty income ratio, household size (number of people), and household density (number of people per rooms) are presented by C. burnetii status along with linearized standard errors. P values are based on Student's t tests.
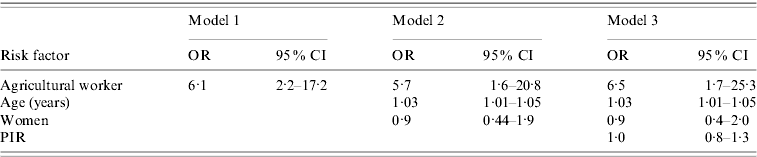
Table 3 displays a comparison of three models of C. burnetii infection. The first presents the bivariate association between C. burnetii infection and agricultural work, with those employed in the agricultural industry six times more likely to demonstrate evidence of infection [odds ratio (OR) 6·1, 95% confidence interval (CI) 2·2–17·2]. Model 2 shows that the strong relationship between infection and agricultural work remained (OR 5·7, 95% CI 1·6–20·8) after controlling for age and gender. In the final model, model 3, the association between infection and agricultural work persisted (OR 6·5, 95% CI 1·7–25·3) after further controlling for PIR. Age itself was also strongly associated with C. burnetii infection (OR 1·03, 95% CI 1·01–1·05), while gender (OR 0·9, 95% CI 0·4–2·0) and PIR (OR 1·0, 95% CI 0·8–1·3) were not. As described in the Methods section, ethnicity and birthplace were not controlled for in the final model in Table 3 since all participants employed by the agricultural industry, who were also positive for C. burnetii infection, were of White ethnicity and born within the USA, so including these can produce unstable results. Nevertheless, adding ethnicity to the model had little effect on the association between infection and agricultural work (OR 5·8, 95% CI 1·4–23·6).
Table 3. Independent effects of risk factors on prevalent C. burnetii Footnote *
OR, Odds ratio; CI, confidence interval; PIR, poverty income ratio.
* Multiple logistic regression was used to obtain odds ratios and 95% confidence intervals for risk of prevalent C. burnetii infection. Three models are compared. Model 1 shows the bivariate association between agricultural work and infection. Model 2 shows the association between agricultural work and infection while controlling for age (in years) and gender (women relative to men). Model 3 shows the association between agricultural work and infection while controlling for age, gender and PIR.
DISCUSSION
This study follows an earlier report [Reference Anderson5] describing the prevalence of Q fever in the USA and its unequal distribution across ethnicity identifying a preponderance in Mexican Americans. Using a reduced sample with available occupational data, this current report demonstrates that agricultural work is strongly associated with C. burnetii infection. These workers were six times more likely to demonstrate prevalent antibodies to the infection. These findings hold implications for the potential occupational hazards experienced by those working in the agricultural industry.
While this is the first report to identify occupational exposure as a potential risk for C. burnetii infection in a nationally representative sample of the US population, it is certainly not the first to demonstrate the relevance of agricultural work for Q fever. C. burnetii is a zoonotic organism that is prevalent in livestock, particularly cattle, goats and sheep [Reference Parker, Barralet and Bell1–Reference Woldehiwet3, Reference Maurin and Raoult9]. These animals act as reservoirs for the organism and are the primary sources of exposure relevant to human transmission [Reference Maurin and Raoult9]. These animals shed the bacterium into the environment, primarily through the birthing process and the release of the placenta. Once shed the organism is extraordinarily robust and can resist wide environmental variation [Reference Maurin and Raoult9]. The agent is typically aerosolized within dust and can become transmitted by air over substantial distances beyond the immediate area of initial shedding by livestock [Reference McQuiston4, Reference Hartzell10]. Because the surveillance of Q fever in the USA has identified very low levels of disease [Reference McQuiston4], the close relationship between agricultural work has been more clearly defined by analyses in Europe and Australia [Reference Hartzell10]. For example, the Brisbane Southside Public Health Unit (Australia) reported that 38% of Q fever incident cases were due to occupational exposure to the agricultural industry, and a further 39% due to incidental outdoor exposures (visits to farms, saleyards) [Reference Palmer11].
The earlier report by Anderson and colleagues identified an important disparity in infection prevalence by ethnicity [Reference Anderson5], which this current report reproduces albeit non-significantly due to the reduced sample size. Moreover, the Anderson et al. study further described the higher prevalence in those participants born outside the USA, and the current report reproduces this strong and significant association even with the reduced sample [Reference Anderson5]. The current report adds to the previous study by identifying a significant occupational exposure, agricultural work, associated with prevalent C. burnetii infection. Moreover, this association was stronger than that between infection and ethnicity or infection and birthplace. Therefore, while ethnic minorities, and Mexican Americans in particular, and those born outside the USA may be at significant risk for Q fever, these risk profiles may pale in comparison to the potential occupational exposures experienced in the agricultural industry. Nevertheless, the cross-sectional nature of the data, and the small sample sizes in sub-populations preclude causal conclusions and simply emphasize an important need for longitudinal studies to more clearly define agricultural-worker risk.
Gender was not significantly associated with infection; however, other studies have shown differences in infection between men and women due to the greater representation of men in agricultural work [Reference Richardus12, Reference Raoult, Marrie and Mege13]. As expected, age was a robust indicator of Q fever prevalence, with each increasing year of age corresponding to a 3% increase in the likelihood of infection. Age has also been shown to be associated with infection in previous work [Reference Pascual-Velasco14]. PIR was consistently not associated with C. burnetii infection unlike the previous report by Anderson et al. [Reference Anderson5]. This is due to the fact that the current study uses the continuous spectrum of PIR rather than the dichotomized classification of poor vs. not poor used in the previous study. It was felt that the diversity of the population under study with respect to occupation would be better represented by the more nuanced classification of economic status rather than a dichotomy.
The study has some limitations that are worth further discussion. First, it must be recognized that this study design is cross-sectional. As such, no direct claim for causality can be made of the association between agricultural work and C. burnetii infection. Another limitation of this study is the small sample size used to analyse an uncommon disease. The sample was limited because antibodies to C. burnetii were only identified for the 2003–2004 cycle of the NHANES survey, and due to the fact that since the study was considering an occupational exposure, all participants were required to have complete data regarding this exposure. Finally, this report is limited by its generalization of occupational exposure within the agricultural industry. This analysis was unable to distinguish between varying levels of exposure in workers employed in different jobs across the agricultural industry. Nevertheless, another study showed that those employed in the agricultural industry with great variation in exposure to livestock, did not show any difference by high, medium or low exposure, and, moreover, all exposure groups had very high prevalence of infection relative to the general population employed in non-agricultural industries [Reference Reid and Malone15].
In conclusion, this study has added to the important work of Anderson and colleagues by showing that agricultural work has the potential to constitute a genuine occupational hazard for Q fever in the USA. More directed longitudinal analyses will be required to support this finding, identify which job-types and associated exposures are associated with greatest risk within the agricultural industry, and to determine appropriate interventions for workers.
DECLARATION OF INTEREST
None.





