Streptococcus agalactiae (group B streptococcus; GBS) is a leading cause of invasive neonatal infections associated with high morbidity and mortality. Infection usually presents either as early onset (within 6 days of life) with bacteraemia, pneumonia or meningitis or late-onset (7–89 days of life) [Reference Edwards, Baker, Mandell, Bennett and Dolin1]. Early onset neonatal disease is associated with maternal intrapartum GBS colonization and transmission is thought to occur vertically, either through ascending spread of the organism into the amniotic fluid or through the birth canal at time of delivery [Reference Edwards, Baker, Mandell, Bennett and Dolin1]. Genital and lower gastrointestinal tract colonization of GBS occurs in about 10–40% of pregnant and non-pregnant women [Reference Edwards, Baker, Mandell, Bennett and Dolin1]. A previous study from Malaysia reported that the genital carriage rate of GBS in parturients was 9·7% and the average annual incidence of neonatal GBS septicaemia in babies born in hospital was 0·4/1000 live births [Reference Lim2]. Women with prenatal GBS colonization have a 25-fold higher risk of delivering a baby with early onset GBS disease compared to non-colonized women [Reference Boyer and Gotoff3].
There are at least nine recognizable GBS serotypes based on capsular polysaccharide antigens, i.e. serotypes Ia, Ib and II–VIII [Reference Johri4]; with serotype IX proposed recently [Reference Slotved5]. The serotype distribution of GBS varies with geographical region and time and hence conjugate vaccines developed for major disease-causing GBS serotypes may not be universally optimal [Reference Johri4, Reference Croak6, Reference Lachenauer7].
A Medline search revealed no recently reported study from Malaysia describing serotypes of GBS in pregnant women and data from South East Asia are scarce. Therefore, the current study was undertaken to determine the serotypes and antimicrobial susceptibility of GBS in pregnant women attending the University of Malaya Medical Centre (UMMC). This laboratory-based prospective study was conducted from March to August 2008. Two hundred non-duplicate GBS isolates were collected from vaginal swabs from pregnant women attending as outpatients or in-patients at UMMC. The samples, in either Amies or Stuart's transport media were directly plated onto blood agar, chocolate agar, modified Thayer–Martin agar (MTM) and Sabouraud's dextrose agar (SDA). Isolates were identified as GBS by colonial morphology, a positive CAMP test and a positive group B Lancefield antigen detected by a commercial Streptococcus typing kit (Avipath-strep; Omega Diagnostics, Scotland, UK). Isolates were stored at −80°C and serotyped by co-agglutination (Essum GBS serotyping kit; Sweden) with reagents for serotypes Ia, Ib, II–VIII. Isolates not agglutinating with any of the reagents as well as those having non-specific weak agglutination with more than one serotype were termed non-typable (NT). Antimicrobial susceptibility testing was performed and interpreted by the Clinical and Laboratory Standards Institute (CLSI) disc diffusion method [8] and susceptibility to penicillin, erythromycin, vancomycin and clindamycin was recorded. For pregnant women with recurrent GBS isolates, only the first was included for analysis. Ethical approval to conduct this study was obtained from the Research Ethics Committee, UMMC.
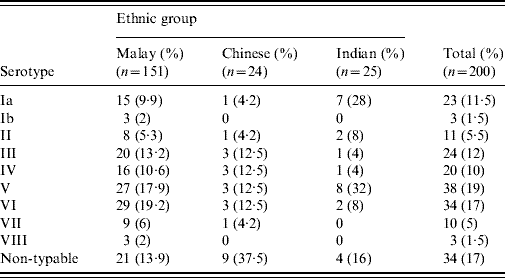
The ethnic distribution was Malays (75·5%), followed by Indians (12·5%) and Chinese (12%). The mean age of the women was 28·9 (±4·7) years. Of the 200 GBS isolates, serotypes V (19%) and VI (17%) were predominant followed by serotypes III, Ia and IV which were isolated at almost similar frequencies (12%, 11·5% and 10%, respectively). Serotypes II, VII and VIII constituted 12% of the isolates (Table 1) and 17% were NT. Serotypes V (17·9%) and VI (19·2%) isolates were the most prevalent types in Malay women whereas in Chinese women, 37·5% were serologically NT; serotypes V (32%) and Ia (28%) predominated in Indian women.
Table 1. Serotype distribution of GBS isolates from pregnant women
All isolates were susceptible to penicillin and vancomycin, but eight isolates (4%) were resistant to erythromycin and five (2·5%) were resistant to clindamycin. Four of the erythromycin-resistant strains, were also resistant to clindamycin. Two each of the erythromycin-resistant strains belonged to serotype Ib and NT, respectively, and one each were of serotypes II, III, IV and V.
The epidemiology of GBS serotypes not only varies in different geographical areas but also changes over time. There is no previous reported study from Malaysia describing serotypes of GBS in pregnant women. However, a study reported from UMMC about two decades ago [Reference Ngeow and Puthucheary9], mainly involving genitourinary isolates from adults and some neonatal isolates, found serotype III to be predominant (50·9%), followed by serotype II. Serotyping was performed with a limited set of antisera (Ia, Ib, Ic, II and III) and so is not directly comparable with the current study. Nevertheless, the number of serologically NT isolates found in 1987 comprised only 4·9% of all isolates and 5·3% of genitourinary isolates [Reference Ngeow and Puthucheary9]. The serotype distribution of GBS in antenatal women is clearly quite different and more diverse. Two serotypes (V and VI) accounted for 36% of the isolates with 33% falling in serotypes III, Ia and IV. The predominance of serotypes V and VI in pregnant Malaysian women is in contrast to the high prevalence of serotypes II and III in a study from China [Reference Shen10] and serotype Ib in Korea [Reference Uh11]. Serotype V has become increasingly frequent in pregnant women in the USA, New Zealand and Kuwait [Reference Croak6, Reference Grimwood12, Reference Al-Sweih13]. By contrast, serotype VI appears to be rare in pregnant women in other countries with the exception of Japan and Kuwait [Reference Lachenauer7, Reference Al-Sweih13]. Interestingly serotype VIII, which is common in pregnant Japanese women [Reference Lachenauer7], represented only 1·5% of our isolates. Serotype IV, noted in 10% of our subjects, is also uncommon in most studies, with the exception of United Arab Emirates [Reference Amin, Abdulrazzaq and Uduman14]. The proportion of NT isolates in the current study (17·1%) is similar to that reported from Kuwait [Reference Al-Sweih13].
An earlier study from UMMC found all GBS genital tract isolates to be susceptible to penicillin with 1·7% resistance to erythromycin [Reference Parasakthi, Ngeow and Tan15]. The resistance rates to erythromycin (4%) and clindamycin (2·5%) found in the current study were lower compared to studies from Taiwan which had resistance of 33% to erythromycin and 41·6% to clindamycin [Reference Ko16]. Relatively high rates of resistance to erythromycin and clindamycin were also noted in a study in the USA [Reference Croak6] where resistance was 12% and 8%, respectively. Resistance to these antibiotics was not associated with serotype V strains as reported by Grimwood et al. [Reference Grimwood12].
In summary, this study provides current data on the common serotypes and antimicrobial susceptibility of GBS vaginal isolates from antenatal women in Malaysia. The emergence of new GBS serotypes and as yet low rate of resistance to erythromycin are findings with important implications for prevention and treatment strategies. Realizing the limitations of our study, further studies and surveillance data from Malaysia are required to monitor the current disease burden of GBS as well as monitoring colonizing and invasive serotypes in both antenatal women and neonates. This information is necessary to implement an effective global vaccine strategy and intrapartum antibiotic prophylaxis, which are the key preventive strategies for neonatal GBS infections.
ACKNOWLEDGEMENTS
This study was supported by research grant provided by Monash University, Sunway Campus, Malaysia (M-1-07). The authors acknowledge Teng Fui Enn for technical assistance.
DECLARATION OF INTEREST
None.



