Postprandial hypotension (PPH), defined as a decrease in systolic blood pressure (SBP) of ≥ 20 mmHg within 2 h of the start of a meal(Reference Jansen and Lipsitz1), occurs frequently in older adults and can result in significant morbidity, including an increased risk of falls and syncope(Reference Jansen and Lipsitz1–Reference Aronow and Ahn3), coronary events, stroke and increased mortality(Reference Aronow and Ahn4). One approach to the prevention and treatment of PPH may be to alter the type of food eaten in favour of macronutrients, which minimise the fall in blood pressure. In the elderly, there is some evidence that carbohydrate ingestion reduces postprandial blood pressure more than other macronutrients(Reference Jansen and Lipsitz1, Reference Potter, Heseltine and Hartley5). Reports of the effects of fat ingestion on blood pressure in older subjects have been inconsistent, with some studies finding no fall(Reference Heseltine, Potter and Hartley6–Reference Sidery, Macdonald and Cowley9) or an increase(Reference Potter, Heseltine and Hartley5) or a delayed but similar fall in blood pressure compared with those of carbohydrate ingestion(Reference Visvanathan, Horowitz and Chapman10). If fat ingestion has a less pronounced effect on blood pressure than carbohydrate ingestion, an approach to the management of PPH in affected older people may be to increase fat at the expense of carbohydrate in the diet.
Digestion of fat and carbohydrate is necessary for the full slowing of gastric emptying, stimulation of gut hormone release and suppression of appetite that follows the ingestion of these macronutrients in food(Reference Pilichiewicz, O'Donovan and Feinle11, Reference Feinle, Rades and Otto12). It is not known, however, whether the digestion of carbohydrate and fat plays a role in the blood pressure fall that follows the ingestion of these macronutrients. This possibility is supported by the observation that acarbose, which inhibits disaccharide digestion to glucose, attenuates the blood pressure-lowering effect of oral sucrose in both elderly subjects and patients with PPH(Reference Gentilcore, Bryant and Wishart13, Reference Jian and Zhou14).
In the present study, orlistat, which inhibits lipase action and thus fat digestion in the gut, was used to examine the role of fat digestion in mediating fat-induced blood pressure decreases. We hypothesised that the products of fat digestion mediate the hypotensive response to fat, and that orlistat would reduce the postprandial fall in blood pressure. If so, this might have therapeutic implications for the management of PPH.
The use of orlistat in such a study is potentially complicated by its effects on gastric emptying and hence on gastric distension. The blood pressure-lowering effects of carbohydrates in food are dependent on small-intestinal nutrient exposure(Reference Gentilcore, Meyer and Rayner15) and are inhibited by gastric distension(Reference Jones, O'Donovan and Russo16). The faster the stomach empties, the greater the fall in blood pressure after oral glucose ingestion(Reference Jones, Tonkin and Horowitz17). Co-administering orlistat with high-fat foods inhibits lipase action and fat digestion, and, as a result, accelerates gastric emptying and reduces gastric distension(Reference Pilichiewicz, O'Donovan and Feinle11, Reference Borovicka, Schwizer and Guttmann18–Reference O'Donovan, Horowitz and Russo21). Both effects might be expected to enhance fat-induced postprandial falls in blood pressure and thus oppose a blood pressure-raising effect of the inhibition of fat digestion if one was present. To bypass the effects of fat and orlistat on both gastric distension and gastric emptying, we also administered lipid (with and without orlistat) intraduodenally.
Methods
Subjects
Part A – oral fat with or without orlistat
Of the healthy older subjects, nine (eight men and one woman), with a mean age of 73·2 (sem 2·0) years (range 66–85 years) and a BMI of 25·9 (sem 0·6) kg/m2 (range 23·0–28·8 kg/m2), were recruited by advertisement.
Part B – intraduodenal fat (with or without orlistat) infusion
A total of ten healthy older subjects (five men and five women), with a mean age of 72·6 (sem 1·1) years (range 66–77 years) and a BMI of 25·1 (sem 0·8) kg/m2 (range 21·4–28·8 kg/m2), were recruited by separate advertisement.
All subjects were non-smokers, and none had a history of gastrointestinal disease or surgery, diabetes mellitus, significant respiratory, renal, hepatic or cardiac disease, autonomic dysfunction, chronic alcohol abuse or epilepsy. No subject was taking medication known to influence blood pressure or gastrointestinal function.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Research Ethics Committee of the Royal Adelaide Hospital. Written informed consent was obtained from all subjects. For part B of the study, we calculated that a minimum of five subjects per group would be required to detect a mean difference in a SBP of approximately 13 mmHg with a power of 0·80, assuming a significance value < 0·05(Reference Visvanathan, Horowitz and Chapman10).
Study protocol
Part A – oral fat with or without orlistat
Each subject was studied on three occasions, separated by at least 48 h, in a single-blinded fashion. On each day, subjects attended the Discipline of Medicine, Royal Adelaide Hospital, at 08.30 hours following an overnight fast (12 h for solids and 8·5 h for liquids). An intravenous cannula was placed in the left antecubital vein for blood sampling, and subjects were seated comfortably on a bed at approximately 90°, to mimic normal physiological conditions during a meal. An automated blood pressure cuff was placed around the right arm for the measurement of blood pressure and heart rate (HR).
On each of the study days, at t = 0 min, subjects consumed the following equivolaemic drinks in randomised order: (1) water (control), 300 ml; (2) fat drink, 300 ml total, comprising 110 ml rich cream blended with 190 ml full-fat milk (88 % fat, 7 % carbohydrate, mostly lactose, 5 % protein; total energy 2732 kJ or 653 kcal), with low-energy flavouring; (3) fat-orlistat drink, 300 ml total, made up of 110 ml rich cream blended with 190 ml full-cream milk, with low-energy flavouring, with crushed and dispersed contents of one 120 mg capsule of orlistat (Xenical®; F. Hoffmann-La Roche Limited, Basel, Switzerland). All of the drinks were consumed within 3 min at room temperature. The dose of 120 mg orlistat was chosen, as it reduced SBP when administered with a meal in a previous study of patients with type 2 diabetes(Reference O'Donovan, Horowitz and Russo21).
Venous blood samples were obtained at baseline immediately before the ingestion of the drink (t = − 5 and − 2 min), at 15 min intervals for the first 60 min, and then every 30 min until t = 181 min. Blood samples were collected in ice-chilled dipotassium EDTA tubes containing 400 KIU (kallikrein inactivator units) aprotinin/ml of blood (Trasylol; Bayer Australia Limited, Pymble, NSW, Australia). Plasma was separated by centrifugation (3200 g, 15 min, 4°C) within 30 min of collection and stored at − 70°C until assayed.
Part B – intraduodenal fat with or without orlistat infusion
Each subject was studied on two occasions, separated by a minimum of 7 d, in a single-blinded fashion. On each day, the subject attended the Discipline of Medicine, Royal Adelaide Hospital, at 08.30 hours following a fast (10·5 h for solids and 8·5 h for liquids)(Reference Gentilcore, Bryant and Wishart13, Reference Gentilcore, Doran and Meyer22, Reference Gentilcore, Visvanathan and Russo23). At that time, a silicone rubber catheter (external diameter approximately 4 mm; Dentsleeve International Limited, Mui Scientific, Mississauga, ONT, Canada) was introduced into the stomach via an anaesthetised nostril(Reference Gentilcore, Doran and Meyer22, Reference O'Donovan, Feinle and Tonkin24). The assembly included an infusion channel (internal diameter approximately 1 mm) and was positioned so that the infusion port was located approximately 10 cm distal to the pylorus (i.e. in the duodenum), as well as two other channels that were positioned in the antrum (2·5 cm proximal to the pylorus) and duodenum (2·5 cm distal to the pylorus), respectively, and were perfused with 0·9 % saline. The correct positioning of the catheter was maintained by continuous measurement of the transmucosal potential difference between the antral ( − 40 mV) and the duodenal (0 mV) channel(Reference Heddle, Collins and Dent25). For this purpose, an intravenous cannula filled with sterile saline was placed subcutaneously in the left forearm and used as a reference electrode(Reference Heddle, Collins and Dent25). The tip of the catheter passed into the duodenum by peristalsis, which took between 20 and 165 min. Once the catheter was in position, the subject was placed in the recumbent position, and an automated blood pressure cuff was placed around the right arm(Reference Gentilcore, Doran and Meyer22, Reference O'Donovan, Feinle and Tonkin24). Approximately 30 min after the catheter had been positioned correctly (at t = 0 min), an intraduodenal infusion of fat (10 % Intralipid®; Fresenius Kabi AB, Uppsala, Sweden) with or without 120 mg orlistat in a volume of 243 ml was begun and continued at a rate of 2·7 ml/min for 90 min. On the two study days, saline (0·9 %) was infused intraduodenally at the same rate between t = 90 and 150 min(Reference Gentilcore, Hausken and Meyer26). The fat infusions resulted in an energy delivery of 12·6 kJ/min (3 kcal/min). Intraduodenal infusions were performed using a volumetric infusion pump (Gemini PC-1; IMED Corporation, San Diego, CA, USA). At t = 150 min, the catheter and the intravenous cannula were removed, the subject was given a light meal and then allowed to leave the laboratory.
Measurements
Blood pressure and heart rate
SBP, diastolic blood pressure (DBP) and HR were measured using an automated oscillometric blood pressure monitor (DINAMAP ProCare 100; GE Medical Systems, Milwaukee, WI, USA). For part A of the study, two baseline measurements were taken at t = − 5 and − 2 min, before the ingestion of the drink at t = 0 min, and subsequently, every 3 min, between t = − 2 and 91 min, and then at 15 min intervals until t = 181 min. For part B, measurements were taken at t = − 9, − 6 and − 3 min before the commencement of the intraduodenal infusions and, subsequently, every 3 min, between t = 0 and 150 min(Reference Gentilcore, Doran and Meyer22, Reference O'Donovan, Feinle and Tonkin24).
‘Baseline’ (i.e. t = 0 min) blood pressure and HR were calculated as the mean of measurements taken at t = − 5 and − 2 min for part A of the study, and at t = − 9, − 6 and − 3 min for part B of the study. PPH was defined as a fall in SBP of ≥ 20 mmHg that was sustained for at least 30 min(Reference Jansen and Lipsitz1).
Gastric emptying
For part A of the study, two-dimensional measurements of the antral area were performed using an Aloka SSD-650 CL Ultrasound Machine (Aloka Company, Limited Tokyo, Japan) with a 3·5–5 MHz sector transducer, as described and validated previously(Reference Hveem, Jones and Chatterton27, Reference Jones, Doran and Hveem28). The area recorded during the fasted state was subtracted from the subsequent measurements made after a meal. Gastric emptying was expressed at any time point as A C(t) = 100 − ((A (t)/A max) × 100), where A C(t) is the corrected antral area at a time point, A (t) is the area measured at a given time point, and A max is the maximum antral area recorded after meal ingestion(Reference Hveem, Hausken and Berstad29). The antral area was measured immediately before the drink (t = 0 min), and at 5 min intervals until 15 min, and then every 15 min until t = 180 min.
Superior mesenteric artery flow
For part B of the study, superior mesenteric artery flow was measured by Duplex ultrasonography (i.e. B-mode and Doppler imaging) using a Logiq™ 9 ultrasonography system (GE Healthcare Technologies, Sydney, NSW, Australia), as described previously(Reference Perko30). The subject was scanned using a 3.5C broad spectrum 2·5–4 MHz convex transducer(Reference Gentilcore, Hausken and Meyer26, Reference Perko30) at t = − 2, 5 and 10 min and then at 15 min intervals between t = 0 and 150 min. Blood flow (ml/min) was calculated immediately using the formula: π × r 2 × TAMV × 60, where r is the radius of the superior mesenteric artery and TAMV is the time-averaged mean velocity(Reference Perko30).
Total TAG concentrations
Plasma total TAG concentrations were measured in stored ( − 70°C) plasma samples. Samples obtained at t = − 2, 90 and 150 min were analysed. Plasma was processed on an Olympus 5400 analyser using TAG-liquid reagent (Integrated Sciences Private Limited, Willoughby, NSW, Australia) at the Institute of Medical and Veterinary Science Laboratories in Adelaide, SA, Australia(Reference Visvanathan, Horowitz and Chapman10).
Cardiovascular autonomic nerve function
In both parts A and B of the study, cardiovascular autonomic nerve function was evaluated at the end of one of the study days, using standardised cardiovascular reflex tests(Reference Piha31, Reference Ewing and Clarke32). Parasympathetic function was evaluated by the variation (R–R interval) of the HR during deep breathing and upon standing (ratio of the R–R interval at approximately beat 30 to the R–R interval at approximately beat 15). Sympathetic function was assessed by the fall in SBP in response to standing. Each test result was scored according to age-adjusted criteria as 0 = normal, 1 = borderline or 2 = abnormal, for a total maximum score of 6. A score of 3 or more was considered to indicate definite autonomic dysfunction(Reference Piha31, Reference Ewing and Clarke32).
Statistical analysis
The overall effects of treatment and time and the treatment × time interactions on SBP, DBP and HR changes from baseline and from t = − 2 to 181 min for part A, and from t = − 2 to 90 min and t = 90 to 150 min for part B were assessed using two-way ANOVA. Superior mesenteric artery flow was assessed from t = − 2 to 90 min and from t = 90 to 150 min. Total TAG concentrations were analysed as changes from baseline. The effects of time on SBP, DBP, HR, superior mesenteric artery flow, blood glucose and total TAG concentrations were analysed using one-way ANOVA. Post hoc, paired comparisons, using Student's t test, adjusted for multiple comparisons by Bonferroni's correction, were performed if ANOVA showed significant effects. The maximum fall in blood pressure and maximum rise in HR were defined as the greatest mean change from baseline in each subject at any given time point for each treatment. In part A, comparisons of the effects of the drinks on blood pressure and HR in older subjects were analysed using repeated-measures three-way ANOVA, with time and treatment as factors. Relationships between the maximal decrease in SBP and the 50 % gastric emptying time (T50), and between the maximal increase in HR and T50 were assessed by Pearson's correlation analyses. All analyses were performed using Statview version 5.0 (SAS Institute, Inc., Cary, NC, USA). Data are expressed as means with their standard errors. A P value of < 0·05 was considered statistically significant.
Results
Part A – oral fat with or without orlistat
A total of twelve people were screened, of which nine were recruited. Of the excluded subjects, two were not willing to cease antihypertensive medications temporarily and one was enrolled in another research study at the time of screening. None of the subjects had definite autonomic neuropathy; the median score for autonomic nerve dysfunction was 0 (range 0–2); two subjects had a score of 1 and one subject had a score of 2.
The study drinks were well tolerated. After the fat-orlistat drink, oily stools and mild flatulence were reported by one subject. After the high-fat drink, one subject reported mild abdominal discomfort and nausea. In both cases, symptoms were mild and had resolved spontaneously within 19 h of drink ingestion.
Systolic blood pressure
There were no significant differences in baseline SBP between the three study days (fat drink 132·8 (sem 4·8) mmHg v. fat-orlistat drink 131·6 (sem 5·5) mmHg v. water 132·6 (sem 4·8) mmHg; Fig. 1(a)). However, one subject had PPH (i.e. a fall in SBP of ≥ 20 mmHg sustained for ≥ 30 min) following both the fat and fat-orlistat drinks. For SBP (change from baseline), there was no significant treatment effect (P = 0·07), but a significant time (P = 0·0001) effect and treatment × time interaction (P = 0·017) were found. SBP decreased progressively after the fat (P = 0·0001) and fat-orlistat (P = 0·0001) drinks, but not after the water drink (P = 0·3). There was a greater reduction in SBP after the fat-orlistat drink than after the fat drink, which was significant between t = 73 and 88 min (P < 0·05). The maximum fall in SBP during the fat (16·3 (sem 2·1) mmHg) and fat-orlistat (20·1 (sem 3·9) mmHg) drinks did not differ significantly (P = 0·27), and there was also no significant difference in the time of maximum fall between the fat and fat-orlistat drinks (66·3 (sem 14·5) v. 68·3 (sem 10·6) min, P = 0·9).
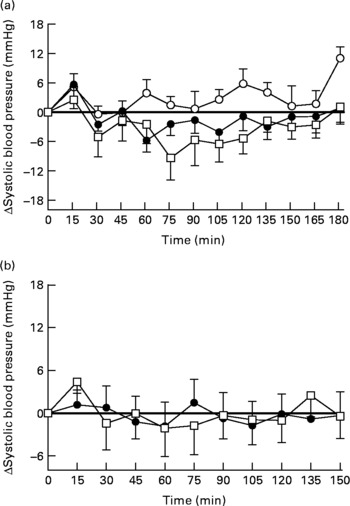
Fig. 1 Changes in systolic blood pressure from baseline in response to (a) the oral ingestion of water (○), fat (●) and fat-orlistat (□) drinks, and in response to (b) the intraduodenal infusion of fat and fat-orlistat in older subjects. Values are means, with standard errors represented by vertical bars.
Diastolic blood pressure
There were no significant differences in baseline DBP between the three study days (fat drink 74·2 (sem 2·4) mmHg v. fat-orlistat drink 75·2 (sem 2·6) mmHg v. water 75·6 (sem 2·5) mmHg). For DBP (change from baseline), there were significant treatment (P = 0·007) and time (P = 0·0001) effects and treatment × time interaction (P = 0·0001) over the duration of the study. DBP decreased after the high-fat (maximum decrease of 9·6 (sem 1·2) mmHg, P = 0·0001) and the fat-orlistat (11·1 (sem 2·9) mmHg, P = 0·0001) drinks, but not after the water drink (P = 0·31). There was a slight, but significantly greater, reduction in DBP following the ingestion of the fat-orlistat drink than after the fat drink (P < 0·05), with a maximal fall in DBP of 11·1 v. 9·6 mmHg, respectively.
Heart rate
There were no significant differences in baseline HR between the three study days (fat drink 59·7 (sem 1·4) beats per min (bpm) v. fat-orlistat drink 57·8 (sem 1·8) bpm v. water 59·2 (sem 1·1) bpm; Fig. 2(a)). For HR (change from baseline), there were significant treatment (P = 0·0001) and time (P = 0·0001) effects, and treatment × time interaction (P = 0·0001). HR increased after both the fat (P = 0·0001) and fat-orlistat (P = 0·0001) drinks, and decreased non-significantly after the water drink (P = 0·09). The increase in HR was slightly greater following the fat drink than after the fat-orlistat drink, but only at 55 and 58 min (P < 0·03). The maximum rise in HR during the fat (14·8 (sem 2·6) bpm) and fat-orlistat (10·9 (sem 1·6) bpm) drinks did not differ significantly (P = 0·13), and there was also no significant difference in the time to maximum rise in HR between the fat and fat-orlistat drinks (79·3 (sem 16·5) v. 71·7 (sem 13·9) min, P = 0·63).
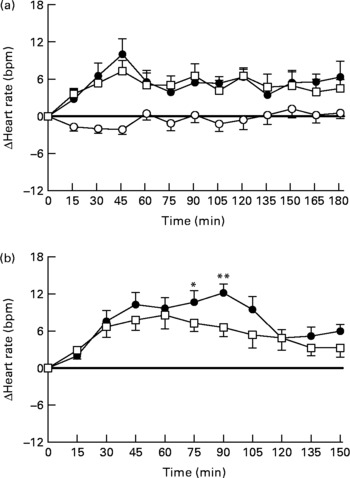
Fig. 2 Changes in heart rate (beats per min (bpm)) from baseline in response to (a) the oral ingestion of water (○), fat (●) and fat-orlistat (□) drinks, and in response to (b) the intraduodenal infusion of fat and fat-orlistat in older subjects. Values are means, with standard errors represented by vertical bars. Mean values were significantly different for treatment × time effect (fat v. fat-orlistat): * P < 0·05, ** P < 0·001.
Gastric emptying
Gastric emptying of water was faster than that of the fat-orlistat drink, which was in turn faster than that of the fat drink (treatment effect, P = 0·0004; Fig. 3). Gastric emptying was significantly faster after the fat-orlistat drink than after the fat drink from t = 45 to 120 min (P < 0·04). There was no difference before that time. The T50 for the fat drink was greater compared with water (60·3 (sem 7·1) v. 24·7 (sem 5·8) min, respectively, P = 0·0003) and non-significantly greater than the fat-orlistat drink (47·4 (sem 5·0) min, P = 0·24).

Fig. 3 Gastric emptying of water (○), fat (●) and fat-orlistat (□) drinks. Values are means, with standard errors represented by vertical bars. Mean values were significantly different for treatment × time effect (fat v. fat-orlistat): * P < 0·04.
Relationships between gastric emptying, blood pressure and heart rate
There were no significant relationships between the maximum fall in SBP and the T50 after either the fat-orlistat drink (R − 0·24, P = 0·54) or the fat drink (R − 0·08, P = 0·85). There was a significant relationship between the maximum rise in HR and the T50 after the fat-orlistat drink (R 0·83, P = 0·006), but not after the fat drink (R 0·17, P = 0·65).
Total TAG concentrations
There was no difference in baseline total TAG concentrations between the three study days (P = 0·72). TAG concentrations did not change from baseline after the water drink. There was a rise in total TAG concentrations after both the fat and treatment fat-orlistat (P < 0·001) infusions. At 90 min, change from baseline TAG concentrations was not significantly different between the fat and fat-orlistat treatment days, although it was non-significantly greater on the fat treatment day (0·46 (sem 0·14) v. 0·11 (0·14) mmol/l, P>0·05).
Part B – intraduodenal fat with or without orlistat infusion
A total of ten people were screened, and all were recruited. The median score for autonomic nerve dysfunction was 1·0 (range 0–4); one of the ten subjects had definite autonomic dysfunction. While the studies were well tolerated, eight of the ten subjects reported adverse effects after completion of the intraduodenal infusions. Loose stools or diarrhoea were experienced by six subjects after completion of the fat (n 4) or fat-orlistat (n 3) infusion. Of the ten subjects, one reported abdominal cramps and two experienced fatigue after completion of the fat-orlistat infusion. In all cases, adverse effects were mild and resolved spontaneously by the following day. PPH (i.e. a fall in SBP of ≥ 20 mmHg sustained for at least 30 min) was evident in two subjects; in one subject during both infusions and in the other during the fat infusion only. In one subject, total TAG concentrations could not be measured as intravenous cannulation was not possible.
Systolic blood pressure
There was no significant difference in baseline SBP between the 2 d (fat v. fat-orlistat infusion): 125·8 (sem 5·4) v. 126·2 (5·9) mmHg (P = 0·85). Between t = 0 and 90 min, there was no difference in SBP between the 2 d (P = 0·94). There were also no significant changes in SBP during the fat (P = 0·62) or fat-orlistat (P = 0·35) infusion. The maximum falls in SBP from baseline during the fat (11·7 (sem 2·8) mmHg) and fat-orlistat (12·7 (sem 3·3) mmHg) infusions were comparable (P = 0·51; Fig. 1(b)).
Between t = 90 and 150 min, there was no significant difference in SBP between the 2 d (P = 0·71), nor were there any significant changes in SBP during the fat (P = 0·83) or fat-orlistat (P = 0·94) infusion. At t = 150 min, SBP was not significantly different from baseline after the fat (P = 0·87) or fat-orlistat (P = 0·94) infusion (Fig. 1(b)).
Diastolic blood pressure
There was no significant difference in baseline DBP between the 2 d (fat v. fat-orlistat infusion): 67·1 (sem 3·4) v. 68·0 (3·2) mmHg (P = 0·41). Between t = 0 and 90 min, there was no significant difference in DBP between the 2 d (P = 0·59). There was a trend (P = 0·08) for a fall in DBP during the fat, and DBP fell during the fat-orlistat (P < 0·02) infusion.
Between t = 90 and 150 min, there was no significant difference in DBP between the 2 d (P = 0·93). There were also no significant changes in DBP during the fat (P = 0·52) or fat-orlistat (P = 0·44) infusion. At t = 150 min, there was no difference in DBP from baseline after the fat (P = 0·98) or fat-orlistat (P = 0·92) infusion.
Heart rate
There was no significant difference in baseline HR between the 2 d (fat v. fat-orlistat infusion): 55·9 (sem 2·4) v. 56·7 (2·2) bpm (P = 0·45). There was a rise in HR during both the fat and fat-orlistat (P < 0·0001 for both) infusions. The maximum increases in HR during the fat (16·0 (sem 1·9) bpm) and fat-orlistat (18·2 (sem 2·9) bpm) infusions were comparable (P = 0·48), but between t = 0 and 90 min, there was a significant treatment × time effect (P < 0·05). HR was higher (P < 0·05) between t = 75 and 90 min during the fat infusion, when compared with the fat-orlistat infusion (Fig. 2(b)).
After 90 min, HR decreased during both infusions. Between t = 90 and 150 min, there was no significant difference in HR between the 2 d (P = 0·11). HR fell (P < 0·0001) during the fat infusion, and there was a trend (P = 0·08) for a fall in HR during the fat-orlistat infusion (P = 0·08). At t = 150 min, HR was greater than baseline after the fat infusion (P = 0·0001), and there was a trend (P = 0·06) for HR to be greater than baseline after the fat-orlistat infusion (Fig. 2(b)).
Superior mesenteric artery flow
There was a trend for a difference in baseline (i.e. t = − 2 min) superior mesenteric artery flow between the 2 d (fat v. fat-orlistat: 588·9 (sem 58·1) v. 708·2 (sem 80·4) ml/min; P = 0·07). There was a rise in superior mesenteric artery flow during both the fat and fat-orlistat infusions (P < 0·0001 for both), which was evident from t = 15 min (P < 0·05 for both). Between t = − 2 and 90 min, there was a significant treatment × time effect (P < 0·006) for superior mesenteric artery flow. Superior mesenteric artery flow was higher (P < 0·05) between t = 60 and 90 min during the fat infusion, when compared with the fat-orlistat infusion (Fig. 4).
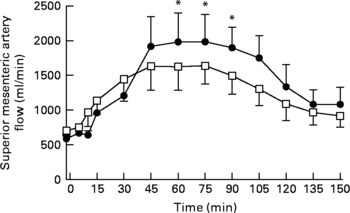
Fig. 4 Superior mesenteric artery flow in older subjects in response to the intraduodenal infusion of fat (●) and fat-orlistat (□) drinks. Values are means, with standard errors represented by vertical bars. Mean values were significantly different for treatment × time effect (fat v. fat-orlistat): * P < 0·05.
Between t = 90 and 150 min, there was no significant difference in superior mesenteric artery flow between the 2 d (P = 0·16). There was a fall in superior mesenteric artery flow during both the fat (P < 0·0001) and fat-orlistat (P < 0·0002) infusions, which was evident from t = 120 min (P = 0·0001) during the fat infusion and from t = 105 min (P = 0·03) during the fat-orlistat infusion. At t = 150 min, superior mesenteric artery flow was greater than baseline after both the fat (P = 0·01) and fat-orlistat (P = 0·05) infusions (Fig. 4).
Total TAG concentrations
There was a trend for a difference in baseline (i.e. t = − 2 min) total TAG concentrations between the 2 d (fat v. fat-orlistat): 1·1 (sem 0·13) v. 0·87 (sem 0·09) mmol/l (P = 0·07).
Between t = − 2 and 150 min, there was no difference in total TAG concentrations between the two study days (P = 0·62). However, there was a rise in total TAG concentrations after both the fat and fat-orlistat infusions (P < 0·0001 for both). At t = 150 min, total TAG concentrations were greater than baseline after both the fat and fat-orlistat infusions (P < 0·0001 for both).
Discussion
We confirmed that orlistat accelerated gastric emptying of fat, as found in previous studies(Reference Pilichiewicz, O'Donovan and Feinle11, Reference Borovicka, Schwizer and Guttmann18–Reference O'Donovan, Horowitz and Russo21). The novel observations are that (1) orlistat potentiated the hypotensive response to oral fat in older subjects, possibly due to faster gastric emptying of fat and (2) the hypotensive effect of fat appeared not to depend on fat digestion.
Oral ingestion of a high-fat drink resulted in an increased HR and significant decreases in both systolic and DBP, with mean falls of approximately 8 and 6 mmHg, respectively. These blood pressure falls are consistent with the results of some(Reference Sidery, Cowley and MacDonald8, Reference Visvanathan, Horowitz and Chapman10), but not all(Reference Potter, Heseltine and Hartley5, Reference Jansen, Peeters and Van Lier7), studies. The increase in HR probably reflects the activation of a baroreceptor reflex; as splanchnic blood flow increases after a meal, thus reducing systemic vascular resistance, there is a compensatory increase in HR. While possible, it seems very unlikely that the fall in blood pressure after fat ingestion was due to the small amount of carbohydrate in the drink (mainly lactose, 7 % of total energy, 192 kJ). Intraduodenal infusion of a greater amount of carbohydrate (glucose at 4·2 kJ/min (1 kcal/min) for 60 min, 251 kJ) than that used in the present study does not decrease blood pressure in older adults, whereas a greater glucose load of 12·6 kJ/min (3 kcal/min) for 60 min has a substantial effect(Reference O'Donovan, Feinle and Tonkin24). Our group has shown that healthy older adults experience comparable blood pressure decreases after oral or intraduodenal fat administration to those after equienergetic oral(Reference Visvanathan, Horowitz and Chapman10) or intraduodenal(Reference Gentilcore, Hausken and Meyer26) carbohydrate administration. It seems unlikely, therefore, that dietary modification, by altering the ratio of carbohydrate to fat content in a meal, has the potential to ameliorate the postprandial fall in blood pressure in older individuals with, or at risk of, PPH.
While the results of the present study do not conclusively answer the question of whether fat digestion is required for its hypotensive effect, they suggest that it is not, although the extent of digestion of fat may modify the cardiovascular response to its ingestion. Fat digestion (lipolysis of TAG to fatty acids) is required for its appetite-suppressant effect(Reference Feinle, O'Donovan and Doran33, Reference Matzinger, Degen and Drewe34), for the stimulation of cholecystokinin, glucagon-like peptide and peptide tyrosine–tyrosine(Reference Feinle, O'Donovan and Doran33, Reference Hildebrand, Petrig and Burckhardt35), suppression of ghrelin(Reference Feinle-Bisset, Patterson and Ghatei36), slowing of gastric emptying(Reference Borovicka, Schwizer and Guttmann18, Reference Schwizer, Asal and Kreiss20, Reference Carney, Jones and Horowitz37) and pancreatic enzyme secretion(Reference Hildebrand, Petrig and Burckhardt35). When orlistat was co-administered with oral fat in part A of the study, there was a slight, but significant, enhancement of the fat-induced decrease in both systolic and DBP, the opposite of what would be expected if products of fat digestion lowered blood pressure. Co-administration of orlistat with intraduodenal fat had no effect on blood pressure in part B of the study. In contrast, orlistat produced a slight but significant inhibition of the fat-induced pulse rate increase in both parts of the study. This is consistent with fat digestion being at least partly responsible for the increase in HR induced by fat ingestion. Similarly, the inhibition of fat-induced increased splanchnic blood flow after intraduodenal orlistat co-administration favours a role of fat digestion in this process.
The results of part A of the study do not exclude a role for fat digestion in fat-induced hypotension, as the hypotensive effect of faster gastric emptying due to orlistat may overshadow a weaker effect of orlistat to block the formation of hypotensive fat digestion products. Gastric emptying was slower after the high-fat drink compared with water, a finding consistent with previous studies(Reference Cunningham and Read38). It has previously been demonstrated that the fall in postprandial blood pressure is directly related to the rate of gastric emptying in individuals with type 2 diabetes(Reference Jones, Tonkin and Horowitz17); when gastric emptying is slowed, the rate of delivery of nutrients to the small intestine, and thus nutrient-driven effects on blood pressure, is delayed. The acceleration of gastric emptying produced by orlistat therefore delivers fat to the small intestine more rapidly, thus presumably enhancing its hypotensive effects, and also reduces gastric distension, which would have the same effect. Although it is known that the stomach empties at a relatively constant rate of 8·4–12·6 kJ/min (2–3 kcal/min)(Reference Horowitz, O'Donovan and Jones39), the fat and fat-orlistat drinks were emptied at faster rates of 16·7–25·1 kJ/min (4–6 kcal/min), suggesting that the intragastric fat may have separated from the aqueous component(Reference Horowitz, Jones and Edelbroek40). Part B of the study allowed examination of the effects of fat digestion more directly, as administration of fat and orlistat directly into the duodenum removed any effects on the rate of gastric emptying. DBP decreased from baseline after intraduodenal fat infusion, as in our previous study in older adults(Reference Gentilcore, Hausken and Meyer26). In the same study(Reference Gentilcore, Hausken and Meyer26), SBP decreased after fat infusion only when compared with the effects of a saline infusion, which we did not include in the present study for logistical reasons. In that study(Reference Gentilcore, Hausken and Meyer26), the effects of fat on DBP were most marked between 90 and 120 min, whereas DBP was maximally reduced before 90 min in the present study. The reason for this difference is not apparent.
The absence in part B of the study of any effect of orlistat co-administration on the fat-induced decrease in DBP argues against a role of fat digestion in this blood pressure effect. This may, however, reflect the limited inhibition of fat digestion produced by orlistat with intraduodenal infusion. The increase in plasma TAG concentrations (the result of fat digestion) produced by fat, administered orally or intraduodenally, was only slightly, and not significantly, inhibited by orlistat co-administration. There are limitations in using plasma TAG concentrations to assess lipase inhibition(Reference Shepard, Jensen and Blotner41–Reference Sahin, Tanaci and Yucel43), and alternative measurements, such as daily faecal fat excretion, may be needed(Reference O'Donovan, Feinle-Bisset and Wishart44, Reference Di Marco, Marier and Ducharme45). It is possible that administration of a higher orlistat dose, or more vigorous mixing with the intralipid solution, would have produced greater lipase inhibition and permitted better examination of the role of fat digestion on blood pressure and HR in part B of the study. Nevertheless, there was no evidence of an orlistat effect on blood pressure, whereas there was a significant inhibitory effect on HR, suggesting a hierarchy of sensitivities, with HR being more readily affected by products of fat digestion than is blood pressure. This warrants further investigation.
In summary, these findings do not support a role for the products of fat digestion in mediating the blood pressure-lowering effect of fat. Orlistat cannot, therefore, be used to attenuate the hypotensive response to a meal in older individuals, while manipulation of the composition of a meal, in particular its carbohydrate and fat content, also seems unlikely to be of benefit.
Acknowledgements
The present study was supported by the National Health and Medical Research Council (NHMRC) of Australia (project grant ID 453650), by an NHMRC of Australia postgraduate scholarship (ID 340310, to K. T.), by a National Heart Foundation of Australia Postdoctoral Fellowship (PR 07A 3309, to D. G.), by an NHMRC/Diabetes Australia Career Development Award (ID 250453, to K. L. J.), by an NHMRC of Australia Career Development Award (grant no. 299074, to C. F.-B.), by an Equipment Grant from the NHMRC of Australia (ID 219354) and by the University of Adelaide and GE Medical Systems Australia for the purchase of the Logiq™ 9 ultrasonography system. We thank Nancy Briggs (Department of Public Health, University of Adelaide) for her assistance with the statistical analyses. The authors' contributions were as follows: K. T. and D. G. were responsible for the acquisition of subjects, data collection, analysis, interpretation and preparation of the manuscript; K. L. J., M. H. and I. M. C. had a primary role in the concept and design of the study, data interpretation and preparation of the manuscript; L. B. and A. J. H. collected the data; O. H. G. supervised the data collection, analysis and interpretation; C. F.-B. assisted in the data interpretation and preparation of the manuscript. None of the authors had any personal or financial conflict of interest.






