The Mediterranean diet has been associated with many health benefits such as a reduced risk of CVD( Reference Martinez-Gonzalez and Bes-Rastrollo 1 – Reference Gardener, Wright and Gu 4 ), cancer( Reference Couto, Boffetta and Lagiou 5 , Reference Toledo, Salas-Salvadó and Donat-Vargas 6 ), type 2 diabetes( Reference Esposito, Maiorino and Ceriello 7 , Reference Salas-Salvadó, Bulló and Estruch 8 ) and neurodegenerative diseases( Reference Psaltopoulou, Sergentanis and Panagiotakos 9 , Reference Sofi, Macchi and Abbate 10 ). The latter includes a slower rate of cognitive decline with age( Reference Tangney, Kwasny and Li 11 ), reduced risk of dementia (particularly Alzheimer’s disease (AD))( Reference Scarmeas, Stern and Mayeux 12 ) and reduced risk of mild cognitive impairment (MCI) and conversion of MCI to AD( Reference Scarmeas, Stern and Mayeux 13 ). These findings are important as dementia is now a leading cause of morbidity and mortality globally with no pharmacologic options available to prevent, slow or reverse its course( 14 ).
More than thirty Mediterranean diet indexes and their variations( Reference Bach, Serra-Majem and Carrasco 15 – Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh 19 ) (including short screeners)( Reference Schroder, Fito and Estruch 20 , Reference Cerwinske, Rasmussen and Lipson 21 ) have been reported in the literature for use in assessing adherence to a Mediterranean dietary pattern. Indexes are popular as they can assess overall dietary patterns, while reducing participant and researcher burden associated with more classical methods of dietary measurement such as long FFQ and weighed food records (FR)( Reference Thompson and Subar 22 ).
However, limitations exist with the currently available Mediterranean diet indexes. For example, while the original and widely used Mediterranean diet score, and its many iterations( Reference Trichopoulou, Kouris-Blazos and Wahlqvist 23 , Reference Trichopoulou, Costacou and Bamia 24 ), includes elements determined a priori, the cut-off points used for this tool vary between the populations studied as they are related to mean or median intakes, which may not reflect ‘traditional’ Mediterranean or optimal intakes. In addition, relatively few Mediterranean diet indexes have been validated directly against an alternate dietary assessment method( Reference Schroder, Fito and Estruch 20 , Reference Hebestreit, Yahiaoui-Doktor and Engel 25 , Reference Sotos-Prieto, Santos-Beneit and Bodega 26 ), especially one not limited by the same recall biases. Further, most Mediterranean diet index scores reported in the literature have been derived indirectly from FFQ (which may or may not be validated), then used to look for associations with health outcomes. Direct validation of dietary tools is now appreciated to be important to reliably interpret results( Reference Freedman, Commins and Moler 27 ).
Importantly, to our knowledge, no existing Mediterranean diet index tools have been validated for use among individuals at various stages of cognitive decline, such as MCI. MCI is considered a pre-dementia stage, defined by subjective concern and mild objective cognitive changes without significant changes in daily functioning related to cognition( Reference Petersen 28 ). In terms of prevention, MCI has been identified as a potential window of opportunity for lifestyle or other interventions as approximately 12 % of individuals with MCI convert to AD per year, compared with an annual conversion rate of 1–2 % in the general population( Reference Petersen, Smith and Waring 29 ). It is therefore important to be able to accurately measure adherence to a Mediterranean dietary pattern in older adults who may have already begun to manifest memory difficulties, or are diagnosed with MCI. A Mediterranean diet index tool, which has been validated in such at-risk populations, would be useful for future interventions investigating the potential of nutrition to slow progression of cognitive decline.
A short Spanish Mediterranean Diet Adherence Screener (MEDAS) used in the largest randomised controlled trial of the Mediterranean diet, that is, PREvención con DIeta MEDiterránea (PREDIMED) in cognitively normal but high CVD risk participants, is the only tool, to our knowledge, that has been associated with clinically demonstrated cognitive benefits. In a sub-cohort of these PREDIMED participants, their MEDAS score out of 14 increased over a 4-year period by approximately two points from a baseline of 8·3–8·6( Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh 19 , Reference Valls-Pedret, Sala-Vila and Serra-Mir 30 ). In addition, an increase in MEDAS scoring has been associated with other benefits, such as reduced risk of obesity and breast cancer( Reference Toledo, Salas-Salvadó and Donat-Vargas 6 , Reference Martínez-González, García-Arellano and Toledo 31 ). However, it is unclear exactly what the cut-off points in MEDAS mean for cognitive and other health outcomes. Also, the score interpretations for what is considered low, medium and high adherence to the diet in relation to studied outcomes vary for this tool( Reference Martínez-González, García-Arellano and Toledo 31 , Reference Hernandez-Galiot and Goni 32 ).
In summary, deficiencies in existing Mediterranean diet index tools may reduce their ability to predict health outcomes and guide lifestyle interventions. In addition, no tools have been developed for, and tested in, a cohort with pre-existing cognitive impairment at higher risk of conversion to dementia. Our aim was to test the reliability and validity of a more comprehensive, newly constructed, Mediterranean diet index tool including elements and cut-off points based on the ‘traditional’ Mediterranean diet, within a cohort of older people with MCI living in a non-Mediterranean country, in order to facilitate clinical research in various at-risk populations.
Methods
Participants
We recruited a convenience sample of community-dwelling participants from Sydney, Australia, who fulfilled MCI criteria( Reference Petersen, Smith and Waring 29 , Reference Gates, Valenzuela and Sachdev 33 ) from an existing clinical trial cohort( Reference Singh, Gates and Saigal 34 ), the Study of Mental and Resistance Training (SMART). The flow of participants from the original SMART trial into this validity study can be seen in Fig. 1. SMART participants had been diagnosed with MCI but without dementia, and 100 were randomised between 2008 and 2011 to resistance training and/or cognitive training for 6 months, with follow-up at 18 months and then annually, where possible, to confirm their ongoing cognitive and health status( Reference Singh, Gates and Saigal 34 ). All SMART participants, except those who were deceased, dropped out, uncontactable, involved in piloting the Mediterranean Diet and Culinary Index (MediCul) tool or known to have reverted to normal cognition or progressed to dementia were invited to participate in this validity study during one of their annual re-assessment visits, which occurred, on average, 78 months from the time they were originally recruited to SMART with the diagnosis of MCI.
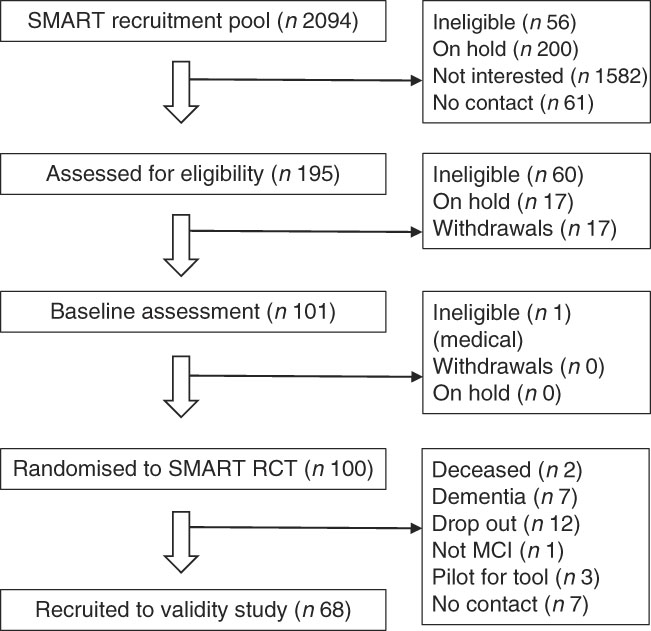
Fig. 1 Participant flow chart. The Study of Mental and Resistance Training (SMART) from which participants were recruited to the Mediterranean Diet and Culinary Index (MediCul) validity study. n, number of participants; RCT, randomised controlled trial; MCI, mild cognitive impairment.
Data administration and collection
The new Mediterranean diet index tool named MediCul was administered at the University clinic site as a paper survey (survey), twice, 1 week apart (time points A and B), with a dietitian observing and available to clarify questions (S. R.-V.). The dietitian also checked responses to ensure no question was missed. Immediately following survey B, participants were instructed to keep a 3-d FR on any two weekdays and one weekend day within a 7-d period, representing usual intake. They were asked to specify brands of foods/drinks, preparation methods and recipes, as well as to use the supplied Australian standard household measures (i.e. metric cups, spoons, jug), to estimate quantities. Participants were not required to weigh foods, although some elected to do so. Returned FR were queried with the participant by the dietitian for potentially missed food categories using a checklist.
Anthropometric data were collected at time point A using calibrated digital scales and a wall-mounted stadiometer at the clinic (in light clothing and no shoes) or portable scales and stadiometer (UC-321PBT (A&D Company Limited) and Seca 213, respectively) if a home visit was required. BMI was calculated by dividing weight (kg) by height (m2).
Additional participant characteristics including education level, marital status, number of chronic diseases, cognitive and physical function scores were sourced from original or follow-up SMART data, selecting the closest time point available for the entire cohort in our validity study. On average, this was 78 months before the validity study for education and marital status, and 59 months earlier for number of chronic diseases, Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), Katz activities of daily living and Bayer informant activities of daily living (Bayer-IADL) scores. ADAS-Cog( Reference Rosen, Mohs and Davis 35 ) and Bayer-IADL( Reference Hindmarch, Lehfeld and de Jongh 36 ) were used as primary outcomes in SMART for global cognitive function and functional independence, respectively( Reference Singh, Gates and Saigal 34 ).
The study was approved by the Ethics Review Committee (RPAH Zone) of the Sydney Local Health District (Protocol no. X08-0064 & HREC/08/RPAH/106).
Tool development
The fifty-item MediCul tool was developed empirically in the form of a short question survey (see online Supplementary Material 1 for elements, cut-off points, scoring and rationale) to (a) reflect a ‘traditional’ Mediterranean dietary pattern and certain aspects of cuisine not assessed by previous tools( Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh 19 , Reference Willett, Sacks and Trichopoulou 37 – Reference Bach-Faig, Berry and Lairon 39 ), (b) include fourteen questions from the validated MEDAS optimised for the English language( Reference Schroder, Fito and Estruch 20 ) and (c) incorporate discretionary foods, commonly consumed in Western populations( 40 ).
After conducting a literature review to identify important Mediterranean dietary elements and existing tools( Reference Radd-Vagenas, Kouris-Blazos and Fiatarone Singh 19 ), draft questions were developed in consultation with Mediterranean diet and survey tool experts. The tool was pilot-tested with five healthy people from the general public aged 50–80 years and three SMART participants with MCI for readability, ambiguity and completion timing, which is 20 min on average, before being finalised.
MediCul includes a blend of frequency and serve questions spanning seventeen main elements and assesses their exposure over the past 6 months: olive oil, vegetables, fruit, nuts, whole grains, legumes, fish/shellfish, eggs, dairy products, white meat, red/processed meats, sweets and sugary drinks, takeaway, water, alcohol, coffee and certain aspects of Mediterranean cuisine. In all, nine of these elements cover desirable features of the ‘traditional’ Mediterranean diet and four cover undesirable features of a Western diet. MediCul is scored from 0 to 100, with a higher score representing increased adherence to a ‘traditional’ dietary pattern (online Supplementary Material 2).
Nutritional analysis
Scoring for both the MediCul and MEDAS tools was operationalised using Excel (MS Office Professional Plus 2013). The FR were coded and entered into FoodWorks 8 Professional Edition: 8.0.3553 (Xyris Software Pty Ltd) selecting AusBrands 2015 and AusFoods 2015 data sources, which map to the AUSNUT 2011–2013 Food Standards Australia New Zealand nutrient database for analysis by S. R.-V. Average intakes for food group outputs were adjusted manually, where required, as FoodWorks draws on the concept of USDA Food Patterns Equivalents Database, which is sometimes contrary to current nutrition guidelines that also consider diet quality (e.g. hot chips are counted in the vegetable group). Missing foods that have become popular in recent times (e.g. paleo bread) were entered into FoodWorks using data from nutrition panels on packaging, and by basing such foods on similar products. Nutrient intakes from supplements were not included, as the aim was to test validity of MediCul based on foods alone( Reference Margetts and Nelson 41 ).
Statistical analysis
The Statistical Package for Social Sciences for Windows version 24 (SPSS Inc.) was used for all analyses. We aimed to have a minimum of fifty participants as recommended by Peat( Reference Peat 42 ) for adequate assessment of repeatability and agreement.
The distribution of MediCul scores, nutrients and food groups was examined for plausibility with the aid of histograms and by considering minimum and maximum values, to identify potential data entry errors. We did not use cut-offs for potential outliers as we were testing the tool among MCI participants and did not want to exclude for possible cognitive influences in reporting.
A comparison was made of MediCul and the derived MEDAS scores, relevant to cognitive outcomes reported in the literature( Reference Valls-Pedret, Sala-Vila and Serra-Mir 30 ). In addition, we estimated the percentage of MCI participants who reached Mediterranean diet thresholds for selected foods/aspects of ‘traditional’ cuisine presumed to be health promoting, and those rarely used traditionally but consumed at significant levels in Western populations and known to be harmful at high or frequent levels of exposure. This was performed using cut-off points for the highest score for relevant questions, from the MediCul tool (online Supplementary Material 1).
Reliability for MediCul across the two administrations, 1 week apart, was assessed using the intra-class correlation coefficient (ICC) and classified as poor (<0·40), fair to good (≥0·4 and <0·75) and very good (≥0·75)( Reference Rosner 43 ). A Bland–Altman plot was used to assess the level of agreement between survey A and B time points, as a high correlation does not necessarily mean good agreement( Reference Bland and Altman 44 ). κ was also used to check percentage agreement within the same category for the seventeen dietary elements. The κ values were characterised as showing almost perfect agreement (0·81–1·00), substantial agreement (0·61–0·80), moderate agreement (0·41–0·60), fair agreement (0·21–0·40), slight agreement (0·00–0·20) and poor agreement (<0·00)( Reference Landis and Koch 45 ).
Validity was assessed by comparing MediCul scores derived from survey A v. MediCul scores from the FR using the Bland–Altman method. The differences between the two methods were plotted against the means of the methods, with limits of agreement (LOA) as 2sd above, and below, the mean difference. Linear regression analysis was used to indicate the direction of bias and whether it was constant across mean scores.
A total of three questions from MediCul relating to growing own vegetables, main meal eaten alone and fasting frequency were unable to be validated as the FR did not include these details. We chose not to score for napping (traditionally conducted immediately after lunch in the Mediterranean); hence, this question was also not validated. Finally, the validation of the MediCul tool was based on scoring out of 97, whereas the reliability analysis was out of 100.
Indirect validity was investigated by examining whether MediCul scores were associated with expected trends in nutrient intakes extracted from the FR. Nutrient values from the FR were checked for normal distribution using graphical methods and skewness, and log 10 transformed where positively/negatively skewed, then re-checked for normality to inform the statistical tests to be used. Normally distributed or normalised nutrients from the FR were compared across tertiles of MediCul score derived from both survey A and the FR using parametric tests (one-way ANOVA), whereas non-normally distributed nutrients were analysed using non-parametric tests (Kruskal–Wallis). When the ANOVA F ratio was significant, variances were checked for equality, and Bonferroni’s test was applied for equal variances or the Games–Howell post hoc test was applied for unequal variances. In addition, first (linear)- and second (quadratic)-order polynomial contrasts were applied to test for nutrient trends across tertiles, as well as the line of best fit.
Means and standard deviations were calculated from FR values for normally distributed nutrients: kilojoules, protein, fat, fat as percentage energy, saturated fatty acids, SFA as percentage energy, SFA as percentage fat, PUFA as percentage fat, MUFA, MUFA as percentage fat, cholesterol, carbohydrate, carbohydrate as percentage energy, sugars, water, dietary fibre, vitamin C, vitamin A, β-carotene, Na, K, Mg, Fe and Zn. Medians and the interquartile range, representing tertiles 1 and 3 of the MediCul score, were calculated for non-normally distributed nutrients: protein as percentage energy, PUFA, n-3 long-chain (LC) PUFA, α-linolenic acid, EPA, docosapentaenoic acid, DHA, ratio of MUFA to SFA, ratio of total unsaturated fatty acids to SFA, vitamin E, vitamin B12, total folate, Se and alcohol.
Linear regression analysis was undertaken to assess precision of the MediCul tool across ADAS-Cog scores, being an index of global cognition (n 67).
Results
We recruited sixty-eight participants from the 100 originally randomised to the SMART trial (Fig. 1). All were included in the reliability study and sixty-five participated in the validity study. A total of two participants did not complete the FR and one had an incomplete FR. The majority of the recruited participants were female (65 %), married/de facto (56 %) and the primary cook at home (71 %). On average, they were aged 75·9 (sd 6·6) years, overweight (BMI=27·3 (sd 5·2) kg/m2), well educated (13 (sd 4) years), had 2·8 (sd 1·6) chronic diseases, good physical function and a confirmed MCI diagnosis based on the most recently available ADAS-Cog scores (Table 1).
Table 1 Characteristics of study participants (n 68) (Mean values and standard deviations; medians, ranges and percentages)
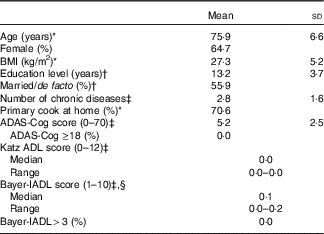
ADAS-Cog, Alzheimer’s Disease Assessment Scale-cognitive subscale, used as the primary test for global cognitive function in Study of Mental and Resistance Training (SMART, higher scores indicate more impairment; cut-off for dementia is ≥18); Katz ADL, Katz index of independence in activities of daily living (higher scores indicate more impairment; cut-off for significant functional impairment is >0); Bayer-IADL, Bayer informant activities of daily living (higher scores indicate more impairment; cut-off for significant functional impairment is >3).
* Assessed at time point A of validity study.
† Assessed at baseline of SMART study( Reference Singh, Gates and Saigal 34 ), on average, 78 months before validity study.
‡ Assessed at 18 months of SMART study( Reference Singh, Gates and Saigal 34 ), on average, 59 months before validity study with n 67 as missing tests for one participant.
§ Participant score substituted for informant score for n 3.
The mean MediCul score for survey A was 54·6/100·0 (sd 13·0; range: 32·5–85·5), with 4·4 % of participants scoring ≥80·0. The mean derived MEDAS score for survey A was 6·1/14·0 (sd 2·2; range: 1·0–11·0). All those with MEDAS scores ≥10·0( Reference Valls-Pedret, Sala-Vila and Serra-Mir 30 ) had a MediCul score ≥81·5.
On the basis of their FR, few participants reached thresholds for Mediterranean foods considered to be protective for cognitive or vascular function, such as olive oil (2 %), legumes (3 %), fruit (14 %) and water (15 %) (Fig. 2). Fewer than half had minimal exposure to potentially harmful foods, used at low levels in the ‘traditional’ diet, such as processed meat (43 %) and red meat (46 %). However, three-quarters of the participants did report that they mostly cooked their main meals at home and 89 % kept sugary drinks to a minimum.
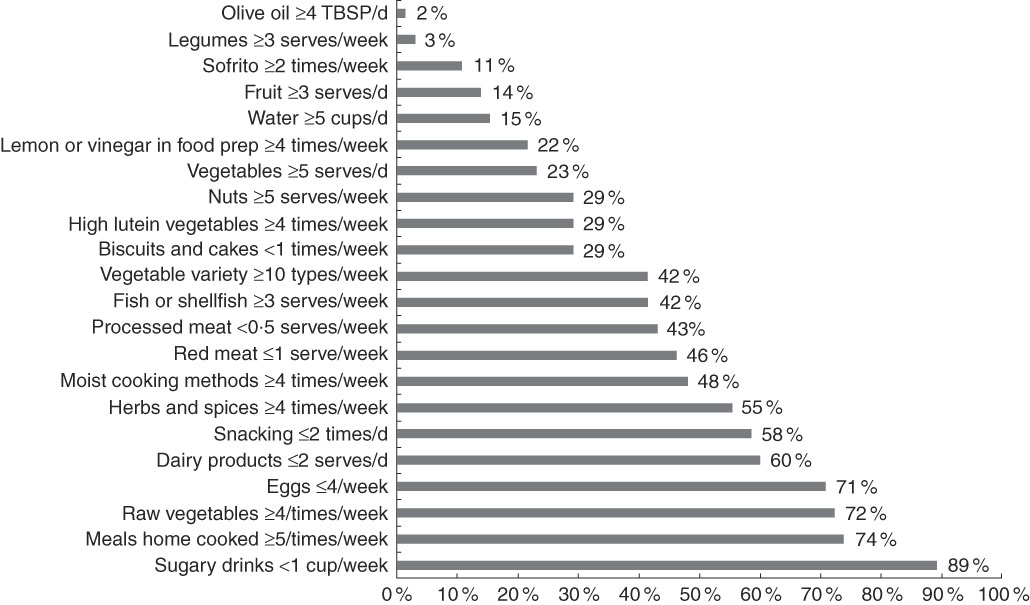
Fig. 2 Percentage of participants who reach Mediterranean diet thresholds according to 3-d food records (n 65). TBSP, tablespoon; prep, preparation.
Reliability
The reliability of the MediCul tool, based on single measures and 95 % CI of the ICC, was very good (ICC=0·93, 95 % CI 0·884, 0·954, P<0·0001), indicating that the total score was measured similarly at the two time points (A and B). The Bland–Altman test for repeated measures showed a mean difference between the two scores of −0·04, with a lower LOA of −9·7 and an upper LOA of 9·6. In all, sixty-six of the sixty-eight (97 %) participants fell within or on the lower and upper LOA with a fairly even distribution across mean scores. There was also no indication of bias according to the regression coefficient (y=−0·79+0·01x) (95 % CI −0·082, 0·109; P=0·778), supporting the null hypothesis that the scores at two time points were equally variable.
Groups that performed well for percentage agreement within the same category at time points A and B were as follows: wholegrains and coffee (almost perfect agreement); fruit, nuts, fish/shellfish, eggs, white meat preference, water and alcohol (substantial agreement); and olive oil, dairy products, red/processed meats, sweets and sugary drinks (moderate agreement). Groups with fair agreement within the same category were vegetables, legumes, takeaway and cuisine( Reference Landis and Koch 45 ) (online Supplementary Material 3). No groups had poor agreement for the proportion within each category at the two time points.
Validity
We assessed paired t tests for scores from the survey administered at time points A, B and the mean of AB v. the FR (n 65). This analysis indicated a very similar mean difference and CI across the three comparisons, which were all significant (P<0·0001) (Table 2). We therefore used survey A time point for the remaining validity testing, given that this represented first time MediCul use as could be applied in future research.
Table 2 Mean difference from paired samples t tests for Mediterranean Diet and Culinary Index (MediCul) scores from surveys A, B, mean AB v. 3-d food record (FR, n 65) (Mean differences and 95 % confidence intervals)
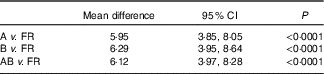
A, first administration of MediCul; B, second administration of MediCul; AB, mean of A and B MediCul administrations.
Bland–Altman analysis showed a positive mean difference of 6·0 between the MediCul score derived from survey A (52·8/97·0, sd 12·4, range: 31·5–83·5) and the FR (46·8/97·0, sd 10·8, range: 21·5–72·0). Scores for all but one participant fell within or on the 95 % LOA, with a lower LOA of −10·7 and an upper LOA of 22·6. There was also no significant linear trend for the fitted regression line (y=−2·30+0·17x) (95 % CI −0·027, 0·358; P=0·091), indicating no systematic bias between the two methods of measurement (Fig. 3).
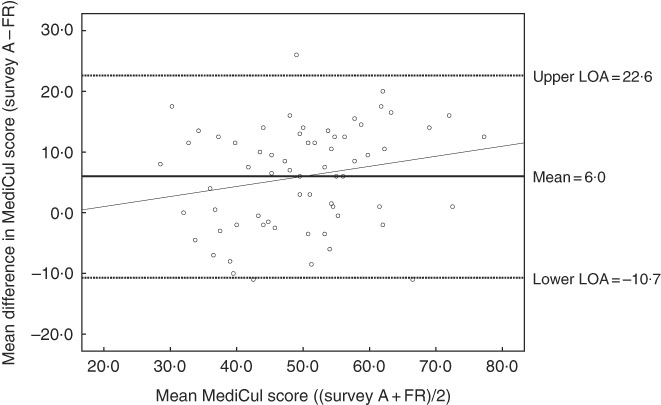
Fig. 3 Bland–Altman plot of the difference between Mediterranean Diet and Culinary Index (MediCul) score measured by survey A (first administration of MediCul) and 3-d food record (FR) and the mean MediCul score of the two methods (n 65). The solid line in the centre indicates the mean difference between the two methods and the dotted lines above and below indicate the limits within which 95 % of the differences between the methods are expected to fall (2sd above, and below, the mean difference). The fitted regression line is (y=−2·30+0·17x) (95 % CI −0·027, 0·358; P=0·091), indicating no systematic bias. LOA, limits of agreement.
Significant linear trends in relation to tertiles of MediCul score were identified for the following nutrients, consistent with what would be expected for a Mediterranean dietary pattern: total fat (g and %), including PUFA and MUFA (g), which increased across tertiles of MediCul score from both the survey and FR (Table 3). This also translated into highly significant trends for ratios of MUFA to SFA and total unsaturated fatty acids to SFA (P<0·0001). Further, dietary fibre, vitamin C, vitamin E and Mg all increased with increasing tertiles of MediCul score from both methods. Conversely, a significant trend for the reduction in carbohydrate as percentage of energy was observed across tertiles of MediCul score from both methods. Protein was either unrelated (g) or significantly decreased (percentage energy) when comparing tertiles from the survey (P=0·041), consistent with the fact that a Mediterranean diet is not a high-protein diet. In some instances, there was a trend for nutrients by tertiles of scores from the FR but not the survey (and vice versa) – for example, n-3 LC PUFA. There was no trend observed for total folate. In all cases linear trends were significant, except for Na when compared with tertiles from the FR, and sugars when compared with tertiles from survey A, where the quadratic trend provided a better fit for the data.
Table 3 Nutrient intakes compared with tertiles of Mediterranean Diet and Culinary Index (MediCul) score (n 65)Footnote * (Mean values and standard deviations; medians and interquartile ranges (IQR))
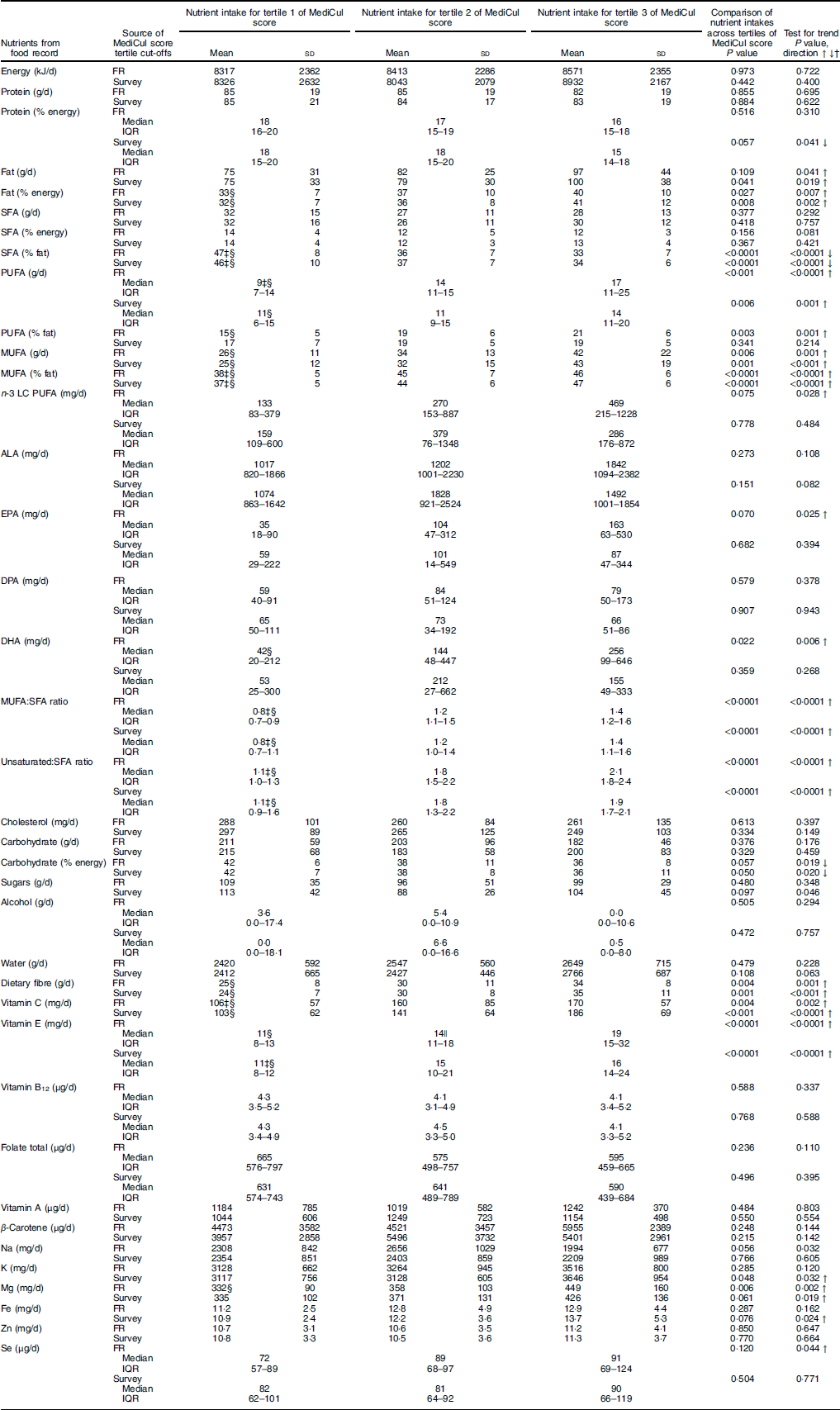
FR, food record; LC, long chain; ALA, α-linolenic acid; DPA, docosapentaenoic acid.
* Tertiles are derived for MediCul index scores from both the FR and survey A (first administration of MediCul). For survey A, the cut-offs for tertiles 2 and 3 were 47·0 and 58·0, respectively. Values are presented as means and standard deviations for normally distributed data or medians and IQR for non-normally distributed data. These data were normalised by logarithmic transformation for use in ANOVA models with the exception of alcohol and DHA, which were not able to be normalised and were therefore analysed using Kruskal–Wallis model. When ANOVA F ratio was significant, variances were checked for equality, and Bonferroni was applied for equal variances or Games–Howell post hoc t test for unequal variances. First (linear)- and second-(quadratic) order polynomial contrasts were applied to test for trends across tertiles, as well as line of best fit. In all cases, linear trends were significant, and there were no significant deviations from normality, except for Na when compared with tertiles from the FR and sugars when compared with tertiles from survey A, where the quadratic trend was positive and the most significant.
† Trend direction indicated as ↑ (increasing) or ↓ (decreasing).
‡ Significant differences between tertiles 1 v. 2.
§ Significant differences between tertiles 1 v. 3.
|| Significant differences between tertiles 2 v. 3.
MediCul precision was stable across a range of cognition (from normal (≤5) to MCI (5–12)) using ADAS-Cog as a measure of global cognitive performance.
Discussion
MediCul is a short survey index tool (takes 20 min to complete, on average) developed to assess adherence to a ‘traditional’ Mediterranean dietary pattern and certain aspects of cuisine, within a Western population. On the basis of our analyses, MediCul has very good reliability and moderate validity relative to a FR, among older individuals with MCI. To our knowledge, this is the first Mediterranean diet index tool to be validated in a group at higher risk of dementia. Our results cannot be generalised to younger or cognitively unimpaired individuals without further testing. In addition, our participants were originally volunteers for a randomised clinical trial and well educated, which may have influenced our results. Although we chose not to exclude participants based on extreme energy intakes, these were nevertheless all within plausible limits( Reference Willett 46 ).
The MediCul tool over-estimated the mean total score compared with its reference method by 6 %, and this was similar to the findings for MEDAS in Spanish (5 %) and German (9 %) cohorts( Reference Schroder, Fito and Estruch 20 , Reference Hebestreit, Yahiaoui-Doktor and Engel 25 ). However, it is well known that questionnaires tend to over-estimate intakes compared with FR( Reference Klipstein-Grobusch, Den Breeijen and Goldbohm 47 ). Although there was a considerable range for LOA from the Bland–Altman method when comparing scores from the MediCul tool v. the FR, and it is unknown whether this may have clinical implications, no systematic bias was found across mean scores. Hence, although the new index tool may be under- and over-estimating the FR-derived MediCul score by 11 and 23 %, respectively, the Spanish cohort MEDAS scores were under- and over-estimated by 43 and 53 %, respectively, compared with FFQ estimates( Reference Schroder, Fito and Estruch 20 ). Further, the under- and over-estimates for the MediCul tool are of a similar range reported for an alternate diet quality index score( Reference Huybrechts, Vereecken and De Bacquer 48 ), and well within limits proposed by Ambrosini et al. ( Reference Ambrosini, Van Roosbroeck and Mackerras 49 ) who classified agreement between an FFQ and FR as being acceptable when LOA were between 50 and 200 %.
MediCul captures wide elements of the Mediterranean dietary pattern as a continuous measure. The cut-off points used and nutrient patterns identified suggest that diet quality may be improving with an increased MediCul score. For example, with increasing tertiles of the MediCul score, there is a significant increase in healthy fats (and ratios of MUFA or total unsaturated fats to SFA), as well as dietary fibre, vitamin C and vitamin E, whereas carbohydrate as percentage energy declines correspondingly, and protein remains the same or decreases slightly. These directions are as anticipated for a ‘traditional’ Mediterranean diet, and macronutrient levels in the third tertile approximate a Mediterranean diet model proposed in Australia( Reference George, Kucianski and Mayr 50 ). For example, the macronutrient proportions in the third tertile of MediCul score from the index tool were as follows: fat, 41 % of energy; protein, 15 % of energy; carbohydrate, 36 % of energy; and MUFA, 47 % of total fat. No trend was observed for total folate, probably a result of fortification in the Australian food supply, making interpretation of folate intakes difficult without additional and specific questions to assess this nutrient.
In our cohort of MCI participants, few reached Mediterranean diet thresholds for adequate intake of certain protective foods, such as olive oil, legumes, fruit and water, and under half met our criterion for high vegetable variety, adequate fish intake and limited red/processed meat intake (Fig. 2). The mean scores from the MediCul tool and the derived MEDAS were also moderately low: 54·6/100·0 (sd 13·0) and 6·1/14·0 (sd 2·2), respectively. As a MediCul score of ≥81·5 was equivalent to a MEDAS score of ≥10·0, a level associated with cognitive benefit in the PREDIMED trial( Reference Valls-Pedret, Sala-Vila and Serra-Mir 30 ), it is of concern that only 3/68 (4·4 %) of older participants with MCI included in our study scored in this range. These findings suggest that individuals with MCI living in Western countries, even those who are well-educated, may not be optimally protected by a Mediterranean dietary pattern, which is recommended for chronic disease prevention by US( 51 ) and Australian( 52 ) dietary guidelines and the National Health Service( 53 ) in the UK. Future studies, however, are required to determine the direction of this relationship, as reverse causality is possible.
Limitations
Individual diets are complex and tend to vary over time, making measurement errors inevitable for all dietary methods( Reference Willett 54 ). The best methods for assessing populations at risk of dementia are yet to be elucidated( Reference Bowman, Shannon and Ho 55 ). The MediCul tool relies on self-reported data that could bias our results, especially given the cohort investigated. Yet there is limited research on cognitive status impact on the integrity of self-reported dietary data( Reference Zuniga and McAuley 56 ). One small study, including MCI participants of a similar age to our participants, found that cognitive impairment may inflate reliability and decrease validity of a FFQ( Reference Bowman, Shannon and Ho 55 ), which is not inconsistent with our findings. Further, MediCul has not yet been validated against disease risk factors and health outcomes or using biochemical measures of food intake, as has been reported for MEDAS( Reference Hebestreit, Yahiaoui-Doktor and Engel 25 , Reference Díez-Espino, Buil-Cosiales and Serrano-Martínez 57 – Reference Schröder, Marrugat and Vila 59 ), and there has generally been limited use of biomarkers to investigate the relationship between diet and cognitive function( Reference Zuniga and McAuley 56 ). However, this type of validation may be most relevant for the assessment of absolute nutrient intakes rather than an index for an overall dietary pattern. Although most FFQ solicit information about intake over the past year( Reference Thompson and Subar 22 ), the MediCul tool asks participants about their last 6 months, which together with specific questions relating to cooking methods for both warmer and cooler weather may address some seasonal variation. However, this time period may still be problematic for information retrieval among individuals with MCI, although it has been reported that if the information recalled is considered inadequate, respondents rely on general knowledge of what they routinely eat( Reference Zuniga and McAuley 56 ). We also had a dietitian present, available to answer questions and check that responses were complete, limiting conclusions about other types of administrations or if the tool is entirely self-administered. Finally, the primary measure of global cognition (ADAS-Cog), assessed at the same time point for the whole cohort in our validity study, was taken, on average, 59 months earlier and it is possible that some participants may have reverted to normal cognition, inflating our results.
To reduce participant burden, we required only a 3-d FR using household measures, which is not ideal for foods that are not consumed daily. However, 3- to 4-d records appear acceptable as it has been reported that the validity of collected information decreases in the latter days of a 7-d record, with recording periods of more than 4 d thought to be unsatisfactory owing to fatigue/disinterest, creating reactivity bias( Reference Thompson and Subar 22 ). In common with most other indexes, no energy adjustment was made for age or sex, which is unavoidable with tools designed for easy use. The FoodWorks nutritional analysis programme has some limitations, with missing foods and categorisation used for some food groups; however, we adjusted for this manually. FoodWorks also contains Australian compositional data but this is unlikely to vary in ways that would influence reliability and validity of MediCul for use in other countries.
Strengths
Small-scale indexes such as screeners may not capture extreme levels of intakes, leading to over-estimation of associations with health outcomes( Reference Panagiotakos, Pitsavos and Stefanadis 60 ). More comprehensive surveys may also have higher validity( Reference Martinez-Gonzalez, Fernandez-Jarne and Serrano-Martinez 61 ), although a ceiling of validity may exist( Reference Willett 54 ). MediCul may be likened more to the larger-scale modified MedDietScore index tool, which has scoring from 0 to 130( Reference Panagiotakos, Kalogeropoulos and Pitsavos 62 ), yet it is relatively quick to complete and compute scoring for, compared with a typical FFQ. MediCul also measures some unique aspects of Mediterranean cuisine such as high-moisture, lower-temperature cooking methods; frequent use of herbs and spices; and exposure to fermented foods such as olives. Such elements have been recommended to improve calculation of Mediterranean diet scores( Reference Hoffman and Gerber 63 ). In its development, MediCul considered various best practice guidelines for dietary assessment( Reference Cade, Burley and Warm 64 ) now advised by The DIETary Assessment Tool NETwork( Reference Cade, Warthon-Medina and Albar 65 ). The fact that a MEDAS score can also be derived from MediCul improves its utility so that comparisons with different studies, for various outcomes, can also be made.
Conclusions
Preventing or slowing cognitive decline may have a significant impact on the lives of individuals, families and carers, as well as future public health budgets. The Mediterranean diet is a promising lifestyle modality based on current evidence. Accurate measurement of adherence to the ‘traditional’ dietary pattern, among at-risk individuals or those with existing cognitive impairment, is vital to progress the field. We found that MediCul is a reliable and moderately valid tool to assess adherence to a Mediterranean dietary pattern among individuals with MCI who are at higher risk of converting to dementia. In our cohort of older Australians with MCI, the mean MediCul score was moderately low, suggesting poor compliance to this dietary pattern. MediCul may be a useful tool for future studies testing a Mediterranean diet intervention for various stages of cognitive decline, including MCI.
Acknowledgements
The authors thank Kenneth Daniel and Yian Noble for assistance with operationalising MediCul scoring. The authors are grateful to Fiona O’Leary and Lynne Cobiac for comments on a draft of the MediCul tool. The authors also acknowledge Adela Yip for help with data entry and greatly appreciate the involvement of all SMART participants.
The original SMART trial was funded by a National Health and Medical Research Council (NHMRC) of Australia Dementia Research Grant, project grant ID no. 512672 from 2008 to 2011. Additional funding for a research assistant position was provided by NHMRC Program grant ID no. 568969, and the project was supported by the University of Sydney and University of New South Wales. M. V. was supported by a University of New South Wales Vice Chancellor’s Fellowship and consecutive NHMRC Clinical Career Development Fellowships. The original trial fulfilled a portion of the degree requirements for PhD for N. G. and C. S. This validity study did not receive financial support from any organisation and fulfilled a portion of the degree requirements for PhD for S. R.-V.
The authors’ contributions are as follows: S. R.-V. contributed to study design, data collection, data analyses, interpretation of findings and wrote the manuscript; S. R.-V. in conjunction with V. M. F. prepared the first draft of the MediCul tool; V. M. F. and M. A. F. S. contributed to study design, statistical analyses and interpretation of findings. M. A. F. S. and all other authors with the exception of S. R.-V. and V. M. F. contributed to the funding acquisition, study design and/or data collection/management and statistical analysis for the original SMART trial and the follow-up assessments of participants. All authors provided critical review and approved the final version of the manuscript.
The authors declare no personal or financial conflicts of interest.
Supplementary materials
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002428









