Recent cross-sectional population studies have reported an approximately 20 % prevalence of hypovitaminosis C (typically defined by a plasma concentration < 23–28 μm) in adults in the Western world, even though vitamin C (VitC) deficiency is easily prevented by ingestion of fresh fruit and vegetables( 1 – Reference Schleicher, Carroll and Ford 4 ). Currently, the only recognised clinical consequence of VitC deficiency is scurvy, i.e. the potentially fatal manifestation of prolonged and severe deficiency (plasma concentration < 11·4 μm), preventable with relatively low intakes of VitC( 5 ). However, reports from both experimental and human studies have suggested that long-term adverse effects may result from more moderate states of deficiency( Reference Tveden-Nyborg, Johansen and Raida 6 , Reference Myint, Luben and Welch 7 ) (reviewed in Higdon & Angelo( Reference Higdon and Angelo 8 ) and Tveden-Nyborg & Lykkesfeldt( Reference Tveden-Nyborg and Lykkesfeldt 9 )).
Increasing focus has been devoted to the function of VitC in the brain. The brain maintains a particularly high level of VitC compared with other organs during states of deficiency, suggesting a central role within the brain( Reference Lykkesfeldt, Trueba and Poulsen 10 , Reference Hughes, Hurley and Jones 11 ), which has been supported by experimental studies indicating that reduced VitC levels in the brain may lead to reduced neuronal quantities and thus functional consequences( Reference Tveden-Nyborg, Johansen and Raida 6 , Reference Harrison, Dawes and Meredith 12 ). Serving as a low-molecular-weight, water-soluble antioxidant, VitC is a main contributor to general redox homeostasis together with intramembranous, lipophilic tocopherols( Reference Reiber, Martens and Prall 13 , Reference Tanaka, Hashimoto and Tokumaru 14 ). The micronutrient is also associated with specific enzymatic pathways. This includes acting as an electron donor for Fe2+-2-oxoglutarate-dependent dioxygenases involved in the formation of stable triple helix collagen( Reference Hara and Akiyama 15 – Reference Yoshikawa, Takahashi and Imamura 17 ). Mice lacking the principal transporter of VitC to the brain( Reference Sotiriou, Gispert and Cheng 18 ) do not survive beyond birth, and display cerebral haemorrhages ascribed to decreased levels of collagen IV in the basement membranes of cerebral microvessels( Reference Harrison, Dawes and Meredith 12 ). A role as a cofactor in the hydroxylation of hypoxia-inducible transcription factors has also been suggested, associating VitC with angiogenesis and normal brain development( Reference Tomita, Ueno and Sakamoto 19 – Reference Kuiper, Dachs and Currie 22 ). VitC is connected to specific neurotransmitters, serving as a reductant in the dopamine β-hydroxylase-catalysed conversion of dopamine to norepinephrine( Reference Diliberto and Allen 23 – Reference Meredith and May 27 ), and has been linked to the reuptake of glutamate( Reference Lane and Lawen 28 , Reference Cammack, Ghasemzadeh and Adams 29 ). Furthermore, VitC is associated with the preservation of reduced tetrahydrobiopterin( Reference Heller, Unbehaun and Schellenberg 30 – Reference Baker, Milstien and Katusic 32 ), which may be involved in the regulation of monoamine neurotransmitter metabolism( Reference Meredith and May 27 , Reference Ward, Lamb and May 33 ). Finally, VitC has been associated with epigenetic regulation of DNA transcription by functioning as a cofactor for ten-eleven translocation dioxygenase enzymes, thereby possibly affecting multiple genes related to brain function (reviewed in Harrison et al. ( Reference Harrison, Bowman and Polidori 34 )).
The uptake of VitC to tissues and cells is dynamic. The principal transporters are the active specific Na-dependent vitamin C transporters (SVCT)( Reference Sotiriou, Gispert and Cheng 18 , Reference Tsukaguchi, Tokui and Mackenzie 35 ), of which isoform 1 (SVCT1) has low affinity/high capacity for ascorbate (ASC)( Reference Wang, Dutta and Huang 36 , Reference Daruwala, Song and Koh 37 ) and is thought to mediate whole-body homeostasis; and isoform 2 (SVCT2) has high affinity/low capacity( Reference Daruwala, Song and Koh 37 , Reference Godoy, Ormazabal and Moraga-Cid 38 ) and is responsible for organ-specific distribution and reuptake of ASC. Facilitated diffusion of dehydroascorbic acid (DHA) – the two-electron oxidation product of ASC – by GLUT 1–4 occurs with varying efficiency, and is affected by competitive inhibition from glucose( Reference Rumsey, Kwon and Xu 39 – Reference Agus, Gambhir and Pardridge 41 ). Passive diffusion is believed to be of minimal importance for overall VitC homeostasis under normal physiological conditions (reviewed in Lindblad et al. ( Reference Lindblad, Tveden-Nyborg and Lykkesfeldt 42 )). The distribution and activity of different VitC transporters vary between tissues and cell types, affecting the ability to adapt to changes in the availability of VitC via both a regulated uptake of the micronutrient and accumulation and recycling of oxidised VitC (for a comprehensive review, please see Lindblad et al. ( Reference Lindblad, Tveden-Nyborg and Lykkesfeldt 42 )).
Human studies of dietary requirements are often complicated by differences in lifestyle and a resulting plethora of uncontrollable factors( Reference Lykkesfeldt and Poulsen 43 ). In observational studies, the precision of VitC intake estimates is affected by dietary recall errors( Reference Frei, Birlouez-Aragon and Lykkesfeldt 44 ), as well as by the effects of season, preparation and storage on the VitC content of different food items( 45 ). VitC intervention studies performed so far have often lacked baseline measurements of VitC and appropriate inclusion and exclusion criteria (reviewed in Lykkesfeldt & Poulsen( Reference Lykkesfeldt and Poulsen 43 )). As an additional challenge in human studies, several tissues (including the brain) have reduced accessibility for sampling in vivo, and due to the non-linear kinetics of VitC, tissue concentrations are not easily predicted from plasma levels. Here, controlled animal experiments constitute a valuable supplement. The guinea pig is a natural model of diet-induced VitC deficiency, since as with humans, it lacks the ability to synthesise the vitamin due to a defective gene coding for l-gulono-γ-lactone oxidase that catalyses the final step of d-glucose conversion to VitC( Reference Burns and Evans 46 – Reference Nishikimi, Fukuyama and Minoshima 50 ).
Although previous studies have attempted to clarify the bioavailability and tissue distribution of VitC in guinea pigs( Reference Hughes, Hurley and Jones 11 , Reference Berger, Shepard and Morrow 51 ), data describing the distribution of the vitamin to the brain during chronic exposure to various dietary regimens are scarce. In the present study, a dose-dependent distribution of VitC was examined in a guinea pig model to elucidate saturation kinetics in the plasma, cerebrospinal fluid (CSF), selected brain areas (frontal cortex (FC), hippocampus (HP) and cerebellum (CB)) and other tissues relative to dietary availability. The characterisation of the dose-dependent tissue levels of VitC in a model with similar dependency on dietary supplementation may prove valuable in the future discussions of dose ranges recommended for humans.
Materials and methods
In vivo study
The present experiment was approved by the Danish Animal Experiments Inspectorate under the Ministry of Food, Agriculture and Fisheries (license no. 2011/561-50). A total of sixty female Dunkin–Hartley guinea pigs (HA-SIFE150200; Charles River Laboratories), 7 d of age, had a 12 mm microchip implanted subcutaneously in the neck for identification (Pet-ID), were blocked according to weight, and subsequently randomised into six dietary groups (n 10) upon arrival to the animal facility. All groups received a purified diet (Research Diets, Inc.) produced to meet the nutritional requirements of guinea pigs (the only difference being the quantity of VitC), and also received water and dried hay ad libitum. Diets with final concentrations of 100, 250, 500, 750, 1000 and 1500 mg VitC/kg were titrated weekly from feeds containing 0 mg/kg (D11091304), 727·6 mg/kg (D11091305) and 2128·4 mg/kg (D11091306) of phosphorylated VitC by analysis. Diet composition is presented in Table 1. Pellets used for titration were stored at − 18°C to diminish oxidation of VitC. The lowest dose 100 mg VitC/kg has previously been shown to be non-scorbutic( Reference Tveden-Nyborg, Johansen and Raida 6 , Reference Tveden-Nyborg, Vogt and Schjoldager 52 ). The animals were group-housed in floor pens in an enriched environment at 22 ± 2°C with a 12 h light–12 h dark cycle, inspected daily by trained personnel and weighed twice per week. At 60–64 d of age, guinea pigs were anaesthetised by inhalation with isoflurane (Isoba Vet; Intervet). Following thoracotomy, an intracardial blood sample was obtained using a syringe with an 18G, 40 mm needle that had previously been flushed with 15 % K3-EDTA (03 664; Sigma-Aldrich)( Reference Lykkesfeldt 53 ). With the heart in situ, CSF was collected from the cerebellomedullary cistern by puncture of the dorsal atlanto-occipital membrane using a micro glass pipette (mean sample volume 56 μl and range 14–110 μl). CSF sampling was completed within < 2 min, after which guinea pigs were euthanised by decapitation. In the majority of the animals, the heart was beating throughout the procedure; however, in a few cases, the heart stopped immediately before or during sampling. The brain was excised from the cranium case and divided into hemispheres by sagittal section through the corpus callosum below the cerebral longitudinal fissure. One hemisphere was randomly assigned for biochemical analysis, and the HP, the rostral part of the cerebrum (coronal section rostral to the corpus callosum, denoted as the FC) and the CB were sampled. Furthermore, the liver, left kidney and left adrenal gland were taken out.
Table 1 Composition of guinea pig diets used for titration*

* Six experimental diets containing vitamin C concentrations ranging from 100 to 1500 mg/kg were titrated weekly from the diets #D11091304, #D11091305 and #D11091306. #D11091304 and #D11091305 were mixed to make diets containing 100, 250 and 500 mg/kg of vitamin C, while #D11091305 and #D11091306 were mixed to make diets containing 750, 1000, and 1500 mg/kg of vitamin C. The unit of amounts stated in the table is g, unless otherwise specified.
† Phosphorylated vitamin C had 33 % activity. Source of vitamin C was Rovimix Stay-C 35.
‡ Analysis performed by Covance Laboratories, Inc.
Biochemistry
Plasma and CSF samples were immediately stabilised by acidic deproteinisation with equal amounts of 10 % (w/v) meta-phosphoric acid (239275; Sigma-Aldrich) in 2 mm-disodium EDTA (Na2-EDTA, 1.08 418.1000; Merck) after collection, to minimise ex vivo conversion of ASC to DHA( Reference Lykkesfeldt, Loft and Poulsen 54 ), and stored at − 80°C. After excision, tissues were flushed in ice-cold Dulbecco's PBS (pH 7·4), frozen on dry ice and stored at − 80°C until analysis. The frozen tissue specimens were homogenised in 4°C Dulbecco's PBS (pH 7·4) and stabilised with equal amounts of 10 % (w/v) meta-phosphoric acid in 2 mm-Na2-EDTA. All samples were analysed using reversed-phase ion-pairing HPLC with coulometric detection, as described previously( Reference Lykkesfeldt 55 , Reference Lykkesfeldt 56 ). The method used has been shown to have excellent analytic reproducibility (within- and between-day CV 1·5 and 3·5 %, respectively) and specificity, as well as adequate sample stability to preserve labile VitC compounds and in vivo ASC–DHA equilibrium for up to 5 years( Reference Lykkesfeldt 56 , Reference Lykkesfeldt 57 ).
Curve fitting
Based on the non-linear appearance of basic data plots (see Figs. 1 and 2), a three-parameter Hill equation was fitted to data from the CSF, brain, liver and kidneys to give a description of the dose–concentration relationship for VitC in guinea pigs. Model fitting was performed in R (versions 3.0.2 and 3.1.0, available at http://www.r-project.org/), and model assumptions were checked with raw residual plots.

Fig. 1 Distribution of vitamin C (VitC) to the plasma (a), cerebrospinal fluid (CSF) (b) and brain (c). The concentration of VitC (ascorbate +dehydroascorbic acid) in the plasma (○, μm) (reproduced from Mortensen et al. ( Reference Mortensen, Hasselholt and Tveden-Nyborg 58 )), CSF (●, μm) and different parts of the brain (nmol/g) after ingestion of diets with specified concentrations of the micronutrient for approximately 55 d is presented. Values are means, with standard deviations represented by vertical bars. The corresponding three-parameter Hill equation fits to the data from the CSF and brain are indicated. Parameter estimates for the algorithms are presented in Table 3. The vertical bars indicate dietary dose ranges leading to saturation of the target tissue. (c) Saturation of the cerebellum (□) and the frontal cortex (▲) is represented by bar A, while bar B indicates saturation of hippocampus (Δ). Plasma and brain: n 10 pigs per group. Hippocampus (100 mg VitC/kg): n 9. An outlier was excluded (depicted in the plot in grey). CSF: n 9, 8, 9, 9, 8 and 9 per group for doses of 100, 250, 500, 750, 1000 and 1500 mg VitC/kg, respectively.

Fig. 2 Distribution of vitamin C (VitC) to the liver (a), kidneys (b) and adrenal glands (c). The concentration (nmol/g) of VitC (ascorbate+dehydroascorbic acid) in tissues after ingestion of diets with specified concentrations of the micronutrient for approximately 55 d is presented. Values are means (n 10), with standard deviations represented by vertical bars. The corresponding three-parameter Hill equation fits to the data from the liver and kidneys are indicated. Parameter estimates for the algorithms are presented in Table 3. It was not possible to reach convergence for the three-parameter Hill equation fit to the data from the adrenal glands.
Statistical analyses
With regard to sample size, a detectable difference of >30 % was assessed to be of potential biological relevance with an expected CV of about 20 % based on previous data from this laboratory. This yielded a sample size of seven when using a power of 80 % and a significance level (α) of 0·05. A few more animals were included due to the duration of the study (n 10). Statistical analyses were performed in SAS JMP (version 10.0.0; SAS Institute, Inc.) and R. ANOVA was used for overall effect tests followed by Tukey's honest significant difference post hoc test provided significant differences. Where test assumptions were not fulfilled (assessed with normal QQ, studentised and raw residual plots) and data transformation was unsuccessful, permutation tests were performed. For growth data, an ANOVA with random effect of animal was performed, with the variance heteroskedasticity incorporated into the model. Random effect of animal was also included in the comparison of VitC levels in different brain parts performed within dietary groups. Spearman's correlation coefficient (ρ) was used for correlation analysis. An α of 0·05 was chosen for all calculations.
Results
Body weights
The growth of guinea pigs in the different dietary groups was comparable. A significant interaction was found between time and diet (P< 0·001); however, the final mean body weights following 49 d on the diet (467 (sd 31), 470 (sd 28), 465 (sd 40), 465 (sd 40), 479 (sd 24) and 465 (sd 48) g for 100, 250, 500, 750, 1000 and 1500 mg VitC/kg, respectively) did not differ between the groups (P>0·05).
Biochemistry
The results from the biochemical analysis of VitC content in the CSF and tissues are presented in Table 2 and Figs. 1 and 2. The plasma VitC data have been published previously( Reference Mortensen, Hasselholt and Tveden-Nyborg 58 ). As expected, the concentration of VitC in all the fluids and tissues examined was affected by the diet (P< 0·001 for all).
Table 2 Cerebrospinal fluid (CSF, μm) and tissue levels (nmol/g) of vitamin C (VitC, ascorbate+dehydroascorbic acid) following the dietary regimen in guinea pigs (Mean values and standard deviations, n 10)

a,b,c.dMean values within a row with unlike superscript letters were significantly different (P< 0·001).
* Indicates the ratio between the concentrations on the target site resulting from the highest and the lowest dietary dose used.
† For plasma and CSF measurements, no post hoc test was performed as groups were compared with a permutation test.
‡ Reproduced from Mortensen et al. (58).
§ n 9.
∥ n 8.
For all dietary doses, the highest tissue concentration of VitC was found in the adrenal glands, where organ saturation had been reached at 250 mg VitC/kg and the mean concentration (approximately 6400 nmol/g) was several times higher than that in all the other tissues examined. At the highest dose of VitC (1500 mg VitC/kg), the liver had the second highest tissue concentration (1880 nmol/g) followed by the brain, kidneys and CSF in decreasing order. However, for the low-dose group (100 mg VitC/kg), all concentrations in the brain surpassed those in the liver, and all tissues had significantly lower concentrations of VitC than that observed in the other dose groups.
Also, for 100 mg VitC/kg, the concentration of VitC varied between all the brain regions examined (P< 0·001 for overall effect and post hoc tests). For higher doses, the HP had lower concentrations of VitC than the FC and CB, which did not differ significantly (P< 0·01 for all post hoc comparisons).
Distribution kinetics
Dose–concentration plots for all tissues examined are shown in Figs. 1 and 2, with three-parameter Hill model fits to the data from the CSF, brain, liver and kidneys. Parameter estimates for the Hill equation are listed in Table 3. Due to the uncertainty of the parameters, the Hill equation curves were not extrapolated beyond the applied VitC doses. In the brain, plateaus on dose–concentration curves at 250–400 and 150–250 mg VitC/kg were observed for the HP, and the FC and CB, respectively, indicating saturation (see Fig. 1(c)). In the CSF and kidneys, a saturation point at 250–400 mg VitC/kg was found; however, visually, the plots suggested that a continued increase in the concentration of VitC may be observed for higher dietary doses. In the liver, a transient plateau was observed for intermediate dietary doses (250–750 mg VitC/kg diet), after which the concentration continued to increase with dose. A considerable inter-individual variation in the correlation between VitC intake and the CSF and tissue concentrations was observed.
Table 3 Parameter estimates for the Hill equation* (Mean values with their standard errors)

CSF, cerebrospinal fluid; C max, maximal concentration on target site; D 50, dose 50 (dietary dose of vitamin C resulting in 1/2 C max); n, Hill coefficient.
* Three-parameter Hill equations
![]() $$( C _{target} = ( c _{max}\times D _{diet}^{ n })/( D _{50}^{ n } + D _{diet}^{ n })) $$
were used to describe the relationship between dietary dose of vitamin C (mg/kg) and concentration in the CSF (μm) and tissues (nmol/g), where C
target is the concentration on the target site and D
diet is the dose of vitamin C in the diet.
$$( C _{target} = ( c _{max}\times D _{diet}^{ n })/( D _{50}^{ n } + D _{diet}^{ n })) $$
were used to describe the relationship between dietary dose of vitamin C (mg/kg) and concentration in the CSF (μm) and tissues (nmol/g), where C
target is the concentration on the target site and D
diet is the dose of vitamin C in the diet.
† For the CSF, the unit is expressed as μm.
The concentrations of VitC in plasma were positively correlated with VitC levels in all the tissues and CSF, as shown in Fig. 3. The correlation was markedly stronger between plasma and concentrations in the liver (ρ = 0·83, P< 0·001), kidneys (ρ = 0·89, P< 0·001) and CSF (ρ = 0·91, P< 0·001) than between plasma and concentrations in the adrenal glands (ρ = 0·46, P< 0·001), FC (ρ = 0·60, P< 0·001), CB (ρ = 0·62, P< 0·001) and HP (ρ = 0·64, P< 0·001), as indicated by the higher ρ values. The visual presentation of correlations (Fig. 3) also suggested that the adrenal glands and the brain regions examined were less correlated with plasma VitC concentrations under sufficient conditions, whereas concentrations in the liver, kidneys and CSF continued to increase with increasing plasma levels. Concentrations in the brain were also only moderately correlated with those in the CSF, with ρ values being 0·67, 0·68 and 0·64 for the HP, FC and CB, respectively (dataset for the calculation of CSF–brain correlations contained only complete data pairs; n 51 for the HP (excluding the data pair containing the outlier indicated in Fig. 1) and n 52 for the FC and CB).

Fig. 3 Correlation between the levels of vitamin C (VitC) in the plasma, cerebrospinal fluid (CSF) and tissues. A positive correlation was found between the levels of VitC (ascorbate+dehydroascorbic acid) in the plasma and all the tissues examined (kidneys (a), adrenal glands (b), frontal cortex (c), liver (d), hippocampus (e), cerebellum (f)) and CSF (g). Spearman's correlation coefficients (ρ) are presented in the table. The association tests have a null hypothesis of no association (i.e. zero correlation, ρ = 0). A total of sixty data pairs were included, except for the hippocampus (n 59, the data pair containing the outlier indicated in Fig. 1 was excluded, depicted in grey) and the CSF (n 52, a useful CSF sample could not be acquired). *** P< 0·001.
The increase in VitC levels occurring from the plasma to the CSF differed between the dietary groups. The CSF:plasma ratio varied from about 4 for the high dietary doses of VitC increasing for lower doses to a maximum of 6·1 for the group receiving 250 mg VitC/kg. For the 100 mg VitC/kg dose group, the ratio was decreased to 3·4 (see Table 4). From the CSF to the brain, the changes in VitC concentration were more pronounced. In the group receiving 1500 mg VitC/kg, a 4- to 6-fold increase was found, whereas the concentration changes in the HP, FC and CB were 22-, 39- and 56-fold, respectively, in the group receiving 100 mg/kg VitC. For intermediary dose groups, the increase of VitC concentrations from the CSF to the brain was 4- to 8-fold depending on group and brain region.
Table 4 Biological variation of vitamin C (VitC) levels in the plasma and cerebrospinal fluid (CSF)*
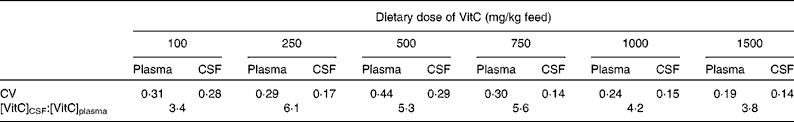
* The biological variation of VitC levels in the plasma and CSF was assessed using the CV. Calculations were based on the formula: CV = sd/mean. All values used for CV and CSF:plasma ratio calculations were obtained from Table 2.
Discussion
In the present study, the distribution kinetics of VitC were dependent on dietary availability and saturation was reached at low doses in the brain compared with other organs. Within the brain, VitC distribution was differential and dose-dependent, and the relationship between dietary dose and tissue concentration was describable with a three-parameter Hill equation. Together with a relatively moderate correlation between the plasma and brain levels of VitC, this indicates the importance of the micronutrient in the brain.
As expected, we found the brain to be favoured during deficiency, reaching levels of VitC surpassed only by concentrations in the adrenal glands. A tight homeostatic control was observed in these organs, minimising fluctuations in the levels of VitC. While the concentration of VitC in other tissues changed more than 6-fold between the lowest and highest doses used in the present study, levels in the brain and adrenal glands changed 4-fold or less. Comparing plasma:target ratios from diets containing 1500 and 100 mg VitC/kg, respectively, indicates that organs are differentially affected by decreased availability of VitC. Concentrations in the plasma and CSF were about 20-fold higher in animals receiving 1500 mg VitC/kg compared with the 100 mg VitC/kg dose group. The low VitC plasma:CSF ratio in the 100 mg VitC/kg group in conjunction with the reported 22- to 56-fold difference in VitC concentration between the CSF and brain regions as well as the corresponding Hill equation, suggests that despite a preferential transport, the CSF is unable to provide the brain with adequate VitC levels in this low dose regimen.
The distribution of VitC within the brain was dependent on dietary dose, and regional concentration differences were observed. At the 100 mg VitC/kg dose, the CB, FC and HP contained different amounts of VitC, whereas the levels in the CB and FC were similar for larger intakes. For all dietary doses, the HP had lower levels of VitC than the CB and FC. At the 100 mg VitC/kg dose, the latter two brain regions also upheld VitC levels closer to saturation compared with the HP. Thus, VitC in the CB and FC was half and one-third of the saturated levels, respectively, after a diet containing 100 mg VitC/kg, whereas the levels in the HP were decreased to less than one-quarter. Moreover, the CB and FC were saturated with a diet containing 250 mg VitC/kg, whereas the HP required 250–400 mg VitC/kg for saturation to occur. This could imply that the HP has lower priority during states of deficiency than the CB and FC, making it more vulnerable to suboptimal levels of VitC. Low levels of VitC in the brain have previously been shown to result in impaired spatial memory and a reduction in neuronal quantities in the dentate gyrus and the cornu ammonis of the HP in the guinea pig( Reference Tveden-Nyborg, Johansen and Raida 6 ). An explanation for the differences in VitC content in the different brain regions could be the varying expression of SVCT2( Reference Tsukaguchi, Tokui and Mackenzie 35 , Reference Mun, Kim and Lee 59 ), the primary transporter of VitC in the brain( Reference Sotiriou, Gispert and Cheng 18 , Reference Tsukaguchi, Tokui and Mackenzie 35 ), or differences in cell-type composition between different brain regions( Reference Harrison, Green and Dawes 60 – Reference Herculano-Houzel and Lent 62 ) as neurons contain higher levels of VitC than glial cells not expressing SVCT2( Reference Mun, Kim and Lee 59 , Reference Rice and Russo-Menna 63 , Reference Berger and Hediger 64 ). Regional differences in the brain concentration of VitC have previously been found in human post-mortem samples, with the HP containing relatively high levels of VitC( Reference Mefford, Oke and Adams 65 ). In C57/BL6 J mice, Harrison et al. ( Reference Harrison, Green and Dawes 60 ) found that the CB and HP have the highest levels of VitC, and unlike in the present study, the FC had significantly lower levels of VitC than the CB. The underlying reason for the discrepancy is presently unknown.
Of the tissues examined, the kidneys consistently had the lowest concentration of VitC, whereas the tissue with the highest concentration of VitC (excluding the adrenal glands having levels of VitC several times higher than all the other organs at all dietary doses) varied depending on dietary dose. Whether this diet-dependent distribution of VitC in the body reflects a concentration-dependent uptake, tissue-specific differences in retention ability or both cannot be elucidated from the present study. However, it has been shown, both here (Table 2) and previously, that the brain has an exceptional retention capacity even during prolonged and severe VitC deficiency( Reference Lykkesfeldt, Trueba and Poulsen 10 , Reference Hughes, Hurley and Jones 11 , Reference Frikke-Schmidt, Tveden-Nyborg and Birck 66 ). Furthermore, the Hill equation fits to the VitC concentration data from brain tissue show a marked increase as a function of dietary dose in the lowest part of the dose range, indicating a highly efficient uptake until saturation is reached.
The levels of VitC reached in the CSF are comparable with those found in humans( Reference Reiber, Ruff and Uhr 67 , Reference Tallaksen, Bohmer and Bell 68 ). In comparison with plasma values, the concentration in the CSF was increased, most probably due to the active transport of VitC over the choroid plexus by SVCT2( Reference Tsukaguchi, Tokui and Mackenzie 35 , Reference Mun, Kim and Lee 59 , Reference Angelow, Haselbach and Galla 69 ). Furthermore, endothelial cells in the choroid plexus contain GLUT that are able to transport DHA into the CSF( Reference Vannucci 70 ). For both plasma and CSF, the CV was high within the individual dose groups (Table 4), demonstrating the challenge of using these biological fluids for diagnostic purposes. A larger CV in plasma compared with the CSF has been proposed to indicate an independent regulation of the CSF( Reference Reiber, Ruff and Uhr 67 , Reference Kuehne, Reiber and Bechter 71 ). The increased ratio during the lower dietary doses of VitC, as reflected by the saturated brain levels shown in Fig. 1(c), may be the result of a compensatory capacity protecting the brain against deficiency when general levels of VitC decrease, and could be due to both increased uptake and retention ability, but may also just reflect normal concentration-dependent changes in active transport processes. For the lowest dietary dose (100 mg VitC/kg), the decline in the CSF:plasma ratio in combination with the large CSF:brain ratio could indicate an inability to uphold sufficient concentrations of VitC to sustain the brain; a similarly low CSF:plasma ratio has been found previously in guinea pigs depleted of VitC( Reference Søgaard, Lindblad and Paidi 72 ). The decreased ratio could be due to changes in the recycling mechanisms of VitC including usage of the available VitC pool in the brain, alterations of intracellular VitC reuptake, and/or changes in CSF flow rate( Reference Reiber, Ruff and Uhr 67 , Reference Kuehne, Reiber and Bechter 71 ). However, the calculated correlation coefficients do not support intrathecal homeostasis as concentrations of VitC in the plasma and CSF were highly correlated, whereas correlations between the CSF and brain levels were moderate as was the case with plasma–brain correlations. The complexity of the correlation between dietary intake of VitC, plasma and CSF levels could explain the inability of the three-parameter Hill equation to describe the distribution pattern.
The adrenal glands, where VitC has been shown to be important for catecholamine synthesis and corticosterone production( Reference Bornstein, Yoshida-Hiroi and Sotiriou 73 ), had the highest tissue concentration of VitC and maintain one-quarter of the VitC content during non-scorbutic deficiency, which is approximately the same as that for the HP. Only a weak positive correlation was found between the levels of VitC in the plasma and adrenal glands. In contrast, a strong positive correlation was found between the concentrations of VitC in the plasma and kidneys. The kidneys contain SVCT1( Reference Tsukaguchi, Tokui and Mackenzie 35 , Reference Wang, Dutta and Huang 36 , Reference Lee, Oh and Mun 74 ), enabling tubular re-absorption of VitC during states of deficiency( Reference Corpe, Tu and Eck 75 – Reference Levine, Wang and Padayatty 77 ). Berger et al. ( Reference Berger, Shepard and Morrow 51 ) found VitC saturation in the kidneys at a dose of 833 mg/kg in young adult (approximately 5 months old), male guinea pigs, whereas a saturation point about 400 mg/kg for female guinea pigs was found in the present study. Whether this could be due to differences in uptake efficiency or re-absorption capacity presently cannot be ruled out. However, it has previously been shown that males and females are equally good at retaining VitC in the brain( Reference Hughes, Hurley and Jones 11 ). The relationship between dietary VitC and liver concentration cannot be described adequately by the three-parameter Hill equation. It follows a different pattern than that observed in the brain and adrenal glands with an initial dose-dependent increase, reaching a plateau for intermediate doses, followed by an additional phase of increase, not reaching saturation within the dose range used in the present study. A partial explanation for this could be the presence of both isoforms of the SVCT in the liver( Reference Wang, Dutta and Huang 36 , Reference Macias, Hierro and de Juan 78 ). SCVT1 and SVCT2 have different K m values( Reference Daruwala, Song and Koh 37 ) (reviewed in Savini et al. ( Reference Savini, Rossi and Pierro 79 )), are expressed by different cell types( Reference Macias, Hierro and de Juan 78 ) and respond differently to VitC deficiency( Reference Reidling and Rubin 80 , Reference Meredith, Harrison and May 81 ).
In contrast to the continuous positive correlation with plasma levels observed in the liver, kidneys and CSF, correlation curves for the brain and adrenal glands indicate a relatively modest correlation at high plasma levels. The moderate correlation coefficients indicate that the brain and adrenal glands are influenced less by the plasma levels of VitC than other organs probably due to a higher priority and favoured saturation of these tissues in relation to the distribution of VitC. The Hill models presented for regional concentration of VitC in the brain, although incomplete approximations, where the parameters dose 50 (D 50, dietary dose of vitamin C resulting in half of the maximal concentration on the target site (C max)) and Hill coefficients (n) are subject to some uncertainty, appear adequate in describing the distribution of VitC to the brain. Hill coefficients reported in Table 3 imply positive cooperativity between different binding sites on SVCT2, which is likely as VitC is transported down a Na gradient maintained by Na+/K+-ATPase( Reference Angelow, Haselbach and Galla 69 ), with a Na+:VitC stoichiometry of 2:1( Reference Godoy, Ormazabal and Moraga-Cid 38 ). However, the distribution kinetics of VitC are intricate, and the data presented here represent the collective ability of a complex system to absorb VitC. The picture may be confounded by the relatively few data pairs on the steep parts of the sigmoidal dose–concentration curves (due to titration limitations). Previously, n values of approximately 2 have been reported for SVCT2( Reference Godoy, Ormazabal and Moraga-Cid 38 ).
Plasma levels of VitC were fluctuating, with large inter-individual variations within groups. A considerable part of the variation may be attributed to guinea pigs not being fasted before blood sampling (due to animal welfare reasons), allowing them to ingest different amounts of VitC that was quickly reflected in the plasma concentration. Other contributing factors to the fluctuation may also include polymorphisms in SVCT1, the Hb-binding protein haptoglobin or glutathione-S-transferase alleles( Reference Cahill and El-Sohemy 3 , Reference Mortensen, Hasselholt and Tveden-Nyborg 58 , Reference Loria, Whelton and Caulfield 82 – Reference Eck, Erichsen and Taylor 84 ) (reviewed in Michels et al. ( Reference Michels, Hagen and Frei 85 )). The curve pattern for plasma VitC in male and female guinea pigs( Reference Berger, Shepard and Morrow 51 , Reference Mortensen, Hasselholt and Tveden-Nyborg 58 ) deviates from that observed in human subjects( Reference Levine, Conry-Cantilena and Wang 76 , Reference Levine, Wang and Padayatty 77 ), as saturation was apparently not reached with the given dietary doses. Based on the observed tissue concentration curves, it is speculated that plasma curves similar to the ones for human plasma, leucocytes and platelets( Reference Levine, Conry-Cantilena and Wang 76 , Reference Levine, Wang and Padayatty 77 ) could be achieved in guinea pigs using higher doses of VitC. However, it is questionable how valuable an extended plasma curve would be as several potential target tissues are already saturated with the doses used in the present study. Whether this reflects the situation in humans remains to be clarified; however, if so, the value of VitC plasma concentration as an indicator of overall VitC status is debatable.
Although the use of ad libitum feeding mimics the natural situation with uptake over the small-intestinal epithelium via SVCT1 and by facilitated diffusion( Reference Tsukaguchi, Tokui and Mackenzie 35 ), this approach poses a challenge in relation to the precision of administered dose, particularly when evaluating the effects of suboptimal VitC in the brain as the dose gap from deficiency to saturation is relatively narrow. In agreement with previous findings, a dose of 100 mg VitC/kg diet did not result in growth arrest and weight loss (as observed in guinea pigs fed lower doses of VitC( Reference Lykkesfeldt, Trueba and Poulsen 10 , Reference Berger, Shepard and Morrow 51 )) and no clinical signs of scurvy were observed( Reference Tveden-Nyborg, Johansen and Raida 6 , Reference Tveden-Nyborg, Vogt and Schjoldager 52 , Reference Williams, Suckow, Stevens and Wilson 86 ), confirming that 100 mg VitC/kg leads to a suboptimal and non-scorbutic VitC status in guinea pigs. Although both mouse and rat models unable to produce VitC endogenously exist( Reference Maeda, Hagihara and Nakata 87 – Reference Mizushima, Harauchi and Yoshizaki 89 ), it seems plausible that uptake efficiency and defence mechanisms during states of deficiency could be different and/or better developed in a species with an evolutionary absolute need for dietary VitC. This is indicated by a differential ability to utilise DHA as a source of ASC in Osteogenic Disorder Shionogi rats and guinea pigs( Reference Ogiri, Sun and Hayami 90 , Reference Cui, Otsuka and Fujiwara 91 ).
In conclusion, the distribution kinetics of VitC in the guinea pig depend on dietary availability favouring VitC retention in specific tissues (brain and adrenal glands) during deficiency while other organs are depleted. Brain and adrenal gland concentrations of VitC were also found to be only moderately correlated with plasma levels. In accordance with previous reports, VitC was found to increase in concentration from the plasma to the CSF and brain where regional differences in VitC levels were observed and saturation was reached at low dietary doses. Obtained dose–concentration curve patterns for the brain regions examined share resemblance with reported human dose–plasma, dose–leucocyte and dose–platelet curves, underlining the validity of the guinea pig model in the study of VitC.
Acknowledgements
The authors thank Annie Bjergby Kristensen, Elisabeth Veyhe Andersen and Joan Elisabeth Frandsen for excellent technical assistance. The authors appreciate the useful suggestions regarding descriptive models from Associate Professor Niels Bindslev, Department of Biomedical Sciences, University of Copenhagen, and gratefully acknowledge Professor Ib Skovgaard, Department of Mathematical Sciences, University of Copenhagen for advice concerning the statistical analyses.
The present study was supported by grants from the Danish National Research Councils (to J. L., grant no. 271-08-0763), and the LIFEPHARM Centre for In vivo Pharmacology. The funding sources had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: S. H., P. T.-N. and J. L. designed the study; S. H. and P. T.-N. conducted the in vivo experiment; S. H. and J. L. performed the data analysis; S. H., P. T.-N., and J. L. wrote the paper.
The authors declare that they have no conflict of interest.










