Introduction
In patients with psychotic disorders, smoking is highly prevalent [Reference De Leon and Diaz1]. Moreover, smoking prevalence in individuals who are at risk for psychosis—also known as ultra-high risk (UHR) individuals—exceeds three times the prevalence rate found in age-matched controls [Reference Fusar-Poli, Tantardini, De Simone, Ramella-Cravaro, Oliver and Kingdon2,Reference Auther, McLaughlin, Carrión, Nagachandran, Correll and Cornblatt3]. In individuals with a psychotic disorder and also in UHR individuals, a widespread impairment of neurocognitive functioning has been observed [Reference Catalan, Salazar de Pablo, Aymerich, Damiani, Sordi and Radua4]. Deficits in verbal learning, visual memory, processing speed, attention/vigilance, and intelligence contribute to the prediction of later transition to psychotic disorder [Reference Catalan, Salazar de Pablo, Aymerich, Damiani, Sordi and Radua4].
A modifiable factor that is probably related to cognition is smoking [Reference Depp, Bowie, Mausbach, Wolyniec, Thornquist and Luke5]. Acute nicotine administration is associated with beneficial effects on cognition by increasing activity in several brain regions [Reference Heishman, Kleykamp and Singleton6]. However, the relationship between nicotine administration and cognitive functioning is complex and not completely disentangled [Reference Valentine and Sofuoglu7]. Distinguishing between cognitive effects of acute or chronic nicotine use is important: concerning the latter, a substantial body of literature in both the general population as in patients with psychosis have found worse cognitive functioning in chronic nicotine users. For example, memory performance accuracy was poorer in adolescent smokers than in nonsmokers [Reference Jacobsen, Krystal, Mencl, Westerveld, Frost and Pugh8]. Furthermore, cognitive impairments were more severe with earlier age of onset of smoking. Another study [Reference Mons, Schottker, Muller, Kliegel and Brenner9] showed that elderly smokers are at a higher risk for cognitive impairment and that this risk increases with the duration and intensity of smoking and subsides with time after smoking cessation. These results are in line with findings from a recent meta-analysis in patients with established psychosis, where chronic smoking was related to impairments across several cognitive domains [Reference Coustals, Martelli, Brunet-Lecomte, Petillion, Romeo and Benyamina10]. Additionally, a longitudinal study of patients with psychosis and their unaffected siblings found that smoking cessation was associated with an improvement in processing speed [Reference Vermeulen, Schirmbeck, Blankers, Van Tricht, Bruggeman and Van den Brink11]. There is a lack of longitudinal studies evaluating the association between nicotine (both acute as well as chronic) and cognitive functioning in UHR individuals. So far, no longitudinal and only one cross-sectional study [Reference Gupta and Mittal12] with a small sample size have been conducted. This is unfortunate, given the presence of cognitive deficits [Reference Seidman, Shapiro, Stone, Woodberry, Ronzio and Cornblatt13], their impact on functional outcome [Reference Lin, Wood, Nelson, Brewer, Spiliotacopoulos and Bruxner14], and the high smoking prevalence [Reference Carney, Cotter, Bradshaw, Firth and Yung15] in this vulnerable group.
Therefore, the aim of the current study is to examine the association between smoking and cognitive performance on several domains across different assessment times in an UHR population and a healthy control group. Based on the findings from populations with established psychosis [Reference Coustals, Martelli, Brunet-Lecomte, Petillion, Romeo and Benyamina10] and unaffected siblings [Reference Vermeulen, Schirmbeck, Blankers, Van Tricht, Bruggeman and Van den Brink11], we hypothesized that smoking status or the number of cigarettes smoked per day would be negatively associated with cognitive functioning in UHR as well as in healthy controls. Furthermore, we aimed to explore whether cessation of smoking was associated with improvements in cognitive functioning.
Methods
Study design
This study was performed within the European network of national schizophrenia networks studying gene–environment Interactions (EU-GEI) cohort [Reference van Os, Rutten, Myin-Germeys, Delespaul and Viechtbauer16,Reference Menghini-Muller, Studerus, Ittig, Heitz, Egloff and Andreou17]. EU-GEI is a multicenter, prospective naturalistic study conducted between May 1, 2010 and April 30, 2015 and consisted of a baseline measurement and three follow-up points (at 6 months, 1 year, and 2 years). The aim of EU-GEI is the identification of clinical, genetic, and environmental interactions in the development, severity, and course of (subclinical) psychotic disorders in participants and their families. The EU-GEI study was conducted in accordance with the Declaration of Helsinki and the protocol of the study was approved by the institutional review boards of all study sites.
Participants
Individuals were recruited from 11 early detection centers (Amsterdam, Den Haag, Vienna, Basel, Cologne, Melbourne, Copenhagen, Paris, Barcelona, Sao Paulo, and London). Further details are also described in Supplementary Material S1. Participants were aged between 14 and 45 years (mean age: 22 years). Participants were included in the study if they met at least one of the three UHR criteria as defined by the Comprehensive Assessment of At-risk Mental State (CAARMS) [Reference Yung, Yuen, McGorry, Phillips, Kelly and Dell’Olio18]: (a) Vulnerability Group: a first-degree relative with a psychotic disorder or diagnosed with schizotypal personality disorder in combination with a significant drop in functioning during at least 1 month in the previous year; (b) Attenuated Psychotic Symptoms (APS) Group: the presence of subthreshold positive psychotic symptoms for at least 1 month during the past year; or (c) Brief Limited Intermittent Psychotic Symptoms (BLIPS) Group: an episode of frank psychotic symptoms that lasted no longer than 1 week, which abated spontaneously. Exclusion criteria were a prior experience of a psychotic episode of more than 1 week as determined by the CAARMS [Reference Yung, Yuen, McGorry, Phillips, Kelly and Dell’Olio18] or an intelligence quotient (IQ) below 60. Control participants were recruited from four different sites (London, Amsterdam, Den Haag, and Melbourne). Exclusion criteria for controls were similar to those for UHR participants. Additionally, controls were excluded if they met the criteria for an ARMS status as defined by the CAARMS [Reference Yung, Yuen, McGorry, Phillips, Kelly and Dell’Olio18]. Individuals were analyzed for whom complete data for smoking status was available at baseline. Assessments at 6 months were scarce in most inclusion sites, however, not due to any patient-specific reasons, as this time point was introduced later in the study. EU-GEI researchers with various backgrounds (research assistants, psychologists, psychiatrists, nurses, and PhD students) were extensively trained to increase inter-rater reliability (IRR). All researchers achieved high IRR scores before permitted to perform assessments [Reference van Os, Rutten, Myin-Germeys, Delespaul and Viechtbauer16,Reference Berendsen, Kapitein, Schirmbeck, van Tricht, McGuire and Morgan19]. All participants provided written informed consent following a full explanation of the study.
Assessment instruments
Smoking behavior
We used the Composite International Diagnostic Interview (CIDI) to assess detailed information on smoking behavior which has previously been found to be reliable in a cross-cultural trial [Reference Cottler, Robins and Helzer20]. Smokers were defined as people who smoked daily for at least 1 month over the past 12 months. Participants were asked how many cigarettes they smoked per day in the time frame they smoked the most during the last/past year.
Cognitive measurements
Each participant was assessed on cognitive performance on baseline, 6 months, 1 year, and 2 years in accordance with the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery [Reference Green, Nuechterlein, Gold, Barch, Cohen and Essock21]. Cognitive performance included the following domains and tasks:
-
1. Speed of processing as measured with the Trail Making Test [Reference Tombaugh22] part A.
-
2. Attention/vigilance as measured with the Digit Span Forward, a subtest of the third version of the Wechsler Memory Scale (WMS-III) [Reference Wechsler23].
-
3. Working memory as measured with the Digit Span Backward, a subtest of the WMS-III.
-
4. Verbal learning as measured with the Rey Auditory Verbal Learning Test (RAVLT) [Reference Brand and Jolles24], including a component of immediate recall (trial 1–5) and delayed recall (trial 7).
-
5. Cognitive flexibility (reasoning and problem solving) as measured with the Trail Making Test part B.
Assessment of covariates
Age and gender were a priori selected covariates. Additionally, socioeconomic status (including social functioning, educational level, and work), cannabis use, childhood trauma, and psychiatric medication (including antipsychotics, antidepressants, and anxiolytics) were also selected as confounders, and psychopathology were selected as covariates as they have been found associated with both smoking and cognition in individuals with psychosis [Reference Vermeulen, Schirmbeck, Blankers, Van Tricht, Bruggeman and Van den Brink11,Reference Quigley and MacCabe25–Reference Keefe, Sweeney, Gu, Hamer, Perkins and McEvoy27]. The general level of social functioning was scored using the disability scale of the Global Assessment of Functioning (GAF) [Reference Goldman, Skodol and Lave28]. Educational level was defined as total years in education. IQ was assessed with the 15-min version [Reference Velthorst, Levine, Henquet, de Haan, van Os and Myin-Germeys29] of the Wechsler Adult Intelligence Scales third editions [Reference Wechsler30], but was not included as a covariate considering the overlap with years in education. Current employment was divided into two subgroups: no paid work and student or paid work. The Cannabis Experience Questionnaire was administered to assess information regarding the current and lifetime use of cannabis (yes or no). The Childhood Trauma Questionnaire was used to explore whether participants had experienced childhood trauma, which has been found to be a valid retrospective assessment instrument [Reference Liebschutz, Buchanan-Howland, Chen, Frank, Richardson and Heeren31]. A total score of all five subscales (emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect) was computed as a covariate for the analyses. Severity of symptoms was measured using the CAARMS [Reference Yung, Yuen, McGorry, Phillips, Kelly and Dell’Olio18] and the following subscales were included in the analyses: general symptoms (in which the majority of items measure affective symptoms), positive, and negative symptoms.
Statistical analyses
We used SPSS Statistics version 26 (Chicago, IL) for all analyses. Baseline differences in demographic characteristics and outcomes between smokers and nonsmoking UHR individuals were assessed using independent t tests and Mann–Whitney U tests for numerical variables and Pearson Chi-square tests for categorical variables. To test the first hypothesis, linear mixed models were applied to assess associations between smoking status, the number of cigarettes smoked per day, and cognitive performance scores across different assessment times over a period of 2 years (multicross-sectional). UHR individuals and controls were included in the analyses if data on the outcome variable of interest was available for at least one time point (baseline, 6 months, 1 year, or 2 years) as mixed modeling allowed us to calculate valid estimates under the assumption of missing at random. Little’s MCAR test [Reference Roderick32] appeared to be nonsignificant indicating that data were missing at least at random, which was in line with visual inspection of missing data patterns on baseline. Models were fitted using maximum likelihood estimation [Reference Harrison, Donaldson, Correa-Cano, Evans, Fisher and Goodwin33,Reference Seltman34] and continuous variables were centered [Reference Bolger and Laurenceau35] to improve model performance and interpretability [Reference Harrison, Donaldson, Correa-Cano, Evans, Fisher and Goodwin33]. As outcome variables, raw scores for each cognitive domain were used. Visual inspection of residual plots of all cognitive measurements revealed no deviations from normality, with exception of the cognitive performance scores on the Trail Making Test (parts A and B). In the first model, smoking status (yes/no), time, age, and gender were entered as fixed effects. In case simple models showed significant results, all other a priori selected covariates were planned to be added en bloc: GAF, education, work, cannabis, trauma scores, and medication. In all models, subjects were added as random intercept and time was added as random slope. In order to investigate a dose–response relationship, linear mixed effect models were run replacing smoking status by the number of cigarettes smoked per day. To answer the second research question regarding the effect of change in smoking behavior, we compared smoking behavior at 1-year follow-up to baseline as well as at 2-year follow-up to 1-year follow-up. We categorized four UHR subgroups between assessments: individuals who never smoked, individuals who continued smoking, individuals who were able to quit, and individuals who started smoking. Change scores between assessments were calculated for all outcome variables. In the first set of mixed model analyses, individuals who never smoked were compared with individuals who started smoking. In a later set, we compared individuals who continued to smoke with individuals who were able to quit. Additionally, linear mixed effect models with change in the number of cigarettes smoked per day as independent variable were performed. Similar fixed and random effects as previously mentioned were entered. No longitudinal analyses regarding change in smoking status and/or cigarettes were performed in healthy controls due to a lack of power (Supplementary Material S5b). In all mixed model analyses, p values were calculated by the Satterthwaite method which has been evaluated in REML-fitted models and produced the most acceptable type I error rates in mixed-effects models [Reference Luke36]. Given the six outcome variables in five independent cognitive domains, Bonferroni correction was used to minimize the risk of type I errors. Therefore, the two-tailed significance threshold was set at 0.008 (0.05/6).
Results
Sample characteristics
In total, 345 UHR individuals and 67 healthy controls participated in the study (Figure 1). For the current analyses, only participants with data on smoking were included (330 UHR and 66 healthy controls). Sociodemographic features and clinical characteristics are presented in Table 1. In total, 11 UHR individuals and 2 healthy controls had assessments dates with extreme deviation (>1,000 days from baseline) on follow-up outcome variables. As we were interested in associations over approximately a 2-year period, these participants were excluded from the mixed model analysis. Data on cognitive performance scores were missing for a maximum of 58 (17.6%) UHR individuals and 23 (34.8%) healthy controls at baseline. See Supplementary Material S2 for detailed information regarding missing data on covariates and cognitive performance scores (Table 2).

Figure 1. Available date per cognitive measurement over time in ultra-high risk (UHR) individuals and healthy controls.
Table 1. Demographic characteristics of smoking and nonsmoking ultra-high risk (UHR) individuals and controls.
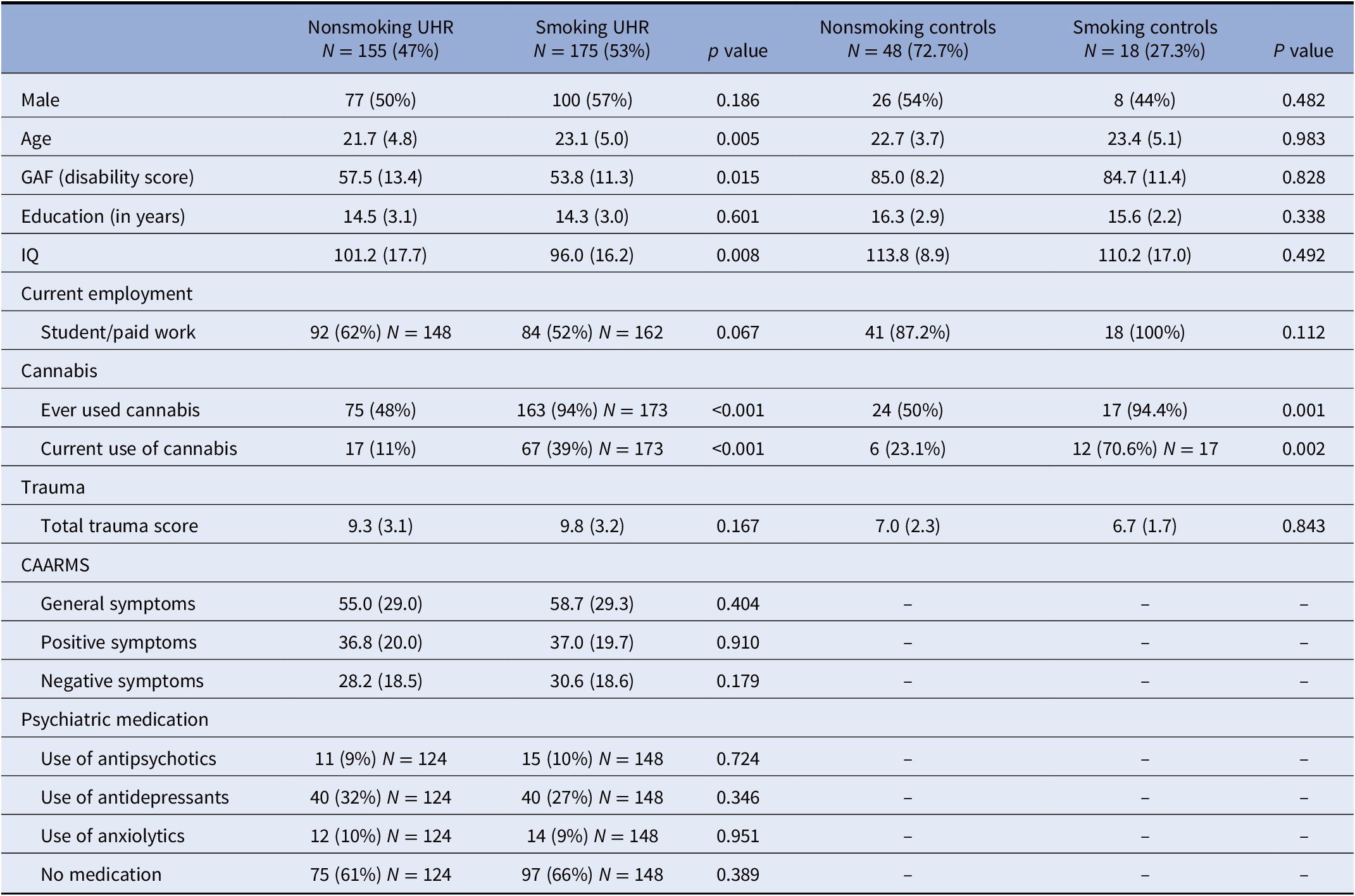
Data are presented as N (%) or mean (standard deviation).
Abbreviations: CAARMS, Comprehensive Assessment of At-risk Mental State; GAF, Global Assessment of Functioning; IQ, estimated Intelligence Quotient.
Table 2. Multicross-sectional results from linear mixed models regarding smoking status and cognitive performance in UHR individuals and controls.
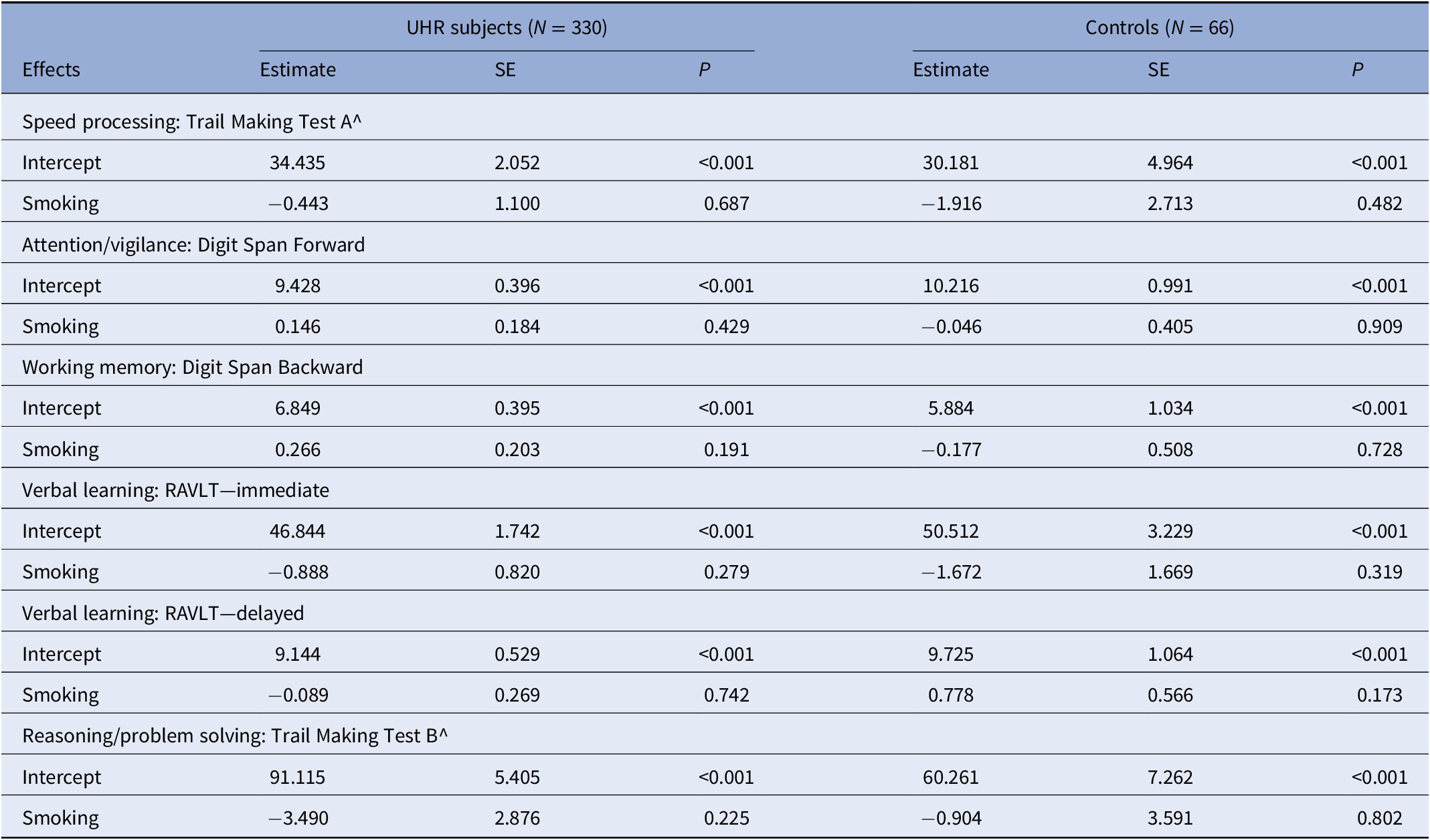
The following fixed effects were added to the model: age + gender (model 1) + time. Subjects were added as random intercept and time was added as random slope.
Abbreviation: RAVLT = Rey Auditory Verbal Learning Test; SE, standard error.
Baseline characteristics of smoking and nonsmoking individuals
At baseline, 175 (53%) UHR individuals and 18 (27%) controls reported daily smoking within the last 12 months. Smoking UHR individuals and controls smoked an average of 12.0 (SD = 8.7) and 9.7 (SD = 8.5) cigarettes per day, respectively. Baseline comparisons showed that smoking UHR subjects were significantly older than nonsmokers, reported more lifetime or current cannabis use, had lower GAF scores, and had lower IQ. Smoking control subjects significantly used more cannabis than nonsmoking controls, as listed in Table 1. In total, 35% of smoking individuals used psychiatric medication (of whom 10% used antipsychotics) compared to 40% of nonsmoking individuals (of whom 9% used antipsychotics). Baseline cognitive performance scores between smoking and nonsmoking UHR and controls are listed in Supplementary Material S3.
Multi-cross-sectional associations between smoking behavior and cognitive performance
Mixed model analyses showed no significant differences between UHR individuals and controls who did or did not smoke on any of the cognitive performance scores, as listed in Table 3. In the second set of mixed model analyses, the number of cigarettes smoked per day was added as an independent variable instead of smoking status. These models revealed no significant associations between the number of cigarettes per day and cognitive performance scores (see Supplementary Material S4).
Table 3. Longitudinal results from linear mixed models regarding change in smoking status and change in cognitive performance in UHR.
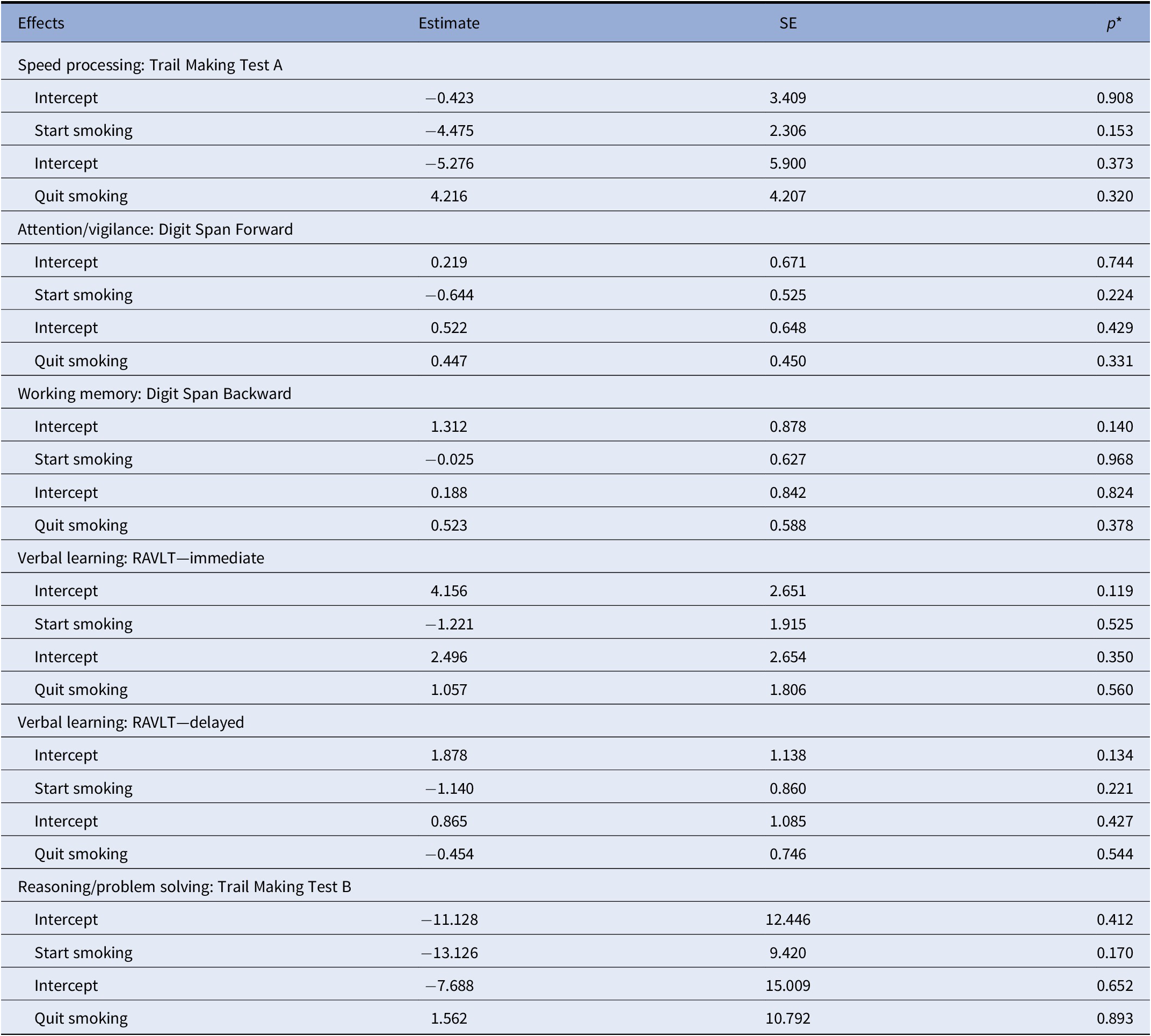
The following fixed effects were added to the model: age + gender (model 1) + time. Subjects were added as random intercept and time was added as random slope. Reference group: no smoking (vs. start) and continue smoking (vs. quit).
Abbreviation: RAVLT = Rey Auditory Verbal Learning Test.
Longitudinal associations regarding change in smoking status and change in cognitive performance
Data on smoking behavior over time was available for 43% of UHR individuals and 46% of controls (Supplementary Material S5). From these, 84.8% of UHR individuals did not change their smoking behavior between two assessments: a total of 37.7% never smoked and 47.3% continued to smoke between assessments. Over time, 10.7% of UHR individuals quit and 6.4% started smoking.
In the second set of mixed model analyses, nonsmoking individuals over time (N = 106) were compared to individuals who started to smoke between baseline and 1-year follow-up, or between 1-year and 2-year follow-up (N = 18). No significant between-group differences regarding change in cognitive performance score were found. A second set was applied between individuals who continued to smoke over time (N = 130), compared to individuals who quit between baseline and 1-year follow-up, or between 1-year and 2-year follow-up (N = 27). No significant differences were found between cognitive performance scores of individuals who continued smoking and those who stopped smoking. See for full details Table 3. Concerning controls, 83.6% did not adjust smoking behavior over time. 67.2% (N = 41) never smoked and 16.4% (N = 10) continued to smoke. Of all controls with available smoking data, 8.2% (N = 5) quit as well did 8.2% (N = 5) initiated to smoke over time.
Longitudinal association regarding change in the number of cigarettes and change in cognitive performance
Explorative analyses were conducted in 40.5% of UHR individuals for whom it was possible to calculate a change score in the number of cigarettes smoked per day between assessments (Supplementary Material S5a). No significant associations were found between change in the number of cigarettes smoked per day and change in any of the cognitive performance scores (Supplementary Material S6).
Discussion
This study showed that in both UHR individuals and healthy controls, none of the tested cognitive domains (speed of processing, attention/ vigilance, working memory, verbal learning, or reasoning/problem solving) revealed multicross-sectional or longitudinal associations with (change) in smoking behavior across a two-year follow-up. Smoking prevalence was high (53%) in this sample of UHR individuals, compared to our control group (27.3%) and findings in the general population [Reference Prochaska, Das and Young-Wolff37]. The cognitive performance of our sample was comparable to UHR samples from previous studies [Reference Lin, Wood, Nelson, Brewer, Spiliotacopoulos and Bruxner14,Reference Allott, Wood, Yuen, Yung, Nelson and Brewer38] and tended to be intermediary between individuals with a first episode and healthy individuals [Reference Whitson, O’Donoghue, Hester, Baldwin, Harrigan and Francey39,Reference Stouten, Veling, Laan, van der Helm and van der Gaag40]. To the best of our knowledge, this is the first prospective study to evaluate the relationship between naturalistic smoking and cognitive functioning on different domains in UHR and healthy controls.
Our multicross-sectional findings are at odds with the findings of Gupta et al. [Reference Gupta and Mittal12] who reported better visual learning, higher processing speed, and improved working memory associated with chronic smoking in 35 UHR individuals. Nonetheless, the effect-sizes of correlations in their study were small (r = 0.33, r = 0.29, and r = 0.30, respectively). Furthermore, they defined smoking behavior differently (i.e., categorically) and different tasks were used to evaluate cognitive functions (except for the Trail Making Test A). More importantly, correlations between smoking and cognitive function were only corrected for IQ. The use of cannabis was not mentioned as a confounding variable, although the history of a substance dependence disorder in the prior 6 months was an exclusion criterion.
Two prior studies [Reference Cadenhead41,Reference De Koning, Bloemen, Van Duin, Booij, Abel and De Haan42] in UHR evaluated smoking in relation to sensorimotor gating using prepulse inhibition (PPI), a biological measure proposed to be associated with cognitive functioning [Reference Potter, Summerfelt, Gold and Buchanan43,Reference Yadon, Bugg, Kisley and Davalos44]. Cadenhead et al. [Reference Cadenhead41] reported greater PPI—reflecting better sensorimotor gating—in chronic tobacco smoking UHR compared to nonsmoking UHR subjects. By contrast, De Koning et al. [Reference De Koning, Bloemen, Van Duin, Booij, Abel and De Haan42] did find a smoking x group interaction effect in the UHR group which was associated with lower prepulse inhibition. These contradictory results underline that further research is required to elucidate the impact of smoking on cognitive functioning as measured by cognitive batteries and biological measures in UHR.
Associations between smoking and worse cognitive functioning have been found repeatedly in patients with psychosis: a recent meta-analysis by Coustals et al. [Reference Coustals, Martelli, Brunet-Lecomte, Petillion, Romeo and Benyamina10] evaluating 18 studies concluded that smoking patients had worse performance on certain cognitive tasks than nonsmoking patients. However, studies focusing on patients with a first-episode psychosis showed contradictory results. Some authors [Reference Grossman, Bowie, Lepage, Malla, Joober and Iyer45] reported worse global cognition in smokers, which is in line with findings in psychosis and the general population. Others [Reference Hickling, Perez-Iglesias, Ortiz-Garcia de la Foz, Balanza-Martinez, McGuire and Crespo-Facorro46–Reference Sánchez-Gutiérrez, García-Portilla, Parellada, Bobes, Calvo and Moreno-Izco49] did not find any differences between smoking and nonsmoking FEP and some authors found cognitive-enhancing effects of nicotine [Reference Zabala, Eguiluz, Segarra, Enjuto, Ezcurra and Gonzalez Pinto50]. To the best of our knowledge, no systematic review nor meta-analyses exist on this topic. In the general population, it has been shown that the longer people smoke (measured by pack-years), the higher the risk of cognitive deficits [Reference Sabia, Elbaz, Dugravot, Head, Shipley and Hagger-Johnson51]. The lack of associations between smoking and cognitive functioning in the current study might be explained by the fact that our UHR subjects and controls were on average younger (22 years) than the subjects included in the meta-analysis of Coustals et al. (27–49 years) [Reference Coustals, Martelli, Brunet-Lecomte, Petillion, Romeo and Benyamina10] and the general population study (56 years) [Reference Sabia, Elbaz, Dugravot, Head, Shipley and Hagger-Johnson51]. Consequently, our UHR subjects might not have smoked for that many years and thus, not suffer yet from cumulative brain damage caused by smoking-induced oxidative stress, inflammation, and white matter lesion progression, which have been associated with cognitive decline [Reference Campos, Serebrisky and Castaldelli-Maia52,Reference van Dijk, Prins, Vrooman, Hofman, Koudstaal and Breteler53].
An alternative explanation for the absence of an association between smoking and cognition in UHR might be that latent psychotic processes caused deterioration of cognitive performance in both smoking and nonsmoking UHR, covering a true smoking effect. However, the fact that we did not find any associations between smoking (and the number of cigarettes) and cognition in our control group argues against this explanation as this group did not suffer from illness-related symptoms. Additionally, one might state that in some individuals, the use of nicotine played an etiologic role [Reference Kendler, Lonn, Sundquist and Sundquist54,Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis and GMJ55] in crossing the threshold to UHR, while others met UHR criteria without any substance-related risk factors and might be more susceptible for (subclinical) and cognitive symptom severity.
A secondary finding of the current study was the fact that 10.3% of UHR individuals were able to quit smoking over a 1-year follow-up period. Cessation rates were almost twice as high compared to an UHR population studied by Ward et al. [Reference Ward, Lawson, Addington, Bearden, Cadenhead and Cannon56] (5.5% cessation) in which smoking cessation was evaluated over a longer follow-up period (2-year). Sustained quitting is associated with a shorter follow-up period [Reference Zeng, Zong, Zhang, Feng, Ng and Ungvari57] which might be a plausible explanation for higher cessation rates in the current study. In a FEP population, 3.9% quitted smoking during a 15-month follow-up period [Reference Wade, Harrigan, Edwards, Burgess, Whelan and McGorry58]. Together, these data suggest that early interventions focused on smoking cessation and relapse prevention in UHR are worthy of investigation. We did not find an association between smoking initiation (N = 19) or smoking cessation (N = 30) and change in any of the tested cognitive domains in these small subsamples. These results are in contrast with studies conducted in patients with psychosis and the general population, which found a positive relationship between smoking cessation and improvements of processing speed [Reference Vermeulen, Schirmbeck, Blankers, Van Tricht, Bruggeman and Van den Brink11] and overall cognitive functioning [Reference Mons, Schottker, Muller, Kliegel and Brenner9], respectively. In the general population study [Reference Mons, Schottker, Muller, Kliegel and Brenner9], a longer time since smoking cessation was associated with higher differences in cognitive performance compared to current smokers. This may explain the lack of an association in our study as cognitive performance was evaluated after a 1-year interval of smoking cessation, compared to a 3-year interval in psychosis [Reference Vermeulen, Schirmbeck, Blankers, Van Tricht, Bruggeman and Van den Brink11] and a 10-year interval in the general population [Reference Mons, Schottker, Muller, Kliegel and Brenner9]. Furthermore, the current study may not have been sufficiently powered to reach the statistic threshold as only 30 individuals were included in the change analysis for smoking cessation, compared to substantially larger samples in the general population (N = 602) [Reference Mons, Schottker, Muller, Kliegel and Brenner9] and in patients with psychosis (N = 517) [Reference Vermeulen, Schirmbeck, Blankers, Van Tricht, Bruggeman and Van den Brink11].
Our results suggest that although the smoking prevalence in UHR is higher [Reference Fusar-Poli, Salazar de Pablo, Correll, Meyer-Lindenberg, Millan and Borgwardt59] and cognitive functioning is poorer compared to healthy controls [Reference Seidman, Shapiro, Stone, Woodberry, Ronzio and Cornblatt13], associations between smoking behavior and cognitive performance were not detected. This might indicate that smoking in UHR individuals is initiated for other reasons than (effective) alleviation of poorer cognitive functioning as suggested by the self-medication hypothesis [Reference Segarra, Zabala, Eguíluz, Natalia, Elizagárate and Sanchez60]. A shared genetic and environmental architecture [Reference Khokhar, Dwiel, Henricks, Doucette and Green61,Reference Peterson, Bigdeli, Ripke, Bacanu, Gejman and Levinson62] between smoking and psychosis could partially explain why these UHR individuals already smoke so frequently and heavily.
Strengths and limitations
The strengths of the current study lie in the prospective design together with the inclusion of a relatively large sample of UHR subjects (N = 330) and a healthy control group (N = 66). Furthermore, several sites across the world took part in this study, which increases its generalizability and importance for public health interventions. This study has also several limitations that should be acknowledged. First, participants were not asked or instructed about smoking behavior before they underwent the cognitive battery. Although smoking was not allowed during the assessment, potential acute pre-assessment effects of nicotine and/or withdrawal effects could have influenced cognitive function. Future studies should carefully consider smoking behavior when applying cognitive measurements. In addition, no data were available regarding levels of carbon monoxide or cotinine. Nevertheless, interviewer-reported questionnaires (such as the CIDI) were shown to produce accurate data regarding the validity of self-reported smoking behavior [Reference Patrick, Cheadle, Thompson, Diehr, Koepsell and Kinne63]. Secondly, the degree of tobacco use was administered using the CIDI [Reference Cottler, Robins and Helzer20], which has a scope of 12 months. Hence, smoking history (i.e., pack-years) was not assessed. Potential brain damage caused by chronic smoking was not taken into account. However, given the fact that our UHR population is relatively young, chronic smoking is less plausible. Third, assessments at 6 months were scarce in most centers due to differences in study design procedures. Also, a substantial number of participants were lost to 1- and 2-year follow-up. Fourth, in our explorative analyses evaluating change in smoking behavior and cognitive performance, our model was not able to calculate a p value of the estimate of the random slope and in some cases, no random intercept. However, sensitivity analyses were done without time as random slope which revealed similar results as the primary model. Lastly, the fact that only help-seeking individuals were included in the study may have led to a selection bias which limits the generalization of our findings.
Conclusion
UHR individuals who smoked did not exhibit cognitive differences compared to nonsmoking UHR. However, the finding that one in every two UHR individuals daily uses tobacco represents a major health issue that demands priority in treatment. Comparisons with previous literature suggest that our UHR sample shows fewer cognitive impairments and higher smoking cessation rates compared to patients with FEP. Implications to promote smoking cessation in the UHR stage needs further investigation.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2021.2233.
Data Availability Statement
Data are not publicly available.
Acknowledgment
The authors thank all participants who took part in the study.
Author Contributions
Conceptualization: F.S. J.M.V., L.d.H.; Data curation: H.S.v.d.H., F.S., J.M.V.; Formal analysis: H.S.v.d.H.; Investigation: H.S.v.d.H.; Methodology: H.S.v.d.H., F.S., J.M.V.; Supervision: F.S., J.M.V., L.d.H.; Writing – original draft: H.S.v.d.H., F.S., L.d.H., J.M.V.; Writing – review & editing: H.S.v.d.H., F.S., L.d.H, J.M.V., M.J.K., M.v.d.G., K.A., B.N., S.R.
Financial Support
The European Network of National Schizophrenia Networks Studying Gene–Environment Interactions (EU-GEI) Project is funded by grant agreement HEALTH-F2-2010-241909 (Project EU-GEI) from the European Community’s Seventh Framework Program. Additional support was provided by a Medical Research Council Fellowship to M Kempton (grant MR/J008915/1).
Conflicts of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.







Comments
No Comments have been published for this article.