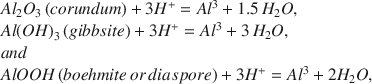Volume 36 - Issue 5 - October 1988
Research Article
Acid Dissolution of Akaganiéite and Lepidocrocite: The Effect on Crystal Morphology
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 385-390
-
- Article
-
- You have access
- Export citation
Relative Solubility of Corundum, Gibbsite, Boehmite, and Diaspore at Standard State Conditions
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 391-396
-
- Article
-
- You have access
- Export citation
Reactions of Polynuclear Hydroxyaluminum Cations with Montmorillonite and the Formation of A 28-Å Pillared Complex
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 397-402
-
- Article
-
- You have access
- Export citation
Modified Clays for the Adsorption of Environmental Toxicants: Binding of Chlorophenols to Pillared, Delaminated, and Hydroxy-Interlayered Smectites
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 403-408
-
- Article
-
- You have access
- Export citation
Hypothetical Structures of Magadiite and Sodium Octosilicate and Structural Relationships Between the Layered Alkali Metal Silicates and the Mordenite- and Pentasil-Group Zeolites
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 409-418
-
- Article
-
- You have access
- Export citation
Cacoxenite in Miocene Sediments of the Maryland Coastal Plain
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 419-424
-
- Article
-
- You have access
- Export citation
Celadonite and its Transformation to Smectite in an Entisol at Red Rock Canyon, Kern County, California
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 425-431
-
- Article
-
- You have access
- Export citation
Effect of Magnesium on the Hydraulic Conductivity of Na-Smectite-Sand Mixtures
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 432-438
-
- Article
-
- You have access
- Export citation
Kaolinization of Bauxite: A Study of the Vlasenica Bauxite Area, Yugoslavia. II. Alteration of Oolites
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 439-447
-
- Article
-
- You have access
- Export citation
Weathering Sequence and Alteration Products in the Genesis of the Graskop Manganese Residua, Republic of South Africa
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 448-454
-
- Article
-
- You have access
- Export citation
Determination of Surface Free Energy of Kaolinite
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 455-461
-
- Article
-
- You have access
- Export citation
Effect of Compaction Pressure and Water Content on the Thermal Conductivity of Some Natural Clays
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 462-466
-
- Article
-
- You have access
- Export citation
Influence of Manganese Oxide Minerals on the Formation of Iron Oxides
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 467-475
-
- Article
-
- You have access
- Export citation
Note
Use of X-ray Powder Diffraction and Linear Dichroism Methods to Study the Orientation of Montmorillonite Clay Particles
-
- Published online by Cambridge University Press:
- 02 April 2024, pp. 476-479
-
- Article
-
- You have access
- Export citation
Book Review
Chemistry of Clays and Clay Minerals, Edited by A. C. D. Newman. Wiley-Interscience, New York, Mineralogical Society Monograph 6, 1987. 469 pp., hardbound, US$110.00 (ISBN 0-471-01141-X.
-
- Published online by Cambridge University Press:
- 02 April 2024, p. 480
-
- Article
-
- You have access
- Export citation