Obesity is a major public health issue in both adult and paediatric populations( Reference Ogden, Carroll and Kit 1 ), particularly as a risk factor for the development of CVD, a leading cause of morbidity and mortality in many countries( 2 ). Increasing adiposity contributes to the development of atherosclerotic CVD, in part through the promotion of a chronic, low-grade inflammatory state and through endothelial dysfunction( Reference Wang and Nakayama 3 , Reference Iantorno, Campia and Di Daniele 4 ), which is typically characterised by reduced nitric oxide (NO) bioavailability( Reference Iantorno, Campia and Di Daniele 4 , Reference Kampoli, Tousoulis and Antoniades 5 ). NO is anti-atherogenic, acting as a key endothelium-derived vasodilator that reduces cellular proliferation, inflammation and platelet activation( Reference Rizvi 6 ). Although clinical manifestations of atherosclerosis are typically not observed in adolescents, CVD has a long asymptomatic phase of development, which can begin early in life( Reference Singhal 7 ). Moreover, the changes in vasculature that are associated with an increased fat mass in older individuals can be observed in overweight and obese paediatric populations( Reference Logue and Sattar 8 – Reference Skinner, Steiner and Henderson 10 ). As an increased BMI in children and adolescents is associated with increased cardiovascular (CV) risk burden( Reference Logue and Sattar 8 ), identification of children who are at risk for atherosclerosis and the introduction of early lifestyle and dietary interventions that reduce this risk are important public as well as personal health goals.
Diets rich in fruits and vegetables can promote health and attenuate, or delay, the onset of various chronic diseases including CVD( Reference Defago, Elorriaga and Irazola 11 , Reference Mink, Scrafford and Barraj 12 ). Besides being a good source of several essential nutrients including numerous vitamins, minerals, fatty acids and fibre, many plant foods are rich in polyphenols – a diverse family of compounds that can provide certain CV benefits( Reference Holt, Heiss and Kelm 13 ). Strawberries are rich in certain polyphenols, which may help explain why the intake of freeze-dried strawberry powder (FDSP) has been reported to improve lipid profiles, insulin sensitivity and attenuate inflammatory and thrombotic responses in adult populations with or without the metabolic syndrome( Reference Basu, Fu and Wilkinson 14 – Reference Ellis, Edirisinghe and Kappagoda 17 ). In the present study, we evaluated the effects of short-term FDSP intake in adolescent males who were at or above the 75th percentile for weight on microvascular function as measured by peripheral arterial tonometry (PAT), as well as effects on platelet reactivity and plasma nitrate/nitrite concentrations.
Methods
Participants
Healthy, male adolescents (14–18 years of age) from the Sacramento, California, metropolitan region were recruited via flyers and newspaper advertisements. Inclusion criteria included a BMI for age and sex at or above the 75th percentile (BMI percentile), based on data from the United States Center for Disease Control, and a willingness to comply with the study protocols. Exclusion criteria included use of medications that affect vascular function, use of dietary supplements, chronic/routine high-intensity exercise or participation in organised sports, and an inability to wear PAT probes and/or to remain still and quiet during test procedures. Participants were instructed to refrain from consuming berries throughout the study period. All analyses were conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of the University of California, Davis, approved the study protocol, and all participants and their parents or their legal guardians were asked to provide written informed assent and/or consent before enrolment.
Study design
A randomised, controlled, double-blind, cross-over study was conducted that examined both the short-term (1 week) and acute (1 h) vascular responses to strawberry intake. Participants were randomised by block design to consume 50 g of FDSP or a control powder for 1 week, and after a 1 week washout period they were instructed to consume the alternate test powder for an additional week. The treatment and washout periods, as well as the 1-h acute sampling point, were chosen on the basis of previous reports of improved vascular function after the intake of flavonoid-rich foods( Reference Heiss, Jahn and Taylor 18 , Reference Balzer, Rassaf and Heiss 19 ), with peak plasma levels of strawberry metabolites obtained approximately 1 h after intake( Reference Mullen, Edwards and Serafini 20 ). Randomisation was performed following a plan formulated via a web-based random number generator (www.randomization.com). The isoenergetically matched powders were produced and provided by the California Strawberry Commission (Table 1), with a daily intake of 50 g of FDSP estimated to provide 500 g in fresh weight of strawberries (equivalent to three servings)( Reference Basu, Fu and Wilkinson 14 ). The control powder was matched for energy content and sugars, was similar in colour and flavour to the test powder, but provided no additional protein or flavonoids, and was significantly reduced in its K and fibre contents. Both powders were pre-packaged in 25 g servings, and mixed with water to produce a shake beverage for consumption. All participants were asked to return any used or unused beverage packages as an assessment of compliance.
Table 1 Powder composition
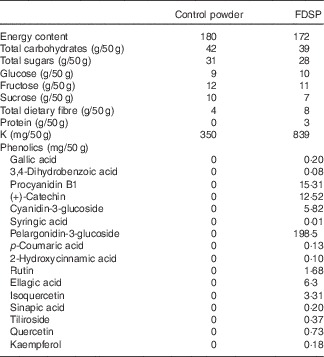
FDSP, freeze-dried strawberry powder.
Study day assessments were conducted before commencement of daily powder intake (Study Visit 1) and at the end of each 1-week period of daily powder intake (Study Visit 2). For each study visit, the participants were asked to arrive in the morning after an overnight fast. After weight and blood pressure were measured, a baseline measurement of microvascular function, assessed by PAT, was carried out, and blood samples were collected. Immediately afterwards, the study participants consumed 50 g of their assigned test beverage; 1 h following this intake, a second PAT measurement was carried out and blood samples were collected, which completed the study visit. Participants were then provided with packets of powder for the 1-week intervention period, and were instructed to consume one packet at breakfast and a second at dinner. Apart from being instructed to refrain from consuming berries throughout the study period, participants were instructed to continue their normal diet and to complete a 3-d food record during each treatment period, which included 2 weekdays and 1 weekend day. Food records were analysed using The Food Processor SQL (version 10.1.0; ESHA Research).
Microvascular function
Microvascular function was assessed by PAT (Endo-PAT2000; Itamar Medical) as previously described( Reference Holt, Yim and Shearer 21 ). In brief, all participants acclimated to a quiet and temperature-controlled room by resting in the supine position for 30 min. Following supine blood pressure measurement, the PAT measurement was performed in the supine position, which involved a 5–10 min baseline recording, followed by a 5-min occlusion period, which was induced by inflating a blood pressure cuff placed on the proximal forearm to approximately 60 mmHg above systolic blood pressure. After 5 min, the pressure was released, and the resulting reactive hyperaemia response was recorded for an additional 3–5 min. The system software automatically calculated the reactive hyperaemia index (RHI), which is the ratio of the average of pulse wave amplitude (PWA) during a 1-min period following 90 s of reactive hyperaemia to the average PWA during a 3·5-min baseline period. In additional, the Framingham reactive hyperaemia index (fRHI) was calculated, which used the natural logarithmic transformation of the RHI ratio during data collection from 90 to 120 s after release of the occlusion. The fRHI has been reported to be strongly correlated with CV risk factors( Reference Hamburg, Keyes and Larson 22 ).
Plasma nitrate/nitrite
Plasma nitrate and nitrite (nitrate/nitrite) were assessed using a nitrate/nitrite colorimetric assay (Cayman Chemical Co.). EDTA-treated plasma was separated from the whole blood by centrifugation (15 min at 1800 g and 4°C) immediately after collection. The separated plasma was stored at −80°C until analysis, processed according to the manufacturer’s protocol and assessed in triplicate.
Assessment of platelet activation markers
The surface expression of platelet activation markers such as P-selectin and the active conformation of glycoprotein IIb/IIIa were assessed as previously described( Reference Pearson, Paglieroni and Rein 23 ). In brief, 50 μl of citrated (3·2 % sodium citrate) whole blood was activated for 5 min with ADP or epinephrine at a final concentration of 20 μmol/l each. Subsequently, the activated blood was diluted with saline, and a 100-μl aliquot was added to a second set of tubes containing the following antibodies: PAC1-FITC (clone PAC-1; BD Biosciences), CD62P-APC (clone AK-4; BD Biosciences) and CD42a-PE (clone ALMA.16; BD Biosciences). After 20 min of incubation, the samples were fixed with 1 % paraformaldehyde; 10 000 CD42a-positive events were collected on a flow cytometer (LSR II; Becton Dickinson). Activated platelets were defined as the percentage of CD42a events expressing PAC-1 (GPIIb/IIIa activated conformation) or CD62P (P-selectin).
Additional blood measurements
Blood samples were analysed for a comprehensive metabolic panel, lipid panel and complete blood count by the University of California Davis Medical Center Pathology Department.
Data analysis
Normally distributed data are expressed as means with their standard errors or standard deviations, as noted. Transformed data are expressed as back-transformed means and 95 % confidence intervals. Data were initially assessed for normality and absence of outliers, and those not normally distributed were determined by the Shapiro–Wilk test, were log-transformed and re-checked for normality. Statistical analyses of the normally distributed data were conducted using the repeated-measures ANOVA (rANOVA) with Bonferroni post hoc CI. Main effects and their interactions were assessed with rANOVA for Treatment (control and FDSP) and Visit (0 h both before, and after 1 week of intake) for short-term intake as well as for the acute change (Variable1 h–Variable0 h) after 1 h of powder intake. Interactive effects for period of intake (Control → FDSP or FDSP → Control) were also assessed for Visit 1 of each treatment group using ANOVA with period as a factor. Data that were not normally distributed were analysed via non-parametric Wilcoxon’s signed-rank tests or Friedman’s two-way ANOVA by ranks test, with post hoc analysis with Wilcoxon’s signed-rank test with a Bonferroni correction. P values of 0·05 or less were considered statistically different. Platelet data are presented as medians and interquartile ranges with all other data presented as means and standard deviations or means and 95 % confidence intervals. Analyses were performed with IBM SPSS software (version 22.0.0.0). Complete data sets were not available for select outcomes because of equipment or analysis failure. Therefore, the final subject total for PAT and nitrate/nitrite analysis was 24 for total cholesterol (TC), HDL and cholesterol:HDL was 25 and for LDL it was 22.
Results
Population characteristics
In all, twenty-eight adolescent males were enrolled between August 2013 and June 2014, of which twenty-five completed the study (Fig. 1). The initial treatment assignment was fifteen for FDSP intake and ten for control powder intake. The mean age of the study population was 16 years. On average, the participants were overweight with normal blood pressure, fasting lipids and blood glucose levels (Table 2). No main or interaction effects for BMI percentile were noted, but a significant Treatment×Visit interaction was observed for BMI z-score (F 1,23=9·91, P=0·005; Table 2). The majority of participants did not return their used and unused treatment. Complete 3-d food records were obtained from seventeen of the subjects. Reported baseline energy and macronutrient intakes were similar between the two groups and did not differ at the end of the study, except for a decrease in the percentage of total kJ (kcal) from fat in the FDSP group (Table 2).

Fig. 1 Participant flow chart.
Table 2 Estimated nutrient intake (Mean values and standard deviations)

FDSP, freeze-dried strawberry powder.
* Significantly different compared with the control group (P<0·05).
Statistical analysis by paired t test or †Wilcoxon’s signed-rank test.
Microvascular function
No acute or short-term effects of daily powder intake on blood pressure were observed (Table 3). Likewise, no statistically significant main or interaction effects for either short-term or acute change in RHI or fRHI were noted.
Table 3 Short-term effects of strawberry powder intake on cardiovascular outcomes (Mean values and standard deviations)

FDSP, freeze-dried strawberry powder; SBP, systolic blood pressure; DBP, diastolic blood pressure; RHI, reactive hyperaemia index; fRHI, Framingham reactive hyperaemia index.
* Significantly different interaction for treatment and visit (P<0·05). Statistical analysis by repeated-measures ANOVA (rANOVA) or †Friedman’s ANOVA on ranks.
Platelet activation markers
The expressions of platelet activation markers were not significantly different at baseline before either FDSP or control powder intake (Table 4). Significant reductions in ADP-induced P-selectin expression were detected with short-term powder intake (P<0·0001; n 24), with a decrease in ADP-induced P-selectin expression after 1 week of powder intake for both control powder (30·0 (22·2, 43·7) % on the first day of the study v. 27·6 (17·3, 40·5) % after 1 week of intake; P=0·01) as well as after FDSP intake (32·4 (21·2, 41·4) % on day 1 v. 26·2 (18·3, 39·3) % after 1 week; P=0·02). No other significant changes in platelet activation were observed with either single serving or acute intake (Table 4).
Table 4 Short-term and acute change in platelet activation markers after 1 week of daily powder intakeFootnote * (Medians and interquartile ranges (IQR))

FDSP, freeze-dried strawberry powder; GPIIb/IIIa-act, activated confirmation of glycoprotein IIb/IIIa; P-Sel, P-selectin.
* Platelet surface activation marker expression (expressed as % CD42a expression) at baseline (0 h) during Visit 1 or after 1 week of powder intake (Visit 2); the effect of acute (1 h) change (Variable1 h–Variable0 h) in marker expression before (Visit 1) or after 1 week (Visit 2) of powder intake. Statistical analysis performed using Friedman’s ANOVA on ranks with Bonferroni correction, with statistical significance of P<0·05.
Plasma lipids
No interactive effects were observed in plasma lipids (Table 3). Significant main effects for Visit were noted for LDL, HDL and cholesterol:HDL, with an overall decrease in LDL (mean difference −0·2 (95 % CI −0·3, −0·08) mmol/l, P=0·002) and cholesterol:HDL (−0·005 (95 % CI −0·01, −0·003) mmol/l, P=0·006), and an increase in HDL (0·0005 (95 % CI 0·00, 0·008) mmol/l, P=0·048) was observed during Visit 2 compared with Visit 1, which was not significantly influenced by the period of intake. In addition, significant main effects for Treatment were observed for the acute 1-h change in cholesterol and non-HDL-cholesterol, with both measures increasing in the FDSP group compared with the control group (0·005 (95 % CI 0·0003, 0·001) mmol/l, P=0·007; and 0·0008 (95 % CI 0·0003, 0·001) mmol/l, P=0·02; respectively).
Plasma nitrate/nitrite concentration
Significant main effects for Treatment and Visit were observed for plasma concentration of nitrate/nitrite after 1 week of intake (F 1,23=5·57, P=0·027 and F 1,23=9·54, P=0·005, respectively), with significantly greater increase in the FDSP group than in the control group (mean difference 0·89 (95 % CI 0·01, 0·17) and Visit 2 v. Visit 1 (0·11 (95 % CI 0·04, 0·19)). Interactions for Visit×Treatment did not reach statistical significance (Table 3, P=0·889). A significant increase in the 1-h acute change in nitrate/nitrite levels (χ 2=58·3, P<0·001) was observed after the intake of FDSP compared with that of the control powder during V1 (16·2 (sd 5·58) v. −1·27 (sd 2·51) μmol/l, respectively; Z=−2·04, P<0·001) and during V2 (−1·63 (sd 2·27) v. 15·9 (sd 6·01) μmol/l, respectively; Z=−1·96, P<0·001). The magnitude of these effects is depicted as the change in plasma nitrate/nitrite (i.e. plasma nitrate/nitrite1 h–plasma nitrate/nitritebaseline before intake) in Fig. 2.

Fig. 2 Significant changes in plasma nitrate/nitrite expressed as the acute change in plasma nitrate/nitrite from baseline. Although the 1 h change in plasma nitrate/nitrite levels was not significant between study visits for either powder (control powder: P=0·655; freeze-dried strawberry powder (FDSP): P=0·502), FDSP intake did significantly increase plasma nitrate and nitrite concentrations during both study visits, compared with control powder intake (Z=−2·04 and Z=−1·96, P<0·001 for both Study Visits 1 (V1) and 2 (V2), respectively). *Significantly different compared with the control group (P<0·05). Friedman’s two-way ANOVA on ranks.
As the above data demonstrate that FDSP intake can induce a significant increase in plasma nitrate/nitrite levels in some individuals, the relationship between the change in plasma nitrate/nitrite levels and microvascular response was further explored. As described above, no significant interaction effect was noted in general for fasting plasma nitrate levels (i.e. Treatment×Visit), which can also be expressed as the short-term change in plasma nitrate/nitrite between the two groups (plasma nitrate/nitrite1 week–plasma nitrate/nitritebaseline; FDSP: 3·31 (sd 7·32) μmol/l v. Control: 1·76 (sd 9·28) μmol/l; Z=−0·44, P=0·6612, n 22). However, when these data were subdivided into those who had a positive 1-week change in fasting plasma nitrate/nitrite levels with FDSP intake compared with the control powder (responders) and those who had a negative change in plasma levels with FDSP intake compared with control (non-responders; Fig. 3(a) and (b)), no changes were noted for the non-responders in RHI or fRHI after 1 week of FDSP intake compared with the control group (RHI: Z=−1·07, P=0·285; fRHI: Z=−0·66, P=0·508). In contrast, the responders showed a significant increase after 1 week of FDSP intake for both RHI and fRHI compared with control powder intake (RHI: Z=−2·51, P=0·012; fRHI: Z=−1·96, P=0·050; Fig. 3(c) and (d)). No other significant effects were observed between nitrate/nitrite responders and non-responders regarding changes in blood pressure, plasma lipids, fasting blood glucose levels, platelet activation markers, baseline PWA and the peak RHI response measured during the first 2·5 min of reactive hyperaemia (data not shown).

Fig. 3 The differential effects of 1 week of freeze-dried strawberry powder (FDSP) intake on fasting plasma nitrate/nitrite levels and microvascular response in adolescent males (14–16 years of age). (a) Individuals who exhibited a significant positive response in fasting plasma nitrate/nitrite levels after 1 week of FDSP compared with intake of the control powder (5·44 (sd 6·54) v. −3·62 (sd 7·31) μmol/l, respectively, n 12); v. (b) who displayed a negative response in plasma nitrate/nitrite after FDSP intake (FDSP: 0·76 (sd 7·72) μmol/l v. Control: 8·23 (sd 7·11) μmol/l, respectively, n 10). An increase in microvascular function was observed in individuals that who positive plasma nitrate/nitrite responders for both (c) reactive hyperaemia index (RHI) (FDSP: 0·07 (sd 0·67) v. Control: −0·45 (sd 0·89), respectively; Z=−2·51, P=0·012, n 12) and (d) Framingham reactive hyperaemia index (fRHI) (FDSP: 0·06 (sd 0·42) v. Control: −0·17 (sd 0·35), respectively; Z=−1·96, P=0·050, n 12). Statistical testing performed using Wilcoxon’s signed-rank test. Each line represents the individual 1-week change in plasma nitrate/nitrite between control and FDSP.
Discussion
An increase in endothelial dysfunction has been characterised in paediatric (<18 years of age) populations, which is a troubling observation, as endothelial dysfunction at early ages has been associated with increased CV risk later in life( Reference Pareyn, Allegaert and Verhamme 9 , Reference Aatola, Hutri-Kahonen and Juonala 24 – Reference Bruyndonckx, Hoymans and Frederix 27 ). A limited number of studies have examined the effects of lifestyle changes on vascular function in obese and overweight children and adolescents. Weight loss has been observed to improve microvascular function( Reference Bruyndonckx, Hoymans and De Guchtenaere 28 ), and the addition of certain nutrients to the diet, such as folic acid( Reference Pena, Wiltshire and Gent 29 ), antioxidant vitamins( Reference Engler, Engler and Malloy 30 ) and certain fatty acids( Reference Engler, Engler and Malloy 31 , Reference Dangardt, Osika and Chen 32 ), may also be of benefit. A limited number of dietary interventions assessing the impact of whole foods have also suggested improved vascular function, and our study adds to this literature by demonstrating improvements in vascular function after strawberry powder intake in adolescents who were on average overweight or obese.
In the present study, we observed a significant positive change in RHI in individuals with increased fasting plasma nitrate/nitrite levels after 1 week of FDSP intake. A recent meta-analysis noted that supplementation with inorganic nitrate was associated with a significant improvement in endothelial function, measured by flow-mediated dilation, and that these effects were dose dependent( Reference Hord, Tang and Bryan 33 ). Inorganic nitrate is present in many vegetables and fruits, and it is thought to be a key constituent providing health benefits associated with these foods( Reference Bondonno, Croft and Hodgson 34 , Reference Susin, Kmecl and Gregorcic 35 ). Strawberries contain high levels of nitrate when compared with other types of fruits( Reference Nabrzyski and Gajewska 36 , Reference Lundberg, Weitzberg and Gladwin 37 ), and this may be responsible, in part, for the favourable changes noted in vascular function in our study. Dietary nitrate may serve as a potential source of the potent vasodilator NO. Dietary nitrate is actively reduced to nitrite by bacteria in the oral cavity, with increasing enterosalivary nitrate/nitrite levels associated with increased plasma levels of nitrite, which under specific conditions can be further reduced to bioactive NO( Reference Cosby, Partovi and Crawford 38 ). The reduction of nitrate to nitrite and subsequently NO is potentially O2 independent( Reference Hobbs, George and Lovegrove 39 ), which is of particular importance in situations of reduced blood flow or increased tissue O2 demand, occurring under conditions such as tissue ischaemia and exercise. Therefore, the intake of nitrate-rich foods and beverages has the potential to improve vascular function in individuals at increased CV risk. Illustrative of this, the consumption of certain nitrate-rich foods, such as beetroot juice and leafy greens, has been reported to have beneficial effects on blood pressure( Reference Vanhatalo, Bailey and Blackwell 40 – Reference Xu, Ikeda and Yamori 43 ).
In addition to nitrate, polyphenols in strawberries may also contribute to favourable vascular effects. In vitro and animal studies have shown that anthocyanin treatments can activate, as well as up-regulate, function and/or production of endothelial NO synthase( Reference Lazze, Pizzala and Perucca 44 – Reference Zhu, Xia and Yang 46 ). A human study with an anthocyanin supplement noted improved endothelium-dependent vasodilation, whereas the addition of a NOS inhibitor abolished the effects( Reference Nohria, Gerhard-Herman and Creager 47 ). Although NO plays a crucial part in regulation of the microvasculature, other factors including input from the sympathetic system and mediators such as adenosine and prostacyclin can contribute to vasodilation/constriction( Reference Hedetoft and Olsen 48 , Reference Zunino, Parelman and Freytag 49 ). Therefore, it is possible that elevated plasma nitrate/nitrite levels may not have been sufficient to act alone in altering the vascular function we report.
In the present study, we did not observe significant positive changes in plasma lipids after 1 week of FDSP intake. Other studies have observed positive changes in plasma lipids after 4 or more weeks of intake( Reference Basu, Fu and Wilkinson 14 , Reference Basu, Wilkinson and Penugonda 15 , Reference Erlund, Koli and Alfthan 50 ). These strawberry feeding trials in adult populations have also reported positive effects on blood lipids, including a decrease in TC and LDL-cholesterol( Reference Basu, Fu and Wilkinson 14 , Reference Basu, Wilkinson and Penugonda 15 , Reference Erlund, Koli and Alfthan 50 ). Similarly, a study that fed mixed berries, which included strawberries, to middle-aged adults at risk for CVD reported an increase in HDL after 8 weeks of intake( Reference Massberg, Brand and Gruner 51 ).
Platelets play an integral role in the initiation and progression of atherosclerosis( Reference Gallistl 52 ), and altered platelet function has been reported in obese children and adolescents( Reference Giordano, Del Vecchio and Cecinati 53 – Reference Alvarez-Suarez, Giampieri and Tulipani 56 ). The effects of strawberries and strawberry drinks made with a similar powder to that used in the present study on platelet function in adults have been examined by several investigations( Reference Ellis, Edirisinghe and Kappagoda 17 , Reference Massberg, Brand and Gruner 51 ). Consumption of two daily portions of berries that included strawberries (100 g strawberry purée) for 8 weeks inhibited platelet function as measured by a platelet function analyser( Reference Massberg, Brand and Gruner 51 ), and 6 weeks of daily intake of a strawberry beverage (approximately 100 g fresh strawberries) significantly attenuated PAI-1 concentrations after a high-carbohydrate/fat meal challenge( Reference Ellis, Edirisinghe and Kappagoda 17 ). A recent study reported that a 30-d intake of 500 g of fresh strawberries was significantly associated with a reduced number of activated platelets( Reference Alarcon, Fuentes and Olate 57 ). Although extracts of strawberry polyphenols have been shown to reduce platelet aggregation and soluble P-selectin levels, in vitro ( Reference Kimura, Lu and Skurnick 58 ), in the present study, we observed similar improvements in platelet function in both groups, which might be attributed to other factors present in both control and strawberry powders, such as the amount of K, which has also been associated with reduced platelet reactivity( Reference Bialasiewicz, Prymont-Przyminska and Zwolinska 59 ).
Limitations of the present study include the short intervention period, which may not have been sufficiently long to elicit positive changes in certain outcomes. Although carryover effects may also be of concern, previous studies have shown that a washout period of 6–10 d is sufficient to return circulating strawberry phenolic levels to baseline( Reference Prymont-Przyminska, Zwolinska and Sarniak 60 ,61). Although our control beverage was isoenergetic, it differed in fibre and K contents, which may have influenced the interaction effects. Our study population contained a mix of normal weight, overweight and obese adolescents, and although the normal-weight participants were on the heavier end of the spectrum, their inclusion could have reduced the statistical power needed to detect significant changes. Further limitations may include the sampling time of the study. Although we initially based the timing of sampling on reports of flavonoid- and anthocyanin-induced changes in vascular function as soon as 1 week and 1 h after intake, recent reports have suggested a biphasic response that may be influenced by the presence of colonic metabolites(62), whether or not a similar response would occur with FDSP intake would be of interest in future trials. Finally, a more rigorous measure of compliance might have been helpful, as most of the teenagers in the present study did not return their used packets for verification, even though they verbally reported consumption. Whether the differential in nitrate and vascular responses can serve as a potential form of compliance is of interest, and will need to be confirmed in subsequent investigations. With the above limitations noted, to our knowledge, the present study is the first to examine the effects of consumption of a strawberry powder-based drink on CV outcomes in overweight adolescents, and supports the concept that strawberries can provide vascular health benefits in heavier adolescent males.
Acknowledgements
The authors thank Matthew Vanness and Grace Lau for their assistance with facilitating study visits, as well as their help with data entry and dietary analysis.
Research funding was provided in part by the California Strawberry Commission (CSC). The CSC had no role in the design, analysis or writing of this manuscript. Partial support was also provided by USDA NIFA National Needs Graduate Fellowship to D. D., and by the Agriculture and Food Research Initiative Competitive grant no. 2012-01370 to R. R. H. The UC Davis Comprehensive Cancer Center Optical Biology Core Laboratory is funded by the UC Davis Comprehensive Cancer Center Support Grant awarded by the National Cancer Institute (NCI P30CA093373).
This manuscript is the result of a team research effort emanating from a study designed by C. L. K., R. M. H. and R. R. H. The study was subsequently conducted by D. D. and Ren, with Shindel serving as study physician. The data were analysed by D. D. and R. R. H., with guidance from R. M. H. and C. L. K., who also contributed to the final interpretation. All authors participated in the writing and critical review of the final manuscript, and have read and approved the final version.
The authors declare that there are no conflicts of interest.









